Abstract
Posaconazole has an important role in the prophylaxis and salvage treatment of invasive fungal infections (IFIs), although poor and variable bioavailability remains an important clinical concern. Therapeutic drug monitoring of posaconazole concentrations has remained contentious, with the use of relatively small patient cohorts in previous studies hindering the assessment of exposure-response relationships. This multicenter retrospective study aimed to investigate relationships between posaconazole concentration and clinical outcomes and adverse events and to assess clinical factors and drug interactions that may affect posaconazole concentrations. Medical records were reviewed for patients who received posaconazole and had ≥1 concentration measured at six hospitals in Australia. Data from 86 patients with 541 posaconazole concentrations were included in the study. Among 72 patients taking posaconazole for prophylaxis against IFIs, 12 patients (17%) developed a breakthrough fungal infection; median posaconazole concentrations were significantly lower than in those who did not develop fungal infection (median [range], 289 [50 to 471] ng/ml versus 485 [0 to 2,035] ng/ml; P < 0.01). The median posaconazole concentration was a significant predictor of breakthrough fungal infection via binary logistic regression (P < 0.05). A multiple linear regression analysis identified a number of significant drug interactions associated with reduced posaconazole exposure, including coadministration with proton pump inhibitors, metoclopramide, phenytoin or rifampin, and the H2 antagonist ranitidine (P < 0.01). Clinical factors such as mucositis, diarrhea, and the early posttransplant period in hematopoietic stem cell transplant recipients were also associated with reduced posaconazole exposure (P < 0.01). Low posaconazole concentrations are common and are associated with breakthrough fungal infection, supporting the utility of monitoring posaconazole concentrations to ensure optimal systemic exposure.
INTRODUCTION
Posaconazole is a triazole antifungal that has gained widespread clinical acceptance in the prophylaxis and salvage treatment of invasive fungal infections (IFIs) due to its broad-spectrum activity and evidence of superiority over itraconazole and fluconazole for the prevention of IFIs in neutropenic patients (6, 40). Posaconazole has low and highly variable bioavailability due to saturable oral absorption, which is influenced by poor dissolution and intestinal pH, resulting in disproportionately increased exposure when administered in divided doses (25). Coadministration with food has been shown to increase posaconazole bioavailability by up to 4-fold (20); conversely, medications such as proton pump inhibitors have been shown to significantly decrease posaconazole exposure due to increased gastric pH (41). Low posaconazole concentrations have been commonly reported in clinical practice (3, 10, 23, 31, 37, 39), with the current lack of an intravenous formulation of the drug complicating efforts to increase posaconazole exposure, particularly in critically ill patients (34).
While therapeutic drug monitoring (TDM) is widely used for other triazole antifungal agents, such as voriconazole and itraconazole (2), the need to monitor plasma posaconazole concentrations has remained contentious (7, 17). Despite this, increasing evidence supports a clinically useful exposure-response relationship for posaconazole, with posaconazole concentrations of >700 ng/ml associated with a reduced risk of breakthrough fungal infections when used as antifungal prophylaxis (9).
Previous studies of posaconazole TDM have typically included small patient cohorts from a single institution (3, 10, 23, 31, 37). Using a multicenter retrospective design, this study aimed to investigate relationships between posaconazole concentration and clinical outcomes and adverse events, while also examining potential drug-drug interactions and clinical factors that may affect posaconazole concentrations.
MATERIALS AND METHODS
Patient enrollment and data collection.
All patients aged 18 years or older who received posaconazole and had at least one posaconazole concentration measured during therapy at six hospitals in Australia between December 2008 and December 2010 were eligible for inclusion. All posaconazole concentration data were collected from a central referral laboratory (SydPath, St. Vincents Hospital, Sydney, Australia). A validated high-pressure liquid chromatography (HPLC) assay was used to measure posaconazole concentrations in blood (5). Patient medical records were individually reviewed using a standardized data collection template at each study site to collect demographic information and clinical data on outcomes of therapy and adverse events as well as posaconazole dosing information and concomitant medications taken during posaconazole therapy. Episodes of mucositis or diarrhea noted in the medical record during posaconazole therapy were documented. This study received multisite ethics approval from the Sydney Local Heath District-Concord Repatriation General Hospital Human Research Ethics Committee.
IFI classification and outcome of therapy.
In patients receiving posaconazole for the treatment of a fungal infection, or with breakthrough fungal infection during posaconazole prophylaxis, IFI was classified as proven, probable, or possible according to the 2008 guidelines from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group (8). Among patients receiving posaconazole prophylaxis, patients with breakthrough fungal infection after at least 7 days of therapy were classified as failing therapy, and patients without breakthrough infection were considered to have successful therapy. In patients who received posaconazole for treatment of an IFI, treatment success was assessed based on partial or complete improvement in clinical (symptoms of infection or fever) and radiological (computed tomography [CT], high-resolution CT, or magnetic resonance imaging findings) signs of infection. Treatment failure was defined as persistent or progressing IFI based on clinical and radiological signs or continuing positive cultures or as death due to IFI after at least 7 days of therapy with posaconazole.
Statistical analysis.
Posaconazole dosing records for each patient were used to verify the time of posaconazole concentration sampling in relation to dose. As posaconazole is known to exhibit a reasonably flat concentration-time profile at steady state, with similar concentrations measured at 4, 8, and 12 h after dose (14), all posaconazole concentrations irrespective of sampling time after dose were included in the analysis. Posaconazole concentrations were excluded from the analysis if measured within the first 7 days of dosing with posaconazole or if the patient's dosing record indicated missed posaconazole dosing prior to sampling for quantitation of posaconazole. Relationships between the median steady-state posaconazole concentration calculated for each patient and outcomes of therapy for patients taking prophylactic posaconazole were assessed using binary logistic regression. Elevation in liver function tests (LFTs) (activities of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and gamma-glutamyl transpeptidase and bilirubin concentrations) was assessed and graded using the U.S. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), v4.03 (29). To examine intrapatient variability in steady-state posaconazole concentrations, the range of posaconazole concentrations observed for each patient was calculated for all patients with more than one posaconazole concentration measurement. A multiple linear regression analysis was used to identify factors that contributed to the variability in posaconazole concentration. Univariate analyses were performed using the Mann-Whitney U test or Wilcoxon signed-rank test as appropriate due to the observed nonnormality of posaconazole concentration. Proportions were compared using the chi-square test or Fisher's exact test as appropriate. Statistical significance was defined by a P value of <0.05. Statistical analyses were performed with PASW Statistics 18 (SPSS Inc., Chicago, IL).
RESULTS
Patient characteristics.
Data from 86 patients receiving posaconazole were included in the study. The majority of patients (72/86; 84%) received posaconazole for prophylaxis against fungal infections rather than for treatment of a fungal infection (proven, probable, or possible IFI or empirical therapy) (14/86; 16%). Information on patient demographics, indications for therapy, and underlying conditions is included in Table 1.
Table 1.
Patient demographic/clinical characteristics and indications for therapy
| Variable | Value for posaconazole patients (n = 86) |
|---|---|
| Demographics | |
| Median age [yr (range)] | 51 (18–79) |
| No. of males/females | 50/36 |
| Median wt [kg (range)a] | 71 (38–122) |
| No. with indication for therapyb: | |
| Antifungal prophylaxis | 72 |
| Proven IFI | 6 |
| Probable IFI | 3 |
| Possible IFI | 3 |
| Empirical therapy | 2 |
| No. with underlying condition: | |
| Acute myeloid leukemia | 38 |
| Acute lymphoblastic leukemia | 11 |
| Non-Hodgkin's lymphoma | 8 |
| Myelodysplastic syndrome | 6 |
| Multiple myeloma | 4 |
| Diabetes mellitus type 2 | 4 |
| Chronic lymphocytic leukemia | 3 |
| Otherc | 12 |
Weight was available for 85/86 patients.
Patients receiving posaconazole for treatment of a fungal infection defined according to the 2008 EORTC/MSG guidelines (8).
Myelofibrosis (2 patients), Hodgkin's lymphoma (1 patient), acute biphenotypic leukemia (1 patient), gray-zone lymphoma (1 patient), T-polylymphocytic leukemia (1 patient), chronic myeloid leukemia (1 patient), aplastic anemia (1 patient), HIV positivity (1 patient), rheumatoid arthritis (1 patient), Crohn's disease (1 patient), and none (histoplasma) (1 patient).
Of the 14 patients who received posaconazole for the treatment of a fungal infection, 10 patients received posaconazole as primary antifungal therapy, with 4 patients receiving concurrent liposomal amphotericin B in addition to posaconazole. Among 4 patients who received posaconazole as salvage therapy, 3 patients were switched to posaconazole due to intolerance of voriconazole, with one patient switched to posaconazole and liposomal amphotericin B due to having an IFI refractory to voriconazole.
Among nine patients who received posaconazole for the treatment of proven or probable IFIs, four had infection caused by members of the order Mucorales; these included Rhizopus spp. (two cases; lung infection and invasive sinusitis with intracranial extension), Mucor spp. (one case; invasive sinusitis and endophthalmitis), and mixed zygomycete and Aspergillus fumigatus infection (one case; lung infection). One patient had Histoplasma capsulatum infection (brain), while the remaining four had disease due to Aspergillus spp. (lung), Scedosporium spp. (lung), Candida albicans (lung), and Fusarium spp. and Paecilomyces lilacinus (disseminated).
In the study cohort, 41 patients (48%) underwent a hematopoietic stem cell transplant (HSCT) for treatment of an underlying hematological disease while receiving posaconazole. Of these patients, 39 underwent an allogeneic transplant and 2 underwent autologous stem cell transplants; 72% (28/39) of allogeneic transplant recipients received a peripheral blood stem cell transplant, while 23% (9/39) received a bone marrow transplant and 5% (2/39) received an umbilical cord blood stem cell transplant. A reduced-intensity conditioning regimen was used in 15% (6/39) of patients who received an allogeneic stem cell transplant.
Posaconazole therapy.
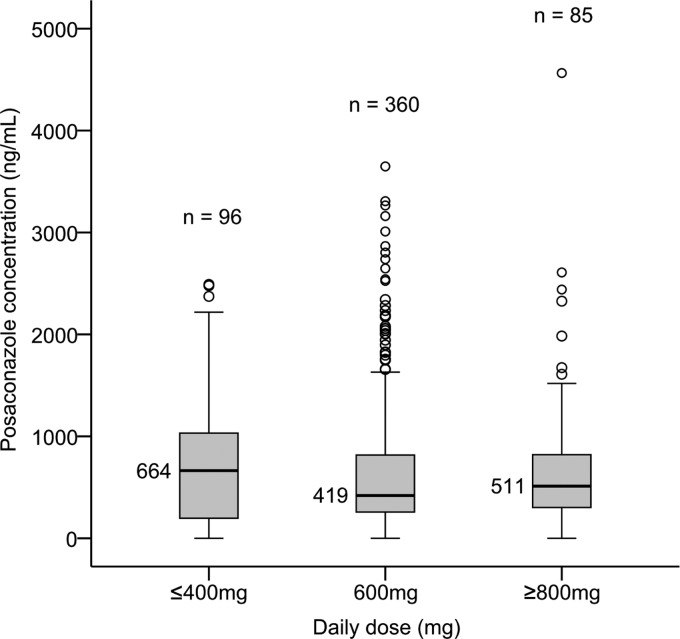
A total of 541 posaconazole concentrations from 86 patients were included in the analysis. The median posaconazole concentration was 466 ng/ml (range, 0 to 4,564 ng/ml). Posaconazole concentrations were highly variable (Fig. 1). Using posaconazole concentration cutoffs that have been associated with poorer treatment outcomes in previous studies (9), 52% (284/541) of posaconazole concentrations were <500 ng/ml, with 66% (355/541) of concentrations being <700 ng/ml. The median posaconazole concentration achieved during therapy was <500 ng/ml in 57% (49/86) of patients; 71% (61/86) of patients had median posaconazole concentrations below 700 ng/ml. The median number of posaconazole concentrations measured per patient was 4 (range, 1 to 43 observations). Among 74 patients with more than one posaconazole concentration measured during therapy, the median intrapatient range of posaconazole concentrations was 526 ng/ml (range, 6 to 4,391 ng/ml). The median dose in patients who received posaconazole prophylaxis was 200 mg three times daily (range, 40 mg twice daily to 300 mg three times daily); in patients who received posaconazole for treatment of a fungal infection, the median dose was 400 mg twice daily (range, 200 mg three times daily to 400 mg three times daily).
Fig 1.
Posaconazole concentration by daily dose (24 h). The black lines and corresponding concentrations represent the medians.
Posaconazole concentration, outcomes of therapy, and adverse events.
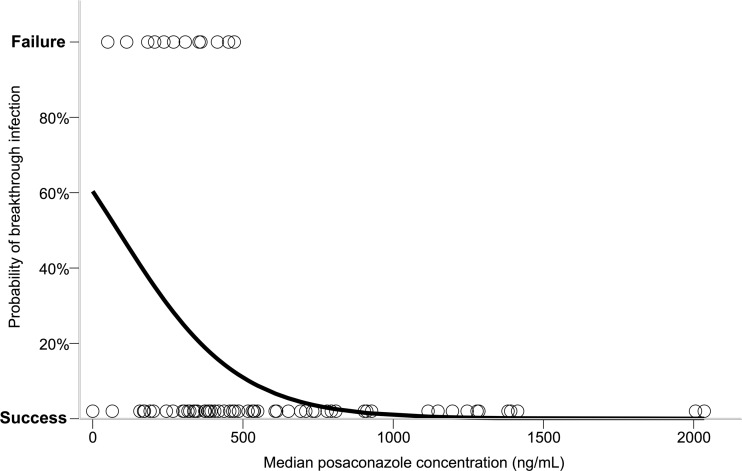
Among 72 patients who received prophylactic posaconazole, 12 patients (17%) developed a breakthrough fungal infection (Table 2). Median posaconazole concentrations in these patients were significantly lower than those in patients who did not develop a breakthrough infection (median, 289 ng/ml versus 485 ng/ml; P < 0.01). Furthermore, all patients who developed a breakthrough fungal infection had a median posaconazole concentration of below 500 ng/ml. Among these patients, 90% of individual posaconazole concentration measurements were <500 ng/ml, with 97% of individual concentrations being <700 ng/ml. The median posaconazole concentration was found to be a significant predictor of prophylaxis outcome (i.e., breakthrough infection versus no breakthrough infection) via binary logistic regression (P < 0.05; Exp(B) 0.995; Nagelkerke r2 = 0.25). The predicted probability of breakthrough fungal infection with increasing posaconazole concentration from the logistic regression analysis is displayed in Fig. 2. Cases of breakthrough fungal infection with posaconazole prophylaxis are summarized in Table 2.
Table 2.
Cases of breakthrough fungal infection on posaconazole prophylaxis
| Case | Sexa (age, yr) | Underlying condition | Median posaconazole concn (ng/ml) | Duration of posaconazole before breakthrough (days) | Site of infection | Infection classificationb | Pathogen | Follow-up of breakthrough infectionc |
|---|---|---|---|---|---|---|---|---|
| 1 | F (59) | Acute myeloid leukemia | 50 | 51 | Lungs | Probable IFI | Candida sp. | Complete response with caspofungin and later itraconazole |
| 2 | F (45) | Myelodysplastic syndrome | 113 | 9 | Lungs | Possible IFI | Unknown | Complete response with voriconazole |
| 3 | M (38) | Acute myeloid leukemia | 183 | 14 | Lungs | Possible IFI | Unknown | Complete response with voriconazole |
| 4 | M (56) | Multiple myeloma | 206 | 21 | Oropharynx | Oral candidiasis (culture proven) | Candida glabrata | NR |
| 5 | M (63) | Acute myeloid leukemia | 237 | 95 | Lungs | Possible IFI | Unknown | Respiratory failure; palliative care due to refractory AML; death |
| 6 | M (46) | Acute myeloid leukemia | 269 | 15 | Bloodstream | Proven IFI | Candida parapsilosis | Complete response with liposomal amphotericin B and later fluconazole |
| 7 | F (60) | Angioimmunoblastic T-cell lymphoma | 308 | 75 | Urinary tract | Urinary tract infection (culture proven) | Candida glabrata | Death due to bacterial sepsis, multiorgan failure |
| 8 | F (45) | Acute myeloid leukemia | 354 | 85 | Eyes | Presumed fungal endophthalmitis | Unknown | Deterioration on aciclovir/prednisolone; improvement on caspofungin; complete response with intravitreal and oral voriconazole |
| 9 | M (21) | Diffuse large B-cell lymphoma | 360 | 10 | Lungs | Possible IFI | Unknown | NR |
| 10 | M (59) | Chronic lymphocytic leukemia | 415 | 11 | Lungs | Possible IFI | Unknown | Complete response with voriconazole and liposomal amphotericin B |
| 11 | F (67) | Acute myeloid leukemia | 452 | 13 | Lungs | Possible IFI | Unknown | Complete response with voriconazole |
| 12 | M (24) | Acute myeloid leukemia | 471 | 15 | Lungs/oropharynx | Possible IFI/oral candidiasis | Unknown (lung)/Candida sp. | Deterioration despite voriconazole; respiratory failure and death due to progressive IFI and AML |
F, female; M, male.
Patients with breakthrough IFIs classified according to the 2008 EORTC/MSG guidelines (8).
NR, not recorded in medical record; AML, acute myeloid leukemia.
Fig 2.
Predicted probability of breakthrough fungal infection determined from the logistic regression analysis. Open circles represent one patient's median posaconazole concentration and outcome of therapy (failure, breakthrough fungal infection; success, no breakthrough infection); logit function = −0.005χ + 0.421, where χ = median posaconazole concentration (ng/ml). Predicted probabilities were calculated from the natural antilog of the logit.
Fourteen patients received posaconazole for treatment of a suspected or confirmed IFI, with treatment outcome being evaluable in 11 patients. Excluding two patients who were treated empirically with posaconazole for persistent febrile neutropenia, four patients failed therapy (4/9; 44%). Median posaconazole concentrations among these patients were numerically lower (median, 436 ng/ml) than those in patients treated successfully with posaconazole (median, 955 ng/ml); however, this difference was not statistically significant (P = 0.19). Median posaconazole concentrations and fungal pathogens in patients failing treatment with posaconazole were 139 ng/ml (zygomycete and Aspergillus fumigatus), 289 ng/ml (Mucor spp.), 583 ng/ml (Fusarium spp. and Paecilomyces lilacinus), and 590 ng/ml (Rhizopus spp.).
In regard to adverse events, data on LFTs measured during posaconazole therapy were available for 86% of patients (74/86); data on baseline LFTs prior to posaconazole dosing were available for 51% of patients (44/86). At baseline, 66% of patients (29/44) had elevated LFTs meeting the criteria of CTCAE grade 1 or higher. During posaconazole therapy, 70% of patients (52/74) had at least one LFT measurement meeting CTCAE grade 1 LFT elevation criteria or higher, with 39% meeting grade 2 criteria, 12% grade 3, and 3% grade 4. Posaconazole concentrations were not significantly different in patients with elevated LFTs (using CTCAE grade 1 to 4 cutoff values) than in patients without LFT elevation.
Factors affecting posaconazole concentration.
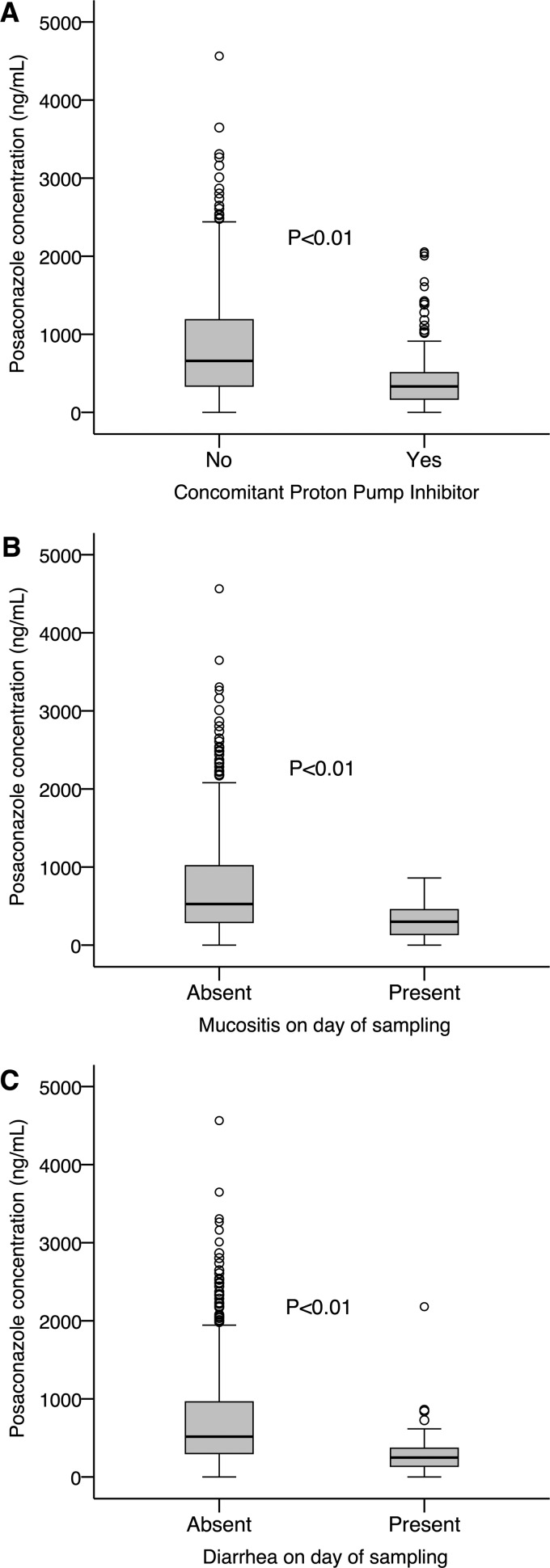
A multiple linear regression analysis identified a number of drug interactions and clinical factors associated with a significant change in posaconazole concentration (Table 3). Drug interactions associated with significantly lower posaconazole concentrations included, in order of highest frequency of coadministration to lowest (percentage of patients receiving medication), concomitant administration of proton pump inhibitors (52%) (Fig. 3A), the H2 receptor antagonist ranitidine (35%), metoclopramide (31%), and phenytoin or rifampin (3%) (P < 0.01). Clinical factors such as mucositis or diarrhea on the day of posaconazole sampling (each affecting 38% of patients) were also associated with significantly reduced posaconazole concentrations (Fig. 3B and C) (P < 0.01).
Table 3.
Factors associated with a significant change in posaconazole concentration identified from the multiple linear regression analysisa
| Model term | Coefficient | 95% confidence interval |
P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Concomitant phenytoin or rifampin | −862 | −1,230 | −494 | <0.01 |
| Concomitant proton pump inhibitor | −589 | −699 | −480 | <0.01 |
| Mucositis on day of sampling | −317 | −459 | −174 | <0.01 |
| Concomitant metoclopramide | −289 | −434 | −145 | <0.01 |
| Diarrhea on day of sampling | −246 | −405 | −87 | <0.01 |
| Concomitant ranitidine | −178 | −304 | −52 | <0.01 |
| Concomitant nutritional supplement | 749 | 430 | 1,068 | <0.01 |
r2 = 0.29; n = 540/541 concentration measurements; weight data were unavailable for one measurement.
Fig 3.
Box plots displaying the effect of a concomitant proton pump inhibitor (A), mucositis (B), or diarrhea (C) on the day of sampling on posaconazole concentration.
Conversely, the administration of a nutritional supplement with posaconazole was associated with significantly increased posaconazole concentrations (P < 0.01). Variables included in the analysis that did not reach statistical significance included patient weight, sex, age, and posaconazole daily dose (P > 0.05). Box plots of the effect of concomitant proton pump inhibitors, mucositis, or diarrhea on the day of posaconazole sampling on posaconazole concentration are displayed in Fig. 3.
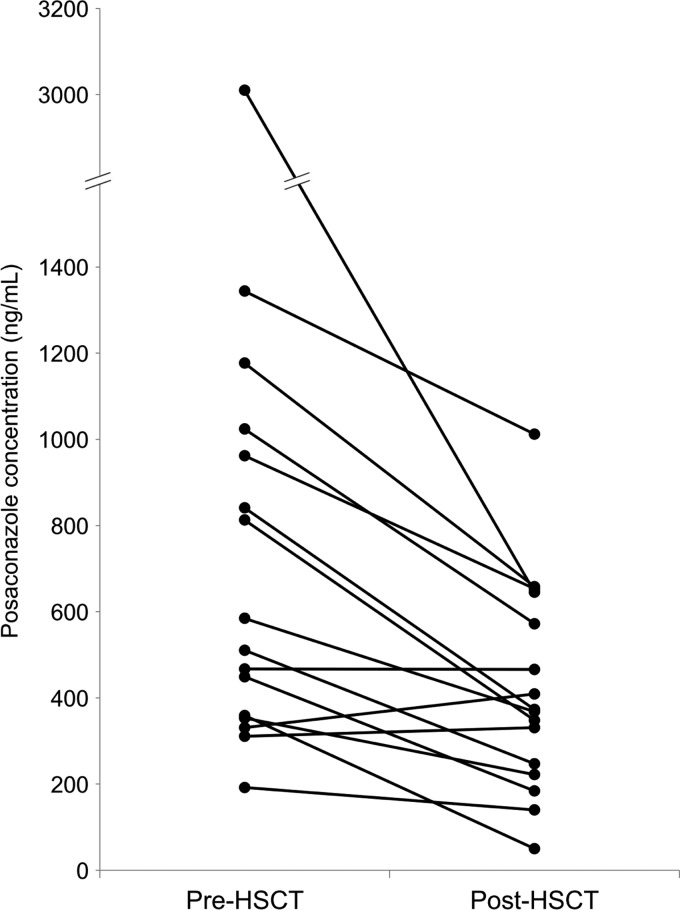
Of the 41 patients who received a hematopoietic stem cell transplant while taking posaconazole, concentration measurements in the period prior to HSCT (within 5 days before HSCT) and in the early posttransplant period (within 5 days after HSCT) were available for 16 patients. In these patients, median posaconazole concentrations were significantly lower in the early posttransplant period (median, 371 ng/ml) than in the period prior to HSCT (median, 548 ng/ml; P < 0.01) (Fig. 4).
Fig 4.
Median posaconazole concentrations measured in 16 patients within 5 days before hematopoietic stem cell transplant (pre-HSCT) and in the early posttransplant period (within 5 days) (post-HSCT).
DISCUSSION
This study is the first multicenter investigation of posaconazole TDM and has found a significant exposure-response relationship for posaconazole in prophylaxis against IFIs, while also identifying significant drug interactions and clinical factors that predict reduced posaconazole concentrations in patients.
A significant exposure-response relationship was observed between plasma posaconazole concentrations and prophylactic efficacy (Fig. 2). All breakthrough fungal infections were observed at median posaconazole concentrations below 500 ng/ml, suggesting that this cutoff value may be a useful concentration target for posaconazole prophylaxis against IFIs. We have recently reviewed the available literature on posaconazole exposure-response relationships and target plasma concentrations (9). A posaconazole concentration target of >500 ng/ml has been recommended in other TDM studies (23, 37); however, Jang et al. identified a number of breakthrough fungal infections in patients with posaconazole concentrations between 500 and 700 ng/ml, suggesting a concentration target of >700 ng/ml (17) when used for antifungal prophylaxis.
In the present study, no relationship was observed between posaconazole concentrations and the incidence of elevated LFTs. This finding is in agreement with pooled analyses of previous posaconazole trials, which have not demonstrated a significant exposure-response relationship for posaconazole-related adverse events (17, 28). While no breakthrough infections were observed at concentrations of between 500 and 700 ng/ml in the present study, in light of the lack of concentration-dependent adverse events with posaconazole and reported risk of breakthrough infections in this concentration range, a posaconazole concentration target of >700 ng/ml is preferable when used for IFI prophylaxis (9).
A limitation of this analysis is the lack of antifungal susceptibility data to determine whether any breakthrough infections were related to a pathogen resistant to posaconazole rather than to subtherapeutic exposure. A number of cases of breakthrough IFI in the present study met the EORTC/MSG criteria only for possible IFIs (Table 2), satisfying the host factor and clinical criteria but not mycological criteria (8). As galactomannan and β-d-glucan tests were not widely available in the hospitals included in this study, it is possible that a number of these cases may have met the criteria of probable IFIs had these tests been available.
Among patients who received posaconazole for the treatment of a proven, probable, or possible IFI, median posaconazole concentrations were numerically lower among patients failing therapy (436 ng/ml) than among those treated successfully (955 ng/ml), although this difference was not statistically significant (P = 0.19), probably due to the low number of patients who received posaconazole for treatment of IFIs in this study. In a cohort of 67 patients who received posaconazole for salvage treatment of invasive aspergillosis, the proportion of clinical responders increased with increasing posaconazole average concentration (Cav) quartiles; the highest rate of response was seen at a mean posaconazole Cav of 1,250 ng/ml (42).
It is also notable that three of the four treatment failures with posaconazole in this study involved fungi from the order Mucorales, which are associated with very high mortality rates (16). In addition, the MIC90 values for posaconazole with Mucorales are substantially higher than those for more common invasive molds, such as A. fumigatus (24), and posaconazole has generally performed poorly in animal models involving these pathogens (35, 38).
Taken together, these findings provide evidence of an exposure-response relationship for posaconazole in the treatment of IFIs; however, future studies of larger patient cohorts with this indication are needed to more accurately define concentration targets for posaconazole in the treatment of IFIs and to determine whether posaconazole is a useful antifungal agent in the treatment of mucormycosis, although initial clinical experience in combination therapy with amphotericin B appears promising (11).
Plasma posaconazole concentrations in this study were low in most patients (median, 466 ng/ml), as has been reported by other authors (3, 10, 23, 37). A multiple linear regression analysis of posaconazole concentrations identified important drug interactions and clinical factors that predict changes in posaconazole concentration (Table 3).
Over half the patients in this study received a proton pump inhibitor while taking posaconazole, resulting in significantly lower posaconazole concentrations (regression coefficient, −589 ng/ml) (Fig. 3A). In a cohort of 17 cardiothoracic transplant recipients, Shields and colleagues found significantly lower posaconazole concentrations in patients receiving proton pump inhibitors (37); a similar result was identified by Neubauer et al. (31). Controlled studies in healthy volunteers have identified reductions in the area under the posaconazole concentration-time curve (AUC) of 32% (20) and 37% (41) with esomeprazole, with the later study also confirming the pH-dependent solubility (and absorption) of posaconazole through monitoring of intraluminal posaconazole concentrations (41).
Few studies have examined the potential impact of H2 receptor antagonists on posaconazole concentrations. A pharmacokinetic subanalysis of a pivotal phase III posaconazole trial did not demonstrate an effect of H2 antagonists on posaconazole concentration (18); however, a pharmacokinetic study by the manufacturer found a 39% reduction in posaconazole peak concentration (Cmax) and total exposure (AUC) when cimetidine was coadministered with posaconazole (27). While this interaction is attributed to altered gastric pH caused by cimetidine reducing posaconazole absorption, the product information states that no posaconazole dosage adjustment is needed when coadministered with H2 antagonists other than cimetidine, without provision of supporting evidence (27).
The present study is the first to demonstrate significantly lower posaconazole concentrations in patients taking ranitidine (regression coefficient, −178 ng/ml). Taking into account the probable mechanism of increased gastric pH, these results suggest that the interaction between posaconazole and H2 antagonists is likely to be a class effect based on pharmacological plausibility, although further investigation with other H2 antagonists, such as nizatidine and famotidine, is needed.
Metoclopramide was coadministered with posaconazole in 31% of patients and was associated with significantly lower posaconazole concentrations (regression coefficient, −289 ng/ml). A previous study with healthy volunteers has demonstrated significant reductions in posaconazole peak and total exposures of 21% and 19%, respectively, when coadministered with metoclopramide due to increased gastric motility (20).
Coadministration of phenytoin or rifampin was associated with the greatest reduction in posaconazole concentration (regression coefficient, −862 ng/ml). While posaconazole is excreted primarily unchanged in feces and is not metabolized by CYP450 enzymes, approximately 20 to 30% is known to be glucuronidated via the phase 2 enzyme UDP glucuronosyltransferase 1A4 (UGT1A4) (12, 26). Both phenytoin and rifamycins have been shown to significantly reduce posaconazole exposure (21, 22), although less information is available for rifampin (15). Posaconazole is also a substrate of P glycoprotein, although the importance of this pathway to posaconazole disposition appears to be limited (36). While the mechanism of these interactions has not been definitively elucidated, an induction of UGT1A4 by phenytoin and rifampin, both of which are known UGT inducers (1, 32), resulting in increased posaconazole metabolism is probable.
Mucositis and diarrhea are common chemotherapy-induced adverse events, each affecting 38% of patients during posaconazole therapy in this study. Both mucositis (Fig. 3B) and diarrhea (Fig. 3C) were associated with lower posaconazole exposure (regression coefficients of −317 ng/ml and −246 ng/ml, respectively), highlighting the important role of gastrointestinal disease in reducing the absorption of posaconazole. Similarly, Lebeaux et al. identified a significant association between low posaconazole concentrations and mucositis or diarrhea (23); diarrhea was also associated with lower posaconazole exposure in a cohort of 241 hematopoietic stem cell transplant recipients with graft-versus-host disease (19).
Hematopoietic stem cell transplant (HSCT) recipients are at high risk of IFIs due to myeloablative conditioning regimens and subsequent periods of profound immunosuppression, with IFIs now recognized to be a leading cause of mortality (30, 33). Low posaconazole concentrations have been reported in HSCT recipients (13); however, no studies have directly examined the impact of HSCT on posaconazole concentration. Among 16 patients with posaconazole concentrations measured both shortly before HSCT and in the early posttransplant period (within 5 days), concentrations were significantly reduced in the early posttransplant period in most patients (Fig. 4) (P < 0.01). As engraftment and neutrophil recovery would not yet be expected in this early period (4), this reduction in posaconazole exposure may further increase the risk of breakthrough IFI in HSCT recipients.
This multicenter study has identified a number of important clinical implications for the use of posaconazole. These results support a significant exposure-response relationship between posaconazole concentration and prophylactic efficacy; a trend toward this relationship was observed in the treatment of IFIs. Numerous commonly coadministered medicines are associated with substantially lower posaconazole concentrations, including the H2 antagonist ranitidine; in addition, HSCT recipients may be susceptible to reduced posaconazole exposure in the early posttransplant period. In conclusion, subtherapeutic posaconazole concentrations are common and are associated with poor clinical outcomes, supporting the need for therapeutic drug monitoring to ensure efficacious posaconazole exposure.
ACKNOWLEDGMENTS
We gratefully acknowledge the support of the medical records and pharmacy departments at Concord Repatriation General Hospital, St. Vincent's Hospital, Royal North Shore Hospital, Royal Prince Alfred Hospital, Westmead Hospital, and St. George Hospital.
Michael Dolton is supported by an Australian Postgraduate Award.
M.J.D., J.E.R., K.N., L.P., and A.J.M. have no conflicts of interest. S.C.-A.C. has been a member of the Antifungal Advisory Boards of Pfizer Australia, Merck, and Gilead Sciences Inc.
Footnotes
Published ahead of print 13 August 2012
REFERENCES
- 1. Anderson GD. 2004. Pharmacogenetics and enzyme induction/inhibition properties of antiepileptic drugs. Neurology 63:S3–S8 [DOI] [PubMed] [Google Scholar]
- 2. Andes D, Pascual A, Marchetti O. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob. Agents Chemother. 53:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bryant AM, Slain D, Cumpston A, Craig M. 2011. A post-marketing evaluation of posaconazole plasma concentrations in neutropenic patients with haematological malignancy receiving posaconazole prophylaxis. Int. J. Antimicrob. Agents 37:266–269 [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention, Infectious Disease Society of America, and American Society of Blood and Marrow Transplantation 2000. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm. Rep. 49(RR-10):1–128 [PubMed] [Google Scholar]
- 5. Chhun S, et al. 2007. Simultaneous quantification of voriconazole and posaconazole in human plasma by high-performance liquid chromatography with ultra-violet detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 852:223–228 [DOI] [PubMed] [Google Scholar]
- 6. Cornely OA, et al. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348–359 [DOI] [PubMed] [Google Scholar]
- 7. Cornely OA, Ullmann AJ. 2011. Lack of evidence for exposure-response relationship in the use of posaconazole as prophylaxis against invasive fungal infections. Clin. Pharmacol. Ther. 89:351–352 [DOI] [PubMed] [Google Scholar]
- 8. de Pauw B, et al. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dolton MJ, Ray JE, Marriott D, McLachlan AJ. 2012. Posaconazole exposure-response relationship: evaluating the utility of therapeutic drug monitoring. Antimicrob. Agents Chemother. 56:2806–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eiden C, et al. 2012. Therapeutic drug monitoring of posaconazole in hematology adults under posaconazole prophylaxis: influence of food intake. Eur. J. Clin. Microbiol. Infect. Dis. 31:161–167 [DOI] [PubMed] [Google Scholar]
- 11. Enoch DA, Aliyu SH, Sule O, Lewis SJ, Karas JA. 2011. Posaconazole for the treatment of mucormycosis. Int. J. Antimicrob. Agents 38:465–473 [DOI] [PubMed] [Google Scholar]
- 12. Ghosal A, et al. 2004. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of posaconazole (Noxafil). Drug Metab. Dispos. 32:267–271 [DOI] [PubMed] [Google Scholar]
- 13. Gubbins PO, et al. 2006. Pharmacokinetics and safety of oral posaconazole in neutropenic stem cell transplant recipients. Antimicrob. Agents Chemother. 50:1993–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heinz WJ, et al. 2011. Relevance of timing for determination of posaconazole plasma concentrations. Antimicrob. Agents Chemother. 55:3621–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hohmann C, Kang EM, Jancel T. 2010. Rifampin and posaconazole coadministration leads to decreased serum posaconazole concentrations. Clin. Infect. Dis. 50:939–940 [DOI] [PubMed] [Google Scholar]
- 16. Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. 2012. Pathogenesis of mucormycosis. Clin. Infect. Dis. 54(Suppl 1):S16–S22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jang SH, Colangelo PM, Gobburu JVS. 2010. Exposure-response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin. Pharmacol. Ther. 88:115–119 [DOI] [PubMed] [Google Scholar]
- 18. Krishna G, et al. 2008. Pharmacokinetics of oral posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Pharmacotherapy 28:1223–1232 [DOI] [PubMed] [Google Scholar]
- 19. Krishna G, Martinho M, Chandrasekar P, Ullmann AJ, Patino H. 2007. Pharmacokinetics of oral posaconazole in allogeneic hematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy 27:1627–1636 [DOI] [PubMed] [Google Scholar]
- 20. Krishna G, Moton A, Ma L, Medlock MM, McLeod J. 2009. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob. Agents Chemother. 53:958–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krishna G, Parsons A, Kantesaria B, Mant T. 2007. Evaluation of the pharmacokinetics of posaconazole and rifabutin following co-administration to healthy men. Curr. Med. Res. Opin. 23:545–552 [DOI] [PubMed] [Google Scholar]
- 22. Krishna G, Sansone-Parsons A, Kantesaria B. 2007. Drug interaction assessment following concomitant administration of posaconazole and phenytoin in healthy men. Curr. Med. Res. Opin. 23:1415–1422 [DOI] [PubMed] [Google Scholar]
- 23. Lebeaux D, et al. 2009. Therapeutic drug monitoring of posaconazole: a monocentric study with 54 adults. Antimicrob. Agents Chemother. 53:5224–5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lewis RE, et al. 2012. How does antifungal pharmacology differ for mucormycosis versus aspergillosis? Clin. Infect. Dis. 54(Suppl 1):S67–S72 [DOI] [PubMed] [Google Scholar]
- 25. Li Y, Theuretzbacher U, Clancy CJ, Nguyen MH, Derendorf H. 2010. Pharmacokinetic/pharmacodynamic profile of posaconazole. Clin. Pharmacokinet. 49:379–396 [DOI] [PubMed] [Google Scholar]
- 26. Lipp H-P. 2010. Clinical pharmacodynamics and pharmacokinetics of the antifungal extended-spectrum triazole posaconazole: an overview. Br. J. Clin. Pharmacol. 70:471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Merck & Co 2010. Noxafil US prescribing information, revised September 2010. http://www.spfiles.com/pinoxafil.pdf
- 28. Moton A, Krishna G, Wang Z. 2009. Tolerability and safety profile of posaconazole: evaluation of 18 controlled studies in healthy volunteers. J. Clin. Pharm. Ther. 34:301–311 [DOI] [PubMed] [Google Scholar]
- 29. National Cancer Institute, National Institutes of Health 2010. Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Department of Health and Human Services, Washington, DC: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf [Google Scholar]
- 30. Neofytos D, et al. 2009. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin. Infect. Dis. 48:265–273 [DOI] [PubMed] [Google Scholar]
- 31. Neubauer WC, et al. 2010. Therapeutic drug monitoring of posaconazole in hematology patients: experience with a new high-performance liquid chromatography-based method. Antimicrob. Agents Chemother. 54:4029–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oesch F, Arand M, Benedetti MS, Castelli MG, Dostert P. 1996. Inducing properties of rifampicin and rifabutin for selected enzyme activities of the cytochrome P-450 and UDP-glucuronosyltransferase superfamilies in female rat liver. J. Antimicrob. Chemother. 37:1111–1119 [DOI] [PubMed] [Google Scholar]
- 33. Post MJ, Lass-Floerl C, Gastl G, Nachbaur D. 2007. Invasive fungal infections in allogeneic and autologous stem cell transplant recipients: a single-center study of 166 transplanted patients. Transpl. Infect. Dis. 9:189–195 [DOI] [PubMed] [Google Scholar]
- 34. Ray J, Campbell L, Rudham S, Nguyen Q, Marriott D. 2011. Posaconazole plasma concentrations in critically ill patients. Ther. Drug Monit. 33:387–392 [DOI] [PubMed] [Google Scholar]
- 35. Salas V, et al. 2012. In vitro and in vivo activities of posaconazole and amphotericin B in a murine invasive infection by Mucor circinelloides: poor efficacy of posaconazole. Antimicrob. Agents Chemother. 56:2246–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sansone-Parsons A, et al. 2007. Effects of age, gender, and race/ethnicity on the pharmacokinetics of posaconazole in healthy volunteers. Antimicrob. Agents Chemother. 51:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shields RK, et al. 2011. Posaconazole serum concentrations among cardiothoracic transplant recipients: factors impacting trough levels and correlation with clinical response to therapy. Antimicrob. Agents Chemother. 55:1308–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spellberg B, et al. 2012. Combination therapy for mucormycosis: why, what, and how? Clin. Infect. Dis. 54(Suppl 1):S73–S78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thompson GR, et al. 2009. Posaconazole therapeutic drug monitoring: a reference laboratory experience. Antimicrob. Agents Chemother. 53:2223–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Torres HA, Hachem RY, Chemaly RF, Kontoyiannis DP, Raad 2005. Posaconazole: a broad-spectrum triazole antifungal. Lancet Infect. Dis. 5:775–785 [DOI] [PubMed] [Google Scholar]
- 41. Walravens J, et al. 2011. Effect of pH and comedication on gastrointestinal absorption of posaconazole. Clin. Pharmacokinet. 50:725–734 [DOI] [PubMed] [Google Scholar]
- 42. Walsh TJ, et al. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin. Infect. Dis. 44:2–12 [DOI] [PubMed] [Google Scholar]