Abstract
Hepatitis C virus (HCV) recurrence is the most important complication in HCV liver transplant patients. Boceprevir with pegylated interferon and ribavirin (PegIFN/RBV) enabled improvement in sustained virological response rates of patients with genotype 1 HCV. Boceprevir interacts with immunosuppressive therapy (IT) by inhibiting the cytochrome P450 3A enzyme. Our aim was to study interactions and assess the safety of boceprevir in the context of HCV recurrence. Boceprevir (800 mg three times a day) initiated after a 4-week lead-in phase was associated with cyclosporine (three patients), tacrolimus (two patients), and everolimus (one patient) in five liver transplant patients with genotype 1 HCV infection who experienced HCV recurrence. The mean follow-up period after HCV therapy was 14.8 ± 3.1 weeks. Estimated oral clearances of IT decreased on average by 50%, requiring reduced dosing regimens. Anemia occurred in all patients, with a mean fall in hemoglobin levels between baseline and week 12 of 3.12 ± 2.27 g/dl. All patients required administration of β-erythropoietin (n = 5), three needed ribavirin dose reduction, and one needed a blood transfusion. A virological response was observed in all patients (mean HCV viral load [HVL] decrease at week 12, 6.64 ± 0.35 log10 IU/ml). These preliminary results in liver transplant patients with HCV recurrence demonstrate the feasibility and safety of coadministration of boceprevir and IT.
INTRODUCTION
End-stage liver disease due to hepatitis C virus (HCV) is still a common indication for liver transplantation (LT) (6). However, a recurrence of HCV infection is the most frequent cause of death and graft loss, accounting for two-thirds of graft failures. The natural history of HCV is accelerated after LT, leading to cirrhosis in 20 to 30% of patients 5 years post-LT (4). The decompensation rate is higher than 70% at 3 years in liver transplant recipients with established cirrhosis, versus less than 10% in immunocompetent patients (4). Progression from decompensation to death is also accelerated after LT, with a 3-year survival rate of <10% following the onset of HCV-related allograft failure (4). Few patients (<5%) experience cholestatic hepatitis, but their prognosis is the poorest (7). To prevent or to treat these occurrences, standard anti-HCV therapy, represented by pegylated interferon and ribavirin, can achieve a sustained virological response (SVR) in 30% of patients, who have a less severe outcome and lower mortality than nonresponders (21). Boceprevir is a novel peptidometic NS3 protease inhibitor that forms a covalent and reversible complex with the NS3 protease in vitro in the HCV replicon system. Recently, two phase III trials showed that combining boceprevir with pegylated interferon and ribavirin increased SVR rates in naive and previously treated nontransplant patients with genotype 1 HCV from 38% to 66% (SPRINT-2) and 21% to 66% (RESPOND-2), respectively (2, 20). The administration of such drugs in the context of HCV recurrence on the liver graft is one of the most important clinical challenges in the field of LT. One limitation is the potential for interaction with calcineurin inhibitors such as cyclosporine and tacrolimus (5). Two distinct pathways extensively metabolize boceprevir, the cytochrome P450 (CYP) (mainly CYP3A) and aldo-ketoreductase pathways (11). Boceprevir is a potent inhibitor of CYP3A4 based on the results of in vitro assessments and of drug-drug interaction studies performed with oral midazolam, with coadministration increasing more than 5-fold the midazolam exposure (17). It was recently demonstrated in healthy volunteers that an intake of boceprevir enhanced a single dose of cyclosporine or tacrolimus (12). However, the effect of such antiviral therapy on cyclosporine, tacrolimus, and everolimus exposure is currently unknown in the context of LT. We describe here the use of boceprevir with the subsequent coadministration of cyclosporine, tacrolimus, or everolimus in liver transplant patients with HCV recurrence on their liver graft.
MATERIALS AND METHODS
Study design and patient characteristics.
This pilot study included five consecutive patients for whom the medical staff in our center (Centre Hépato-Biliaire) decided to introduce boceprevir with pegylated interferon and ribavirin because of HCV recurrence on the graft. Having given them details of the study, we obtained written informed consent from all the patients before initiating therapy. The same regimen of anti-HCV therapy was given to all patients together with boceprevir 800 mg three times daily (TID), which was started after a 4-week lead-in phase of standard therapy with pegylated interferon (α2a or α2b) and ribavirin.
