Abstract
The macrolide-aminoglycoside-streptothricin (MAS) element, an ∼4.2-kb insertion containing erm(B) and aphA3 resistance determinants, distinguishes Streptococcus pneumoniae transposon Tn1545/Tn6003 from Tn6002. Here, it is shown to be an unstable genetic element that, although it lacks recombinase genes, can exploit long, erm(B)-containing direct repeats acting as att sites for spontaneous excision that may result in loss. Consequent to excision, which is RecA independent, Tn1545/Tn6003 changes to Tn6002. In pneumococcal populations harboring Tn1545/Tn6003, the latter appears to coexist with Tn6002.
TEXT
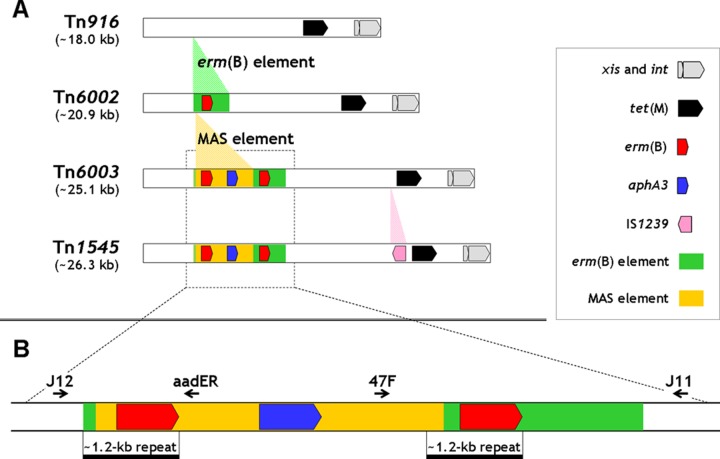
Among the conjugative elements of the Tn916 family (25), named after the prototype Tn916 (16), an ∼18-kb broad-host-range transposon that typically encodes tetracycline resistance via the tet(M) gene, Tn1545 holds a historically prominent place, to the point that the family was initially called Tn916-Tn1545 (7). Tn1545, originally detected in a multiply resistant clinical isolate of Streptococcus pneumoniae, was the first genetic element of the species to be shown to carry the erm(B) gene (at the time called ermAM, encoding coresistance to macrolide, lincosamide, and streptogramin B antibiotics); it was also reported to carry the aphA3 gene, encoding resistance to kanamycin and structurally related aminoglycosides (10, 11). In the years following its discovery, the genetic organization of Tn1545 was essentially analyzed by restriction mapping (4), with only its ends (5) and individual resistance (6, 21, 28) or transposition-related (24) genes being sequenced. The structural organization of Tn1545 has been elucidated quite recently (8), when two new regions were sequenced and the genetic organization of the transposon was compared, and found virtually to match, that of Tn6003. Tn6002 and Tn6003 are two of the most extensively investigated erm(B)-carrying Tn916-related streptococcal elements in recent years (29) (Fig. 1A). Tn6002 (∼20.9 kb; accession number AY898750) results from the insertion of an ∼2.8-kb erm(B)-containing DNA fragment [the so-called erm(B) element] (2, 9, 12, 30) between orf20 and orf19 of Tn916. A related variant, transposon Tn2010, results from the insertion of the mef(E)-carrying mega element into Tn6002 (12, 13). Tn6003 (∼25.1 kb; accession number AM410044) features insertion of the so-called MAS (macrolide-aminoglycoside-streptothricin) element (∼4.2 kb) into Tn6002, within the erm(B) element (8, 9). The MAS element appears to be a rearrangement of part of plasmid pRE25 from Enterococcus faecalis (27) and contains, from upstream to downstream, another erm(B) gene (exhibiting a shifted stop codon) with its leader peptide, an aminoglycoside-streptothricin resistance gene cluster (aadE*, sat4, and aphA3) with a 511-bp deletion in aadE, and an open reading frame (ORF) identical to orf47 of pRE25 (9). A recent reinvestigation (8) found that Tn1545 contains a MAS element virtually identical (99.7% identity; accession number AM903082) to that of Tn6003 as well as a second erm(B), exactly like Tn6003. Tn1545 and Tn6003 thus represent the same element (Tn1545/Tn6003), the only, trivial, difference being an insertion sequence (IS1239) detected in the original Tn1545 between orf13 and orf12. While Tn6002 has been reported present in different streptococcal species, Tn1545/Tn6003 has been reported present only in S. pneumoniae. In this species, however, it appears to be less common than other erm(B)-carrying Tn916-related elements (8), such as Tn6002, ranking first, at least in Europe, and Tn3872 (22), resulting from the insertion of the erm(B)-containing Tn917 transposon into Tn916 (29).
