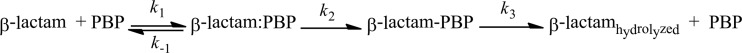Abstract
Acinetobacter baumannii is an increasingly problematic pathogen in United States hospitals. Antibiotics that can treat A. baumannii are becoming more limited. Little is known about the contributions of penicillin binding proteins (PBPs), the target of β-lactam antibiotics, to β-lactam–sulbactam susceptibility and β-lactam resistance in A. baumannii. Decreased expression of PBPs as well as loss of binding of β-lactams to PBPs was previously shown to promote β-lactam resistance in A. baumannii. Using an in vitro assay with a reporter β-lactam, Bocillin, we determined that the 50% inhibitory concentrations (IC50s) for PBP1a from A. baumannii and PBP3 from Acinetobacter sp. ranged from 1 to 5 μM for a series of β-lactams. In contrast, PBP3 demonstrated a narrower range of IC50s against β-lactamase inhibitors than PBP1a (ranges, 4 to 5 versus 8 to 144 μM, respectively). A molecular model with ampicillin and sulbactam positioned in the active site of PBP3 reveals that both compounds interact similarly with residues Thr526, Thr528, and Ser390. Accepting that many interactions with cell wall targets are possible with the ampicillin-sulbactam combination, the low IC50s of ampicillin and sulbactam for PBP3 may contribute to understanding why this combination is effective against A. baumannii. Unraveling the contribution of PBPs to β-lactam susceptibility and resistance brings us one step closer to identifying which PBPs are the best targets for novel β-lactams.
INTRODUCTION
Acinetobacter baumannii is an opportunistic nosocomial pathogen that causes infections in immunocompromised hosts and hospitalized patients (46). Reports of morbidity and mortality associated with A. baumannii infection in recent years are increasing and indicate that A. baumannii is emerging as a major clinical threat (2, 5, 10, 21, 22, 31, 32). In addition, A. baumannii became a foremost cause of morbidity and mortality in wounded soldiers returning from combat in Iraq and Afghanistan (8, 25).
A primary feature complicating the therapy of A. baumannii infections is resistance to antimicrobial agents (36). Clinicians treating patients infected with A. baumannii have antibiotic options reduced to either β-lactam–sulbactam combinations or poorly tested and potentially toxic agents, such as polymyxins B and E (colistin) and tigecycline (3, 26, 38, 41, 47). Regrettably, resistance to β-lactam–sulbactam combinations is also becoming very common (16, 34). Exacerbating this unfortunate situation is a pipeline of antibiotics from pharmaceutical firms that is essentially devoid of agents with promising anti-Acinetobacter activity, at least for the next few years. The recent development of BAL30072 and MC1 monobactams with activity against A. baumannii may offer some hope, although their potency against strains possessing extended-spectrum β-lactamases is still uncertain (23, 35).
In recent years, several studies examining the mechanisms by which A. baumannii becomes resistant to β-lactams were published (1, 9, 11, 12, 50). Most studies focused on the expression of β-lactamases (both intrinsic chromosomal β-lactamases and acquired enzymes) as the primary mechanism of resistance, although there is often a poor correlation between the intrinsic activity of the β-lactamases, the level of their expression, and the degree of resistance observed (40). Some of this variation has been attributed to other mechanisms that may affect the activity of β-lactam antibiotics, including the expression of outer membrane proteins (porins), antibiotic penetration, or the upregulation of multidrug efflux pumps (30).
One of the major mechanisms of β-lactam resistance in bacteria is through modifications in the structure or the expression of penicillin binding proteins (PBPs). PBPs are the transglycosylases, transpeptidases, and carboxypeptidases that manufacture peptidoglycan, the major component of the bacterial cell wall (15, 20). β-Lactam antibiotics inhibit the transpeptidase activity of PBPs by serving as analogues of the natural substrate, the pentapeptide precursors used to cross-link glycan strands.
Acquisition of novel PBPs (e.g., Staphylococcus aureus) or mutations that result in PBPs that confer resistance (Enterococcus faecium and Streptococcus pneumoniae) are major mechanisms of resistance in Gram-positive bacteria (29, 49). However, in Gram-negative bacteria, evidence for PBP involvement in β-lactam resistance is less studied. For species such as Haemophilus influenzae (which shares some characteristics with A. baumannii), β-lactam resistance attributable to changes in PBPs (β-lactamase-negative ampicillin-resistant [BLNAR] strains) has become a significant problem (13, 42). In A. baumannii, earlier studies demonstrated that decreased expression of PBPs and outer membrane proteins (OMPs) is associated with resistance to β-lactams (6, 14, 18, 28, 37, 39). Additionally, loss of binding to β-lactams with PBPs is also correlated with resistance to β-lactams in A. baumannii (17, 19).