The demographic characteristics of the patients are summarized in Table 1. All five patients were men, with a mean age of 62.6 ± 8.3 years (range, 50 to 72) at the time of anti-HCV therapy. The indications for LT were end-stage liver disease caused by a genotype 1 HCV infection (patient 2, genotype 1a; patients 1, 3, 4, and 5, genotype 1b), including hepatocellular carcinoma (patients 2, 3 and 4). Three patients had undergone a previous course of standard anti-HCV therapy (patients 1 and 4, who experienced a relapse, and patient 5, who was a nonresponder). Of the two posttransplant naive patients (patients 2 and 3), one had been a nonresponder before LT (patient 2). Immunosuppression regimens since LT had been based on calcineurin inhibitors: cyclosporine in three patients and tacrolimus in two patients. Baseline trough concentrations were stable. Four patients had received other immunosuppressive drugs: prednisone (patients 1 and 2), mycophenolate mofetil (MMF) (patients 2 and 5), or everolimus (patient 3). The severity of HCV recurrence was assessed by pathological examination, including an analysis of the degree of mononuclear cell infiltration and the degree of fibrosis according to the METAVIR score (3). Patients experienced symptoms of HCV recurrence which included cholestatic hepatitis (CH) (patient 2) and chronic hepatitis with advanced fibrosing stage F2 (patient 3), F3 (patient 5), or F4 (patients 1 and 4). The mean delay between LT and the initiation of anti-HCV therapy was 48.6 ± 37.9 months (range, 3 to 105).
Table 1.
Clinical and biological characteristics of patients at baseline, modalities of anti-HCV therapy, and immunosuppressive regimena
| Characteristic | Result |
||||
|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
| Age (yr), gender | 60, M | 50, M | 64, M | 67, M | 72, M |
| Body mass index (kg/m2) | 28.7 | 23.5 | 20.9 | 24.6 | 26.4 |
| Indication for LT | Cirrhosis | HCC | HCC | HCC | Cirrhosis |
| MELD score at listing | 24 | 10 | 12 | 22 | 28 |
| Calcineurin inhibitor | Cyclosporine | Cyclosporine | Cyclosporine | Tacrolimus | Tacrolimus |
| Other immunosuppressive drug(s) | Prednisone (5 mg QD) | Prednisone (5 mg QD), MMF (500 mg BID) | Everolimus (0.25 mg BID) | None | MMF (250 mg BID) |
| Baseline MELD score | 11 | 10 | 9 | 9 | 7 |
| Interval between LT and antiviral therapy (mo) | 62 | 3 | 36 | 105 | 36 |
| HCV genotype | 1b | 1a | 1b | 1b | 1b |
| Recipient IL-28b genotype | CC | CT | CT | CC | TT |
| Activity (A)/fibrosis stage (F) (METAVIR score) | A2/F4 | CH | A2/F2 | A2/F4 | A2/F3 |
| Baseline total bilirubin (μmol/liter) | 38 | 48 | 12 | 12 | 9 |
| Baseline GGT (IU/liter) | 179 | 3,227 | 391 | 319 | 38 |
| Baseline ALT (IU/liter) | 127 | 389 | 251 | 801 | 59 |
| Baseline INR | 1.2 | 1.0 | 0.9 | 1.1 | 0.9 |
| Baseline creatinine clearance (ml/min) | 111 | 114 | 50 | 57 | 67 |
| Baseline hemoglobin (g/dl) | 12.6 | 12.4 | 11.1 | 14.9 | 13.9 |
| Baseline neutrophil count (g/liter) | 5.9 | 1.6 | 1.8 | 3.2 | 2.3 |
| Baseline platelet count (g/liter) | 103 | 123 | 115 | 147 | 97 |
| Baseline albumin (g/liter) | 25.2 | 33.3 | 32.7 | 29.3 | 37.8 |
| Baseline HCV viral load (log10 IU/ml) | 6.30 | 7.97 | 7.1 | 6.58 | 6.33 |
| Peg-IFN dosage | Alpha-2b 1.4 μg/kg/week | Alpha-2b 1.3 μg/kg/week | Alpha-2a 180 μg/week | Alpha-2b 1.4 μg/kg/week | Alpha-2b 1.3 μg/kg/week |
| Ribavirin dosage | 7 mg/kg/day | 15 mg/kg/day | 12 mg/kg/day | 14 mg/kg/day | 8 mg/kg/day |
ALT, alanine aminotransferase; BID, twice a day (bis in die); CH, cholestatic hepatitis; GGT, gamma-glutamyl transferase; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IL-28B, interleukin 28B; INR, international normalized ratio; LT, liver transplantation; M, male; MELD, model for end-stage liver disease; MMF, mycophenolate mofetil; Peg-IFN, pegylated interferon; QD, once a day (quaque die).