Fig 1.
Schematic representation of Tn916 and Tn916 family erm(B)-carrying transposons Tn6002, Tn6003, and Tn1545 (A), with an enlarged representation of the MAS element/erm(B) element complex (B). The Tn916 backbone is represented as a white rectangle with the genes tet(M), xis, and int in evidence. Faded green, orange, and pink areas between transposons denote distinctive insertions. See the symbols key box for further details. In panel B, the primers used (thin black arrows) and the ∼1.2-kb repeats (horizontal black bars) are shown in the enlarged area.
Long direct repeats have recently been found to play a key role as att sites in the spontaneous excision in circular form of unstable genetic elements lacking recombinase genes (1, 18, 19, 23, 26); besides excision, integration was also experimentally demonstrated in one study (1). From this perspective, the MAS element/erm(B) element complex of Tn1545/Tn6003 contains an ∼3-kb DNA fragment comprising four ORFs (aadE*, sat4, aphA3, and orf47) flanked by two ∼1.2-kb imperfect direct repeats (displaying >99% identity), both including the erm(B) gene and its leader peptide (Fig. 1B). In this study, we show that the MAS element of Tn1545/Tn6003, though lacking recombinase genes, can be excised in circular form by recombination between the flanking repeats acting as attL and attR sites.
Test strains included S. pneumoniae BM4200 (from the Pasteur Institute Collection, Paris, France), i.e., the clinical strain, isolated in France in 1978 (3), in which Tn1545 was originally described (10, 11), and Ar4 and Pg1 (from our laboratory collection), i.e., the clinical strains, both isolated in Italy in 2002 (20), from which we obtained Tn6003 and Tn6002, respectively (8, 9).
Test strains were investigated with PCR and sequencing experiments. A set of four primers (J12, J11, aadER, and 47F), designed from the reported sequence of Tn1545/Tn6003 (Fig. 1B), were used in PCR experiments. Primers J12 and J11 (9), allowing distinction among Tn3872, Tn6002, and Tn1545/Tn6003 (29), are external to the MAS element/erm(B) element complex. Primers aadER (5′-TTCCCGCCTCTCTTCTAT-3′) and 47F (5′-TCAGGAGGGCAAGAACCG-3′) are internal to the MAS element but divergent. Amplification assays were performed using the Ex Taq system (TaKaRa Bio, Shiga, Japan) under standard (extension time, up to 3 min) or long (extension time, 10 min) PCR conditions. Sequencing was carried out using an ABI Prism genetic analyzer (Perkin-Elmer Applied Biosystems, Foster City, CA) with dye-labeled terminators. Sequences were analyzed using the Sequence Navigator software package (Perkin-Elmer). ORFs were predicted using the ORF Finder software (http://ncbi.nlm.nih.gov/gorf/gorf.html).