Knowledge regarding the mechanisms by which PBPs contribute to β-lactam resistance and the role of PBPs in cell wall physiology in A. baumannii is still in its infancy. The importance of this gap in knowledge is highlighted by the observation that resistance to sulbactam, a β-lactamase inhibitor with an apparent affinity for PBP2, is increasing (27, 43), removing an important agent from our therapeutic armamentarium. Previous studies showed that β-lactamase inhibitors (i.e., clavulanic acid, sulbactam, and tazobactam) demonstrate intrinsic activity against A. baumannii (4, 33, 45, 48). In this work, the contribution of PBP1a and PBP3 to β-lactam susceptibility and resistance in A. baumannii and Acinetobacter sp. was investigated. Our data suggest a reason for the efficacy of the ampicillin-sulbactam combination against Acinetobacter spp.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The genes encoding PBP1a from A. baumannii ACICU and PBP3 from Acinetobacter sp. strain ATCC 27244 were cloned with a deletion in the region encoding their membrane anchor (nucleotides 1 to 93 and 1 to 189, respectively) into pET28a(+) with an N-terminal 6×His tag and expressed in Escherichia coli BL21(DE3) RP Codon Plus cells. The atomic structures of PBP1a and PBP3 from A. baumannii ACICU and Acinetobacter sp. strain ATCC 27244 served as model proteins for further study of PBPs in Acinetobacter spp. (23). PBP3 from Acinetobacter sp. strain ATCC 27244 demonstrates 86% amino acid sequence identity and 94% amino acid sequence similarity to PBP3 from A. baumannii ACICU.
PBP purification.
E. coli BL21(DE3) RP Codon Plus cells carrying either the pET28a(+) PBP1a or pET28a PBP3 plasmid were grown to an optical density at 600 nm of 0.6 in superoptimal broth (SOB) supplemented with 1× M9 salts at 37°C with shaking. Next, 100 μM isopropyl β-d-1-thiogalactopyranoside was added and cultures were moved to 16°C with shaking for 18 h. Cells were pelleted and PBPs were extracted using an Ni2+-nitrilotriacetic acid Fast Start system (Qiagen), according to the manufacturer's instructions. The purity of the fractions was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Gels were stained with Coomassie brilliant blue R250. Protein concentrations were determined by measuring the absorbance at a λ of 280 nm and using the proteins' extinction coefficients (Δε; 112,775 M−1 cm−1 for PBP1a and 46,300 M−1 cm−1 for PBP3 at 280 nm), which were obtained using the ProtParam tool at http://us.expasy.org/tools.
Kinetics.
Our methods were adapted from the work of Hujer et al. (24) and Spratt (44). Unlike PBP assays conducted with purified membrane preparations, the PBPs in these assays are soluble and purified, such that host cell β-lactamases do not complicate the assay results (17, 48). Bocillin, a fluorescent β-lactam, was used as a substrate to determine the kinetics of β-lactams and β-lactamase inhibitors with the purified PBP1a and PBP3 proteins (51).
The interaction of a β-lactam with a PBP follows a three-step reaction summarized by Fig. 1. The rate constants for association and dissociation are represented as k1 and k−1, respectively; the acylation and deacylation rate constants are k2 and k3, respectively. The mathematical expression for Michaelis constants (Kms) of a β-lactam for PBP can be represented by Equation 1.
| (1) |
The Kms for the PBPs for Bocillin were determined by incubating 50 nM PBP1a or 25 nM PBP3 with increasing concentrations of Bocillin (250 nM to 40 μM) for 20 min at 37°C in 10 mM phosphate-buffered saline at pH 7.4. The reactions were stopped by the addition of SDS-PAGE loading dye and boiling for 2 min. Samples were then analyzed using SDS-PAGE. Gels were illuminated at a λ of 365 nm and imaged with a Fotodyne gel imaging system. EZQuant gel analysis software was used to assign fluorescence intensity (FI) to the bands on the gel images; background FI was subtracted. Enzfitter software was used to analyze the data for determination of Km using Equation 2.