Drug assay, pharmacokinetic analysis, virological monitoring, and interleukin 28b (IL-28B) genotyping.
This study was performed as part of the routine clinical care of liver transplant recipients requiring close monitoring of their immunosuppressive drugs. The dosing regimens of immunosuppressive drugs were adjusted to reach a therapeutic range that differed as a function of the time elapsing since transplantation. The target trough concentrations ranged from 50 to 150 ng/ml for cyclosporine, from 5 to 10 ng/ml for tacrolimus, and from 3 to 8 ng/ml for everolimus. Blood samples were drawn before immunosuppressive drug intake to measure trough concentrations (Cmin) at a steady state after the liver transplant, at the end of the lead-in phase (week 4), on the day of boceprevir initiation, and every day thereafter. Whole-blood concentrations were assayed using a chemiluminescent microparticulate immunoassay (CMIA) on an Architect autoanalyzer for cyclosporine and tacrolimus and by liquid chromatography coupled to tandem mass spectrometry (LCMSMS) for everolimus. The limits of quantification were 30 ng/ml, 2 ng/ml, and 0.75 ng/ml, respectively, for cyclosporine, tacrolimus, and everolimus. The laboratory was a participant in an international external quality control scheme (Analytical Services International Ltd., London, England). At the steady state, the oral clearances (CLO) of immunosuppressive drugs were roughly estimated from the ratio of dose per unit of time (dose/time interval between 2 doses) over Cmin.
Serum HCV RNA levels (Realtime HCV; Abbott Molecular Inc., May 2011; limit of detection, 12 IU/ml) were determined at baseline and at weeks 2, 4, 6, 8, 10, and 12.
Recipient DNA was tested for the interleukin 28B polymorphism rs12979860 C/T using the ABI TaqMan allelic discrimination kit and the ABI7900HT sequence detection system (Applied Biosystems, Carlsbad, CA).
Safety assessment endpoints.
At baseline and during follow-up, safety was assessed by physical examination and laboratory tests (particularly liver function tests, creatinine clearance, hemoglobin levels, and neutrophil count). Pegylated interferon and ribavirin were initiated in the outpatient clinic. According to our local protocol for the management of transplant recipients, the administration of β-erythropoietin was started if the hemoglobin level dropped below 12 g/dl or the decrease of hemoglobin levels was >1 g/dl/week or if a transfusion had been required during previous anti-HCV therapy. If the hemoglobin level continued to fall, the ribavirin dose was reduced. The transfusion threshold was when the hemoglobin level fell below 8 g/dl. At week 4, patients were hospitalized before the initiation of boceprevir in order to monitor the pharmacokinetics of immunosuppressive drugs and their tolerance of the treatment. For each immunosuppressive drug, the predefined trough concentrations were targeted before starting boceprevir. The daily monitoring of biochemistry parameters was ensured during the first week (between weeks 4 and 5). Patients could be discharged if a steady state for the immunosuppressive drug was achieved and if no adverse events were reported. During the phase of anti-HCV therapy, patients underwent a physical examination and biochemistry follow-up, and spontaneous clinical adverse events were reported during scheduled study visits at weeks 6, 8, and 12.
RESULTS
Management of immunosuppressive drugs after the introduction of boceprevir.