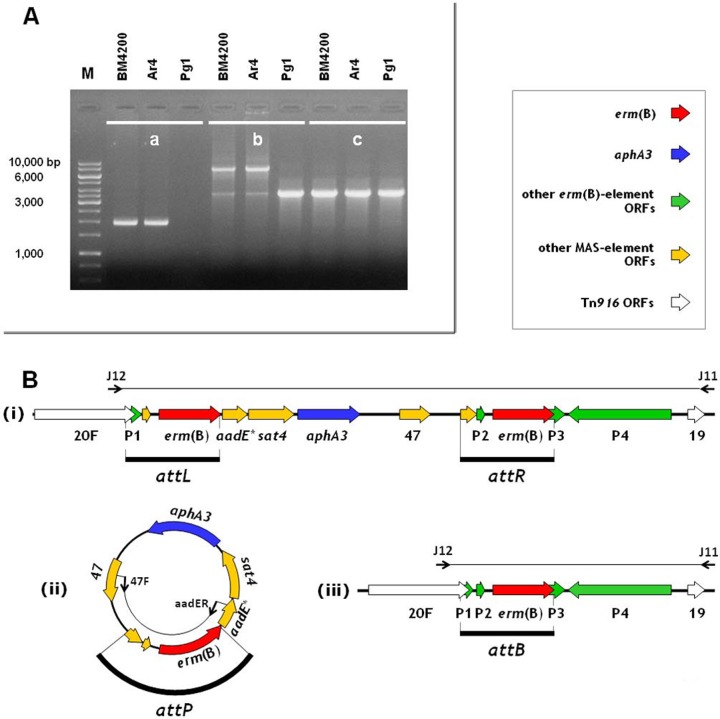
The results of the PCR assays are shown in Fig. 2A, and the relevant genetic organizations are schematically represented in Fig. 2B. With strains Ar4 and BM4200, pairing the two divergent primers yielded an ∼2.0-kb amplicon, indicating a circular MAS element; amplicon sequencing revealed a copy of the above-mentioned ∼1.2-kb repeats. Remarkably, when the same two strains were examined by pairing primers J12 and J11 in long PCR experiments, a faint 3.6-kb amplicon typical of Tn6002 was detected in addition to the ∼7.9-kb amplicon typical of Tn1545/Tn6003 (29). Selective production of the 3.6-kb amplicon was obtained with the two strains by pairing the same primers in standard PCR assays, the results being the same as with strain Pg1; amplicon sequencing demonstrated the absence of an integrated linear MAS element and the presence of an ∼1.2-kb sequence, i.e., an organization consistent with Tn6002. The four ∼1.2-kb segments appeared to represent att sequences: attL and attR, the two direct repeats found in Tn1545/Tn6003; attP, the copy found in the circular form of the MAS element; and attB, the copy found after MAS element excision from Tn1545/Tn6003 and DNA repair (Fig. 2B).
Fig 2.
The MAS element of S. pneumoniae Tn1545/Tn6003 is an unstable multiresistance genetic element exploiting long (∼1.2-kb) erm(B)-containing direct repeats as att sites for its excision. (A) Results of PCR amplification experiments obtained with test strains BM4200, Ar4, and Pg1, by pairing primers aadER and 47F (a), by pairing primers J12 and J11 under long PCR conditions (b), or by pairing primers J12 and J11 under standard PCR conditions (c). Lane M, molecular size markers (1-kb DNA ladder; Fermentas, Burlington, Canada). (B) Genetic organization of the MAS element integrated in Tn1545/Tn6003 (i), the circular MAS element (ii), and transposon Tn6002 (iii), resulting after MAS element excision from Tn1545/Tn6003 and DNA repair. ORFs are indicated as arrows pointing in the direction of transcription (see the symbols key box for further details). The primers used are shown as thin black arrows at the ends of the relevant amplicons (thin lines). The att sites are shown as black bars.
To investigate whether the unstable MAS element may be lost in Tn1545/Tn6003-carrying strains, nonselective serial passages of BM4200 and Ar4 and a search for kanamycin-susceptible colonies were performed by standard procedures (19). One kanamycin-susceptible colony was obtained from each strain after 15 passages. Using appropriate primer pairs (8, 9), both kanamycin-susceptible derivatives yielded a negative PCR for aphA3 and positive reactions for tet(M) and erm(B); more importantly, in long PCR assays using the primer pair J12/J11, the derivatives yielded only the Tn6002-specific amplicon (∼3.6 kb), confirming the loss of the entire MAS element. Sequencing disclosed that the ∼1.2-kb sequence resulting from the excision (attB) was a hybrid of the original attL and attR sequences.
We also demonstrated that the excision mechanism of the MAS element is RecA independent. The recA gene was inactivated by insertion/deletion mutagenesis using a linear construct, with a cat cassette, obtained by a two-step PCR method (14). The construct was used to transform S. pneumoniae strain Ar4 (multiresistant strain BM4200 is also chloramphenicol-resistant [3]). Ar4 recA::cat mutants, with a 959-bp deletion in the recA coding sequence, were verified by PCR using primers external to the region of integration. PCR experiments in Ar4 recA null mutants disclosed both the circular MAS element and the repaired Tn6002, indicating that RecA does not play a role in excision of the MAS element.