| (2) |
The 50% inhibitory concentrations (IC50s) of Acinetobacter PBP1a and PBP3 for β-lactams (e.g., ampicillin, cephalothin, cefotaxime, oxacillin, and doripenem) and β-lactamase inhibitors (e.g., clavulanic acid, sulbactam, and tazobactam) were measured. Here, the IC50 represents the concentration of β-lactam or β-lactamase inhibitor required to reduce the FI of Bocillin upon incubation with PBP1a or PBP3 by 50%. In the development of these assays, we discovered that competition of the β-lactam or β-lactamase inhibitor with the target PBP occurred in a time-dependent manner. We used 5 to 10 μM PBP1a or PBP3 and incubated the proteins with increasing concentrations of a β-lactam or β-lactamase inhibitor. To ensure that equilibrium between the β-lactam ligand and PBP had occurred, we preincubated the PBP and unlabeled β-lactam for 20 min at 37°C before addition of Bocillin (7). At the completion of that time, 20 μM Bocillin was added, reaction mixtures were incubated for an additional 20 min at 37°C, and the reactions were stopped and analyzed as described above. The experiments were conducted so that FI values were inversely related to percent competition. In other words, maximal FI values indicate 0% competition and no FI signal indicates 100% competition. The data were then fit to Equation 3 to determine the IC50. The IC100 value represents the concentration of β-lactam or β-lactamase inhibitor at which competition is at 100% (no FI signal). Each experiment was done in triplicate, and error measurements are shown.
| (3) |
Fig 1.
Interactions of PBPs with β-lactams.
Molecular modeling.
Computer-assisted molecular modeling was performed using the FlexX docking software (BioSolveIT) within the Sybyl platform (Tripos Inc.). The protein of Acinetobacter sp. strain ATCC 27244 with Protein Data Bank accession number 3UE3 was utilized. The following customizations were made to Thr C—C—O—H torsion angles in the active site: Thr526, _cα_cβ_oγ_hγ = 63; Thr528, _cα_cβ_oγ_hγ = 20.
RESULTS AND DISCUSSION
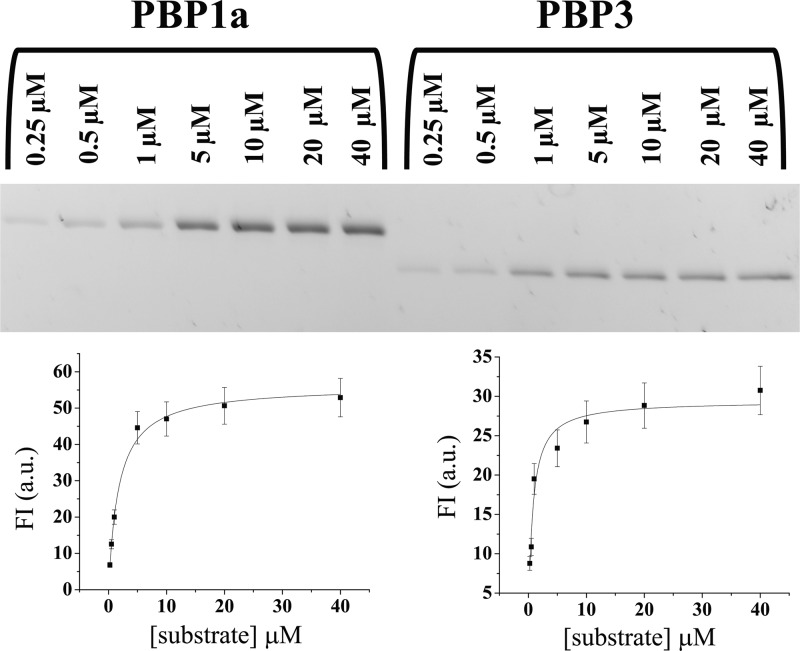
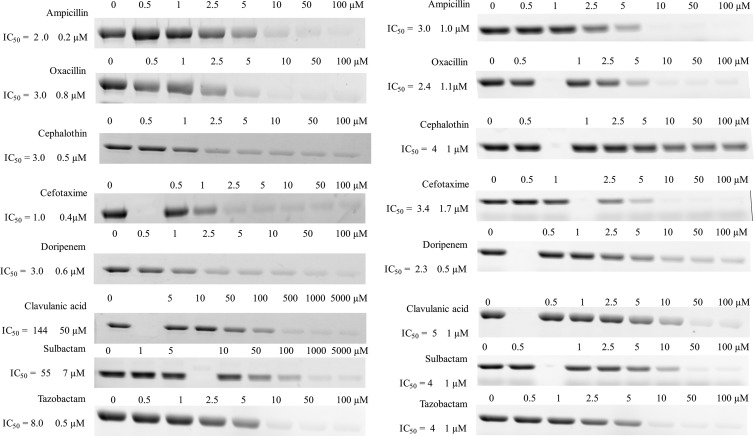
The Km values for Bocillin using 50 nM PBP1a and 25 nM PBP3 were determined (Fig. 2). PBP1a demonstrated a Km value of 1.6 ± 0.2 μM, while the Km value of PBP3 was 0.7 ± 0.1 μM. Using an in vitro assay with Bocillin, we next estimated the ability of β-lactams and β-lactamase inhibitors to interact with these target PBPs. Each β-lactam tested possessed an IC50 between 1.0 ± 0.4 and 3 ± 0.8 μM for PBP1a (Fig. 3). Here, the concentration of β-lactam or β-lactamase inhibitor required to reduce the FI of Bocillin upon incubation with PBP1a or PBP3 by 50% is the IC50. In contrast, β-lactamase inhibitors demonstrated higher IC50s for PBP1a (from 8.0 ± 0.5 to 144 ± 50 μM; Fig. 3). To compare, all β-lactams and β-lactamase inhibitors tested with PBP3 demonstrate similar IC50s between 2.3 ± 0.5 and 5 ± 1 μM. Our data also show that both penicillins and cephalosporins are equally active against both PBP1a and PBP3.