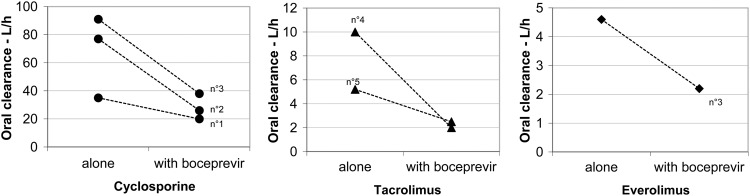
All patients had achieved a steady state of immunosuppressive therapy before the initiation of boceprevir. During the first week after the administration of 800 mg TID boceprevir, the dosing regimen of immunosuppressive drugs in four patients (patients 1, 2, 4, and 5; Table 2) had to be reduced to maintain trough blood levels within the defined target range. The dose remained unchanged at the steady state in one patient (patient 3), despite a rise in immunosuppressive drug concentrations, which nonetheless remained within the target range. A 50% to 80% reduction in estimated oral clearance was observed for all immunosuppressive agents: about 50% for cyclosporine, up to 80% for tacrolimus, and 52% for everolimus (Fig. 1). A steady state was achieved between days 3 and 7 in all patients (mean delay, 4.2 ± 1.8 days). All monitored blood chemistry parameters and concomitant medications remained unchanged, and no clinical events affecting the concentration of immunosuppressive drugs were reported.
Table 2.
Blood concentrations at steady state and dosage regimen of cyclosporine, tacrolimus, and everolimus before and after introduction of boceprevira
| Patient and treatment | Before boceprevir initiation |
After boceprevir initiation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At week 0 |
At week 4/day −1 |
Day 1 |
Day 2 |
Steady state |
At week 12 |
|||||||
| TBC | Change in CI dosage | TBC | Change in CI dosage | TBC | Change in CI dosage | TBC | Change in CI dosage | TBC | Change in CI dosage | TBC | Change in CI dosage | |
| Patient 1, cyclosporine | 42 | 50 mg + 25 mg | 90 | 50 mg + 25 mg | 182 | 25 mg BID | 131 | 25 mg BID | 105 | 25 mg BID | 137 | 25 mg BID |
| Patient 2, cyclosporine | 130 | 125 mg + 100 mg | 122 | 125 mg + 100 mg | 146 | 100 mg BID | NA | 75 mg BID | 241 | 75 mg BID | 149 | 75mg + 50 mg |
| Patient 3, cyclosporine | 20 | 25 mg BID | 23 | 25 mg BID | 30 | 25 mg BID | 41 | 25 mg BID | 55 | 25 mg BID | 30 | 25 mg BID |
| Patient 3, everolimus | 4.2 | 0.5 mg BID | 4.5 | 0.5 mg BID | 6.5 | 0.5 mg BID | 7.5 | 0.5 mg BID | 9.5 | 0.5 mg BID | 4.5 | 0.25 mg BID |
| Patient 4, prolonged-release tacrolimus | 6 | 1 mg QD | 4 | 1 mg QD | 7 | 0.5 mg QD | 11.6 | 0.5 mg QD | 12 | 0.5 mg QD | 5.8 | 0.5 mg 2 days in 3 |
| Patient 5, tacrolimus | 9.7 | 2 mg BID | 8 | 1 mg BID | 14.7 | 1 mg BID | 17.5 | 0.5 mg QD | 8.4 | 0.5 mg QD | 3.9 | 0.5 mg QD |
CI, calcineurin inhibitor; TBC, trough blood concentration (ng/ml); QD, once a day (quaque die); BID, twice a day (bis in die); NA, not available.
Fig 1.
Oral clearance of cyclosporine, tacrolimus, and everolimus when administered alone or concomitantly with boceprevir, at the steady state. A 50% to 80% reduction in estimated oral clearance was observed for all immunosuppressive agents: about 50% for cyclosporine, up to 80% for tacrolimus, and 52% for everolimus.
Virological response.
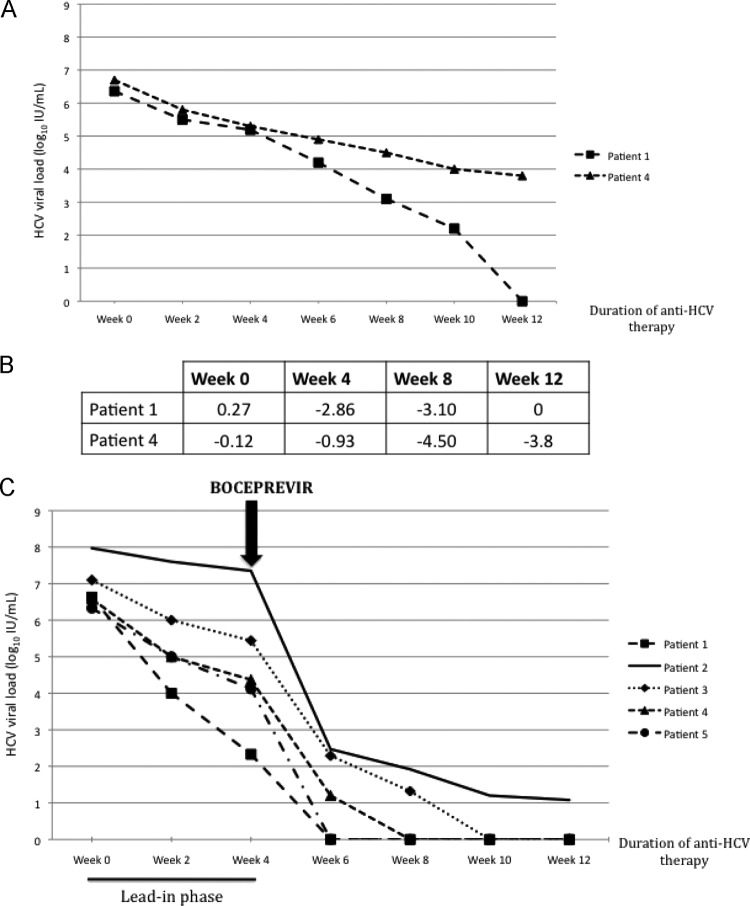
At baseline, the mean HCV viral load (HVL) was 6.87 ± 0.70 log10 IU/ml (range, 6.30 to 7.97) (Fig. 2). The mean period of follow-up since HCV therapy was 14.8 ± 3.1 weeks (range, 12 to 20). At week 4, the mean fall in the HVL was 2.20 ± 1.34 log10 IU/ml (range, 0.62 to 4.30). At week 8, the HVL was undetectable in three patients, with a mean reduction of 6.27 ± 0.36 log10 IU/ml (range, 5.78 to 6.63). At week 12, the HVL was undetectable in four patients (patients 1, 3, 4, and 5), and the HVL was 1.08 log10 IU/ml in one patient (patient 2). The mean fall in the HVL between baseline and week 12 was 6.64 ± 0.35 log10 IU/ml (range, 6.30 to 7.10).
Fig 2.
Kinetics of HCV viral loads between baseline and week 12. (A) Kinetics of HCV viral loads between baseline and week 12 during a previous course of dual therapy. Patients 1 and 4 were relapsers. Patient 5 data are unavailable. (B) Differences in HCV viral load (log10 IU/ml) for patients 1 and 4 between dual therapy and triple therapy from week 0 to week 12. (C) Kinetics of HCV viral loads between baseline and week 12 during triple therapy. At baseline, the mean HCV viral load (HVL) was 6.87 ± 0.70 log10 IU/ml (range, 6.30 to 7.97). Boceprevir was started in week 4, at 800 mg three times a day. The mean decrease in the HVL between baseline and week 12 was 6.64 ± 0.35 log10 IU/ml (range, 6.30 to 7.10).
Safety.
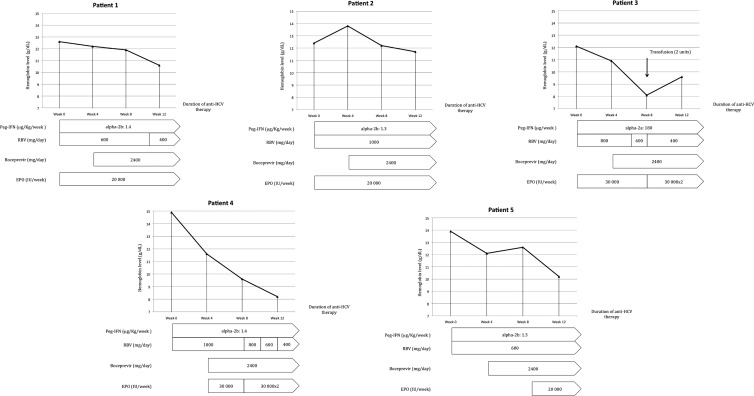
The median duration of hospitalization after the initiation of boceprevir was 7.0 ± 0.7 days (range, 5 to 9). No overdoses of immunosuppressive therapy were reported. The main adverse event was anemia, which occurred in all patients (Fig. 3). At baseline and week 12, the mean hemoglobin levels were 13.18 ± 1.18 g/dl (range, 12.1 to 14.9) and 10.4 ± 1.5 g/dl (range, 8.1 to 12.5), respectively. The mean decrease in hemoglobin levels between baseline and week 12 was 3.12 ± 2.27 g/dl (range, 0.7 to 6.7). All patients required the administration of β-erythropoietin at between 20,000 and 60,000 IU/week. In three patients (patients 1, 3, and 4), the ribavirin dosage was reduced (by between 33% and 75%) after week 8. One patient (patient 3) required a blood transfusion because his hemoglobin level fell below 8 g/dl. No impairment of renal function assessed by creatinine clearance was observed during boceprevir therapy, and no significant modifications to the neutrophil count were reported. The mean variation of creatinine clearance between week 0 and week 12 was −7.5 ± 13.1 ml/min (range, −29.5 to 2.7).
Fig 3.
Kinetics of hemoglobin levels during anti-HCV therapy and management. At baseline and week 12, the mean hemoglobin levels were 13.18 ± 1.18 g/dl (range, 12.1–14.9) and 10.4 ± 1.5 g/dl (range, 8.1 to 12.5), respectively. The mean fall in hemoglobin levels between baseline and week 12 was 3.12 ± 2.27 g/dl (range, 0.7 to 6.7). β-Erythropoietin was introduced in all patients if the hemoglobin level fell below 12 g/dl or if the hemoglobin level decreased by >1 g/dl/week and if a transfusion had been required during a previous anti-HCV therapy. In a second phase, the ribavirin dosage was reduced by between 33% and 75% in patients 1, 3, and 4. Only one patient required a transfusion because of a fall in the hemoglobin level to below 8 g/dl. In patients 2 and 5, MMF was maintained at the same dosage without a reduction of ribavirin. EPO, β-erythropoietin; RBV, ribavirin; Peg-IFN, pegylated interferon.
Patient 1 was hospitalized at week 5 for legionella pneumonia. His neutrophil count had been 1.1 g/liter the previous week (week 4) and 3.0 g/liter at admission. He recovered fully after antibiotic therapy based on ceftriaxone 2 g once a day (QD) for 2 days before bacterial identification, at which point it was combined with ofloxacine 200 mg BID and pursued for 10 days. The patient's anti-HCV therapy was not discontinued. No other clinical or biological events were reported, which enabled the pursuit of anti-HCV therapy in all patients after week 12.
DISCUSSION
We report here on our experience of introducing treatment with boceprevir in the context of LT. Our data showed for the first time that boceprevir can be administered safely in liver transplant recipients experiencing recurrent HCV infection despite a moderate drug-drug interaction with immunosuppressive therapy using either cyclosporine, tacrolimus, or everolimus. Although this study involved only a small number of patients, their virological responses were encouraging given the severity of HCV recurrence.
A number of potent drug-drug interactions have been described with the use of immunosuppressive drugs that are substrates of CYP3A (15, 18). The dosing regimens of these drugs, which display broad interpatient variability in terms of clearance and a narrow therapeutic window, can be optimized using therapeutic drug monitoring (13, 14, 24). Such pharmacological management has been applied successfully to drug-drug interactions between anti-calcineurin inhibitors and azole antifungal agents or HIV1-protease inhibitors (8, 19, 22).
Some data are available on the pharmacokinetics of boceprevir, which is biotransformed by CYP3A4, CYP3A5, and aldo-ketoreductase (17). Managing immunosuppressive drug dosing regimens involving combination with boceprevir can be difficult. Recently, the concomitant administration of immunosuppressive therapy with telaprevir, another HCV protease inhibitor, in healthy volunteers increased cyclosporine exposure approximately 4.6-fold and tacrolimus exposure 70-fold (10). Another recent study, published in abstract form, showed that the concomitant administration of a single dose of boceprevir and cyclosporine or tacrolimus in healthy volunteers increased the maximum concentration (Cmax) of cyclosporine 2-fold, the area under the curve (AUC) of cyclosporine 2.7-fold, the Cmax of tacrolimus 10-fold, and the AUC of tacrolimus 17-fold, while the pharmacokinetics of boceprevir remained unchanged (12). Although these observations in healthy volunteers offer interesting data, the application of such a regimen in a transplant population might produce quite different results (9).
During our study, the reduction in the oral clearance of these drugs indicated that boceprevir inhibits the biotransformation of coadministered immunosuppressive drugs. As previously described with midazolam, a typical CYP3A4/5 substrate, this interaction is most likely a consequence of intestinal and hepatic CYP3A4 inhibition, which is also involved in the metabolism of cyclosporine, tacrolimus, and everolimus (1). From a practical point of view, the interaction between boceprevir and immunosuppressive therapy required an average dose reduction of 50% of the three immunosuppressive drugs that are CYP3A substrates. We recommend follow-up for 7 days, with daily determinations of trough blood concentrations of the immunosuppressive drugs, until a steady state has been achieved. Although the greatest variations are expected in this period, the monitoring has to be prolonged throughout the duration of protease inhibitor therapy.
It should be pointed out that we studied only a small number of patients and that considerable interpatient variations in the potency of drug-drug interactions is a well-known phenomenon. Nevertheless, our data demonstrated mild and manageable drug-drug interactions between cyclosporine, tacrolimus, or everolimus and boceprevir. This early utilization resulted in part from our experience of anti-HCV therapy in severely affected patients and, particularly, in HIV patients, who can experience severe drug-drug interactions (23).
The treatment of anemia, which was the main adverse event, appeared to be manageable if close monitoring of hemoglobin levels was ensured. With respect to this complication, we encourage the early administration of β-erythropoietin, sometimes even before the initiation of anti-HCV therapy, as practiced in our center, to prevent this event. The use of mycophenolate mofetil in two patients did not seem to modify the severity or management of anemia.
We observed a virological response at 12 weeks in all the patients studied; this is a major predictive factor of SVR (21). The patient whose viral load remained positive at 12 weeks (1.08 log10 IU/ml) had displayed the highest HCV viral load at baseline (7.97 log10 IU/ml). Although it should be interpreted with caution, this virological response was encouraging when account was taken of the severity of HCV recurrence in these five patients.
Our findings not only emphasize that the administration of an HCV protease inhibitor such as boceprevir in patients with an HCV recurrence on the liver graft is feasible but also provide new perspectives in terms of new drugs that act directly against HCV. Recent results have indeed been very encouraging, with the combination of different direct-acting antiviral agents (16).
Our preliminary results in transplant patients with HCV recurrence should provide an incentive for the conduct of drug-drug interaction studies aimed at ensuring maximal safety and a sustained virological response.
ACKNOWLEDGMENTS
We disclose no conflicts of interest.
Audrey Coilly was responsible for acquisition of data, analysis and interpretation of data, and drafting of the manuscript; Valérie Furlan was responsible for analysis and interpretation of data and drafting of the manuscript; Bruno Roche was responsible for critical revision of the manuscript for important intellectual content; Caroline Barau was responsible for technical or material support; Coralie Noël was responsible for technical or material support; Laurence Bonhomme-Faivre was responsible for technical or material support; Teresa Maria Antonini was responsible for technical or material support; Anne-Marie Roque-Afonso was responsible for technical or material support; Didier Samuel was responsible for critical revision of the manuscript for important intellectual content; Anne-Marie Taburet was responsible for analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content; Jean-Charles Duclos-Vallée was responsible for study concept and design, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content.
Footnotes
Published ahead of print 20 August 2012
REFERENCES
- 1. Amundsen R, Asberg A, Ohm IK, Christensen H. 2012. Cyclosporine A- and tacrolimus-mediated inhibition of CYP3A4 and CYP3A5 in vitro. Drug Metab. Dispos. 40:655–661 [DOI] [PubMed] [Google Scholar]
- 2. Bacon BR, et al. 2011. Boceprevir for previously treated chronic HCV genotype 1 infection. N. Engl. J. Med. 364:1207–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bedossa P, Poynard T. 1996. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24:289–293 [DOI] [PubMed] [Google Scholar]
- 4. Berenguer M, et al. 2000. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology 32:852–858 [DOI] [PubMed] [Google Scholar]
- 5. Charlton M. 2011. Telaprevir, boceprevir, cytochrome P450 and immunosuppressive agents—a potentially lethal cocktail. Hepatology 54:3–5 [DOI] [PubMed] [Google Scholar]
- 6. Charlton M, et al. 2004. Long-term results and modeling to predict outcomes in recipients with HCV infection: results of the NIDDK liver transplantation database. Liver Transpl. 10:1120–1130 [DOI] [PubMed] [Google Scholar]
- 7. Dixon LR, Crawford JM. 2007. Early histologic changes in fibrosing cholestatic hepatitis C. Liver Transpl. 13:219–226 [DOI] [PubMed] [Google Scholar]
- 8. Dodds-Ashley E. 2010. Management of drug and food interactions with azole antifungal agents in transplant recipients. Pharmacotherapy 30:842–854 [DOI] [PubMed] [Google Scholar]
- 9. Fletcher CV. 2010. Drug interactions should be evaluated in patients. Clin. Pharmacol. Ther. 88:585–587 [DOI] [PubMed] [Google Scholar]
- 10. Garg V, et al. 2011. Effect of telaprevir on the pharmacokinetics of cyclosporine and tacrolimus. Hepatology 54:20–27 [DOI] [PubMed] [Google Scholar]
- 11. Ghosal A, et al. 2011. Characterization of human liver enzymes involved in the biotransformation of boceprevir, a hepatitis C virus protease inhibitor. Drug Metab. Dispos. 39:510–521 [DOI] [PubMed] [Google Scholar]
- 12. Hulskotte EGJ, et al. 2011. Pharmacokinetic interaction between the HCV protease inhibitor boceprevir and the calcineurin inhibitors cyclosporine and tacrolimus, abstr 123. Abstr. 16th Annu. Meet. HepDART, Koloa, HI [Google Scholar]
- 13. Kahan BD, et al. 1995. Reduced inter- and intrasubject variability in cyclosporine pharmacokinetics in renal transplant recipients treated with a microemulsion formulation in conjunction with fasting, low-fat meals, or high-fat meals. Transplantation 59:505–511 [PubMed] [Google Scholar]
- 14. Kovarik JM, Hsu CH, McMahon L, Berthier S, Rordorf C. 2001. Population pharmacokinetics of everolimus in de novo renal transplant patients: impact of ethnicity and comedications. Clin. Pharmacol. Ther. 70:247–254 [DOI] [PubMed] [Google Scholar]
- 15. Kuypers DR. 2008. Influence of interactions between immunosuppressive drugs on therapeutic drug monitoring. Ann. Transplant. 13:11–18 [PubMed] [Google Scholar]
- 16. Lok AS, et al. 2012. Preliminary study of two antiviral agents for hepatitis C genotype 1. N. Engl. J. Med. 366:216–224 [DOI] [PubMed] [Google Scholar]
- 17. Maddur H, Kwo PY. 2011. Boceprevir. Hepatology 54:2254–2258 [DOI] [PubMed] [Google Scholar]
- 18. Manitpisitkul W, McCann E, Lee S, Weir MR. 2009. Drug interactions in transplant patients: what everyone should know. Curr. Opin. Nephrol. Hypertens. 18:404–411 [DOI] [PubMed] [Google Scholar]
- 19. Marfo K, Greenstein S. 2009. Antiretroviral and immunosuppressive drug-drug interactions in human immunodeficiency virus-infected liver and kidney transplant recipients. Transplant. Proc. 41:3796–3799 [DOI] [PubMed] [Google Scholar]
- 20. Poordad F, et al. 2011. Boceprevir for untreated chronic HCV genotype 1 infection. N. Engl. J. Med. 364:1195–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roche B, et al. 2008. Hepatitis C virus therapy in liver transplant recipients: response predictors, effect on fibrosis progression, and importance of the initial stage of fibrosis. Liver Transpl. 14:1766–1777 [DOI] [PubMed] [Google Scholar]
- 22. Saad AH, DePestel DD, Carver PL. 2006. Factors influencing the magnitude and clinical significance of drug interactions between azole antifungals and select immunosuppressants. Pharmacotherapy 26:1730–1744 [DOI] [PubMed] [Google Scholar]
- 23. Teicher E, et al. 2007. Effect of highly active antiretroviral therapy on tacrolimus pharmacokinetics in hepatitis C virus and HIV co-infected liver transplant recipients in the ANRS HC-08 study. Clin. Pharmacokinet. 46:941–952 [DOI] [PubMed] [Google Scholar]
- 24. Zahir H, et al. 2005. Population pharmacokinetic estimation of tacrolimus apparent clearance in adult liver transplant recipients. Ther. Drug Monit. 27:422–430 [DOI] [PubMed] [Google Scholar]