The MAS element of Tn1545/Tn6003 should be regarded as an unstable genetic element that, despite the absence of recombinases, is capable of exploiting long direct repeats for spontaneous excision. The recombination event yields a circular MAS element, which may eventually be lost, whereas the original transposon, after DNA repair, turns into Tn6002 (from Tn6003 [strain Ar4]) or its IS1239-containing equivalent (from Tn1545 [strain BM4200]). Coexistence of Tn6003 with Tn6002 and of Tn1545 with IS1239-containing Tn6002 appears to occur in the bacterial populations of strains Ar4 and BM4200, respectively. The greater prevalence of Tn6002 compared to Tn1545/Tn6003 in S. pneumoniae might reflect the instability of the MAS element and its proneness to spontaneous excision and loss.
Unstable genetic elements like the MAS element may represent a novel class of genetic structures that possibly contribute to the genomic evolution of bacteria. Since they lack recombinase genes, it has been suggested that they employ a parasitic strategy for their mobility (1), by exploiting the host recombination trans-acting functions (1, 18, 26). The details of this mechanism are still unknown, even though our data and those of other groups (1, 26) concur in ruling out the involvement of RecA. The fact that such elements may carry resistance determinants (18, 19, 23) suggests that they may also be involved in the spread of antibiotic resistances. It is worth noting that the erm(B) gene, contained in the att sequences herein reported, has also been found in the att sequences associated with another such unstable genetic element, that carries the multidrug resistance gene cfr in methicillin-resistant Staphylococcus aureus (19). These elements might be not uncommon; for instance, a similar mechanism could account for a deleted form (17) of Tn5398, the best-known erm(B)-carrying element of Clostridium difficile (15), where the deletion might in fact represent excision/loss of a DNA segment flanked, again, by two ∼1.2-kb erm(B)-containing direct repeats.
ACKNOWLEDGMENT
This work was supported in part by the Italian Ministry of Education, University and Research (PRIN 200929YFMK).
Footnotes
Published ahead of print 13 August 2012
REFERENCES
- 1. Azpiroz MF, Bascuas T, Laviña M. 2011. Microcin H47 system: an Escherichia coli small genomic island with novel features. PLoS One 6:e26179 doi:10.1371/journal.pone.0026179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brenciani A, et al. 2007. Genetic elements carrying erm(B) in Streptococcus pyogenes, and association with tet(M) tetracycline resistance gene. Antimicrob. Agents Chemother. 51:1209–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buu-Hoï A, Horodniceanu T. 1980. Conjugative transfer of multiple antibiotic resistance markers in Streptococcus pneumoniae. J. Bacteriol. 143:313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caillaud F, Carlier C, Courvalin P. 1987. Physical analysis of the conjugative shuttle transposon Tn1545. Plasmid 17:58–60 [DOI] [PubMed] [Google Scholar]
- 5. Caillaud F, Courvalin P. 1987. Nucleotide sequence of the ends of the conjugative shuttle transposon Tn1545. Mol. Gen. Genet. 209:110–115 [DOI] [PubMed] [Google Scholar]
- 6. Caillaud F, Trieu-Cuot P, Carlier C, Courvalin P. 1987. Nucleotide sequence of the kanamycin resistance determinant of the pneumococcal transposon Tn1545: evolutionary relationships and transcriptional analysis of aphA-3 genes. Mol. Gen. Genet. 207:509–513 [DOI] [PubMed] [Google Scholar]
- 7. Clewell DB, Flannagan SE, Jaworski DD. 1995. Unconstrained bacterial promiscuity: the Tn916-Tn1545 family of conjugative transposons. Trends Microbiol. 3:229–236 [DOI] [PubMed] [Google Scholar]
- 8. Cochetti I, Tili E, Mingoia M, Varaldo PE, Montanari MP. 2008. erm(B)-carrying elements in tetracycline-resistant pneumococci and correspondence between Tn1545 and Tn6003. Antimicrob. Agents Chemother. 52:1285–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cochetti I, et al. 2007. New Tn916-related elements causing erm(B)-mediated erythromycin resistance in tetracycline-susceptible pneumococci. J. Antimicrob. Chemother. 60:127–131 [DOI] [PubMed] [Google Scholar]
- 10. Courvalin P, Carlier C. 1986. Transposable multiple antibiotic resistance in Streptococcus pneumoniae. Mol. Gen. Genet. 205:291–297 [DOI] [PubMed] [Google Scholar]
- 11. Courvalin P, Carlier C. 1987. Tn1545: a conjugative shuttle transposon. Mol. Gen. Genet. 206:259–264 [DOI] [PubMed] [Google Scholar]
- 12. Del Grosso M, Camilli R, Iannelli F, Pozzi G, Pantosti A. 2006. The mef(E)-carrying genetic element (mega) of Streptococcus pneumoniae: insertion sites and association with other genetic elements. Antimicrob. Agents Chemother. 50:3361–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Del Grosso M, Northwood JGE, Farrell DJ, Pantosti A. 2007. The macrolide resistance genes erm(B) and mef(E) are carried by Tn2010 in dual-gene Streptococcus pneumoniae isolates belonging to clonal complex CC271. Antimicrob. Agents Chemother. 51:4184–4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fadda D, et al. 2003. Characterization of divIVA and other genes located in the chromosomal region downstream of the dcw cluster in Streptococcus pneumoniae. J. Bacteriol. 185:6209–6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farrow KA, Lyras D, Rood JI. 2001. Genomic analysis of the erythromycin resistance element Tn5398 from Clostridium difficile. Microbiology 147:2717–2728 [DOI] [PubMed] [Google Scholar]
- 16. Franke AE, Clewell DB. 1981. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of “conjugal” transfer in the absence of a conjugative plasmid. J. Bacteriol. 145:494–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hussain HA, Roberts AP, Mullany P. 2005. Generation of an erythromycin-sensitive derivative of Clostridium difficile strain 630 (630Δerm) and demonstration that the conjugative transposon Tn916ΔE enters the genome of this strain at multiple sites. J. Med. Microbiol. 54:137–141 [DOI] [PubMed] [Google Scholar]
- 18. Kazimierczak KA, Flint HJ, Scott KP. 2006. Comparative analysis of sequences flanking tet(W) resistance genes in multiple species of gut bacteria. Antimicrob. Agents Chemother. 50:2632–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Locke JB, Rahawi S, Lamarre J, Mankin AS, Shaw KJ. 2012. Genetic environment and stability of cfr in methicillin-resistant Staphylococcus aureus CM05. Antimicrob. Agents Chemother. 56:332–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marchese A, et al. 2005. Antibiotic susceptibility and serotype distribution in Streptococcus pneumoniae circulating in Italy: results of the SEMPRE surveillance study (2000–2002). Int. J. Antimicrob. Agents 26:138–145 [DOI] [PubMed] [Google Scholar]
- 21. Martin P, Trieu-Cuot P, Courvalin P. 1986. Nucleotide sequence of the tetM tetracycline resistance determinant of the streptococcal conjugative shuttle transposon Tn1545. Nucleic Acids Res. 14:7047–7058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McDougal LK, et al. 1998. Detection of Tn917-like sequences within a Tn916-like conjugative transposon (Tn3872) in erythromycin-resistant isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2312–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palmieri C, et al. 2012. Characterization of a Streptococcus suis tet(O/W/32/O)-carrying element transferable to major streptococcal pathogens. Antimicrob. Agents Chemother. 56:4697–4702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poyart-Salmeron C, Trieu-Cuot P, Carlier C, Courvalin P. 1989. Molecular characterization of two proteins involved in the excision of the conjugative transposon Tn1545: homologies with other site-specific recombinases. EMBO J. 8:2425–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roberts AP, Mullany P. 2009. A modular master on the move: the Tn916 family of mobile genetic elements. Trends Microbiol. 17:251–258 [DOI] [PubMed] [Google Scholar]
- 26. Santiviago CA, et al. 2010. Spontaneous excision of the Salmonella enterica serovar Enteritidis-specific defective prophage-like element φSE14. J. Bacteriol. 192:2246–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwarz FV, Perreten V, Teuber M. 2001. Sequence of the 50-kb conjugative multiresistance plasmid pRE25 from Enterococcus faecalis. Plasmid 46:170–187 [DOI] [PubMed] [Google Scholar]
- 28. Trieu-Cuot P, Poyart-Salmeron C, Carlier C, Courvalin P. 1990. Nucleotide sequence of the erythromycin resistance gene of the conjugative transposon Tn1545. Nucleic Acids Res. 18:3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Varaldo PE, Montanari MP, Giovanetti E. 2009. Genetic elements responsible for erythromycin resistance in streptococci. Antimicrob. Agents Chemother. 53:343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Warburton PJ, Palmer RM, Munson MA, Wade WG. 2007. Demonstration of in vivo transfer of doxycycline resistance mediated by a novel transposon. J. Antimicrob. Chemother. 60:973–980 [DOI] [PubMed] [Google Scholar]