Fig 2.
Determination of Kms for PBP1a and PBP3 with Bocillin. (Top) Increasing concentrations of Bocillin (250 nM to 40 μM) with 50 nM PBP1a and PBP3; (bottom) Henri-Michaelis-Menten curves using the data from the gels depicted at the top with PBP1a (left) and PBP3 (right), plotting FI in arbitrary units (a.u.) versus substrate concentration.
Fig 3.
Determination of IC50s for PBP1a (left) and PBP3 (right) with β-lactams and β-lactamase inhibitors. Blank lanes with no concentration heading were empty wells on the SDS-polyacrylamide gel.
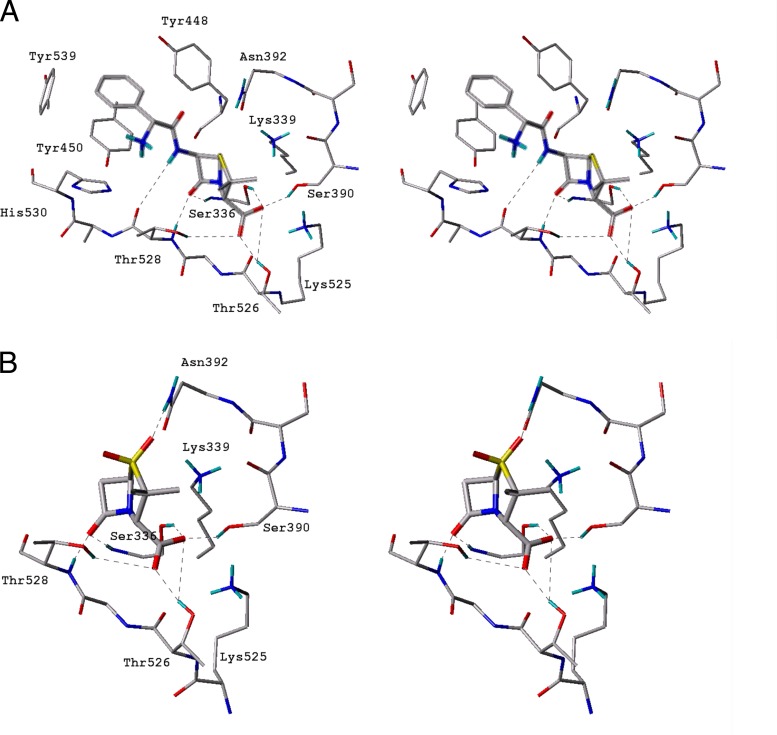
Given the clinical importance of the ampicillin-sulbactam combination against A. baumannii and the fact that the IC50s of β-lactams for PBP3 of Acinetobacter sp. appear to be lower for these compounds than for PBP1a, we generated molecular models of PBP3 with ampicillin and sulbactam. The ampicillin model proposes that the β-lactam carbonyl oxygen is tightly lodged in the oxyanion hole formed by the amide backbone of Ser336 and Thr528, with C O—HN bond distances of 1.71 and 2.00 Å, respectively (Fig. 4A). The C-3 carboxylate oxygens are recognized by a network of hydrogen bonds to the O—Hs of Ser336 and Ser390 (with C
O—HN bond distances of 1.71 and 2.00 Å, respectively (Fig. 4A). The C-3 carboxylate oxygens are recognized by a network of hydrogen bonds to the O—Hs of Ser336 and Ser390 (with C O—HO distances of 1.85 and 2.01 Å, respectively) and to the O—H of Thr526 (with a C
O—HO distances of 1.85 and 2.01 Å, respectively) and to the O—H of Thr526 (with a C O—HO distance of 1.80 Å) and electrostatic interactions with Lys525 (3.97 Å) and Lys339 (4.65 Å). Additional interactions of the aryl group of the acylamido side chain with Tyr539 (edge to face with a distance of 2.92 Å) and with Tyr450 (π stacking interaction with a distance of 3.74 Å) are present. Notably missing is a commonly observed interaction of the carbonyl oxygen of the ampicillin C-6 acylamido side chain with the terminal amido group of Asn392. In this model, we used the apoenzyme as the docking site, and there appears to be nothing preventing rotation of the Asn392 side chain to come into closer interaction with the acylamido C
O—HO distance of 1.80 Å) and electrostatic interactions with Lys525 (3.97 Å) and Lys339 (4.65 Å). Additional interactions of the aryl group of the acylamido side chain with Tyr539 (edge to face with a distance of 2.92 Å) and with Tyr450 (π stacking interaction with a distance of 3.74 Å) are present. Notably missing is a commonly observed interaction of the carbonyl oxygen of the ampicillin C-6 acylamido side chain with the terminal amido group of Asn392. In this model, we used the apoenzyme as the docking site, and there appears to be nothing preventing rotation of the Asn392 side chain to come into closer interaction with the acylamido C O group.
O group.
Fig 4.
Stereoimages of ampicillin (A) and sulbactam (B) docked into the active site of PBP3.
The model of sulbactam suggests that the β-lactam carbonyl oxygen is tightly lodged in the oxyanion hole, formed by the N—Hs of Ser336 and Thr528, with C O—HN bond distances of 1.35 and 1.98 Å, respectively (Fig. 4B). The C-3 carboxylate oxygens are recognized by a network of hydrogen bonds to the O—Hs of Ser336 and Ser390 (with C
O—HN bond distances of 1.35 and 1.98 Å, respectively (Fig. 4B). The C-3 carboxylate oxygens are recognized by a network of hydrogen bonds to the O—Hs of Ser336 and Ser390 (with C O—HO distances of 1.66 and 2.04 Å, respectively) and to the O—Hs of Thr526 and Thr528 (with C
O—HO distances of 1.66 and 2.04 Å, respectively) and to the O—Hs of Thr526 and Thr528 (with C O—HO distances of 1.89 and 3.05 Å, respectively), as well as electrostatic interactions with Lys525 (3.98 Å) and Lys339 (4.56 Å). The additional interaction of one of the two sulfone oxygens (α face) with the terminal N—H of Asn392 (1.96 Å) is particularly noteworthy.
O—HO distances of 1.89 and 3.05 Å, respectively), as well as electrostatic interactions with Lys525 (3.98 Å) and Lys339 (4.56 Å). The additional interaction of one of the two sulfone oxygens (α face) with the terminal N—H of Asn392 (1.96 Å) is particularly noteworthy.
In conclusion, we present an initial study that explores the IC50s of β-lactams and β-lactamase inhibitors for PBPs in A. baumannii and Acinetobacter sp. Surprisingly, the relatively low IC50s of the sulfone β-lactamase inhibitors (sulbactam and tazobactam) for PBP3 lend credence to the clinical observation that certain β-lactamase inhibitors are effective against A. baumannii (4, 45, 48). Most interestingly, molecular modeling proposes that productive interactions between ampicillin and sulbactam with PBP3 occur and potentially explain on a chemical basis why this combination may be potent against A. baumannii; the similarity of the intermolecular interactions with Thr526, Thr528, and Ser390 is striking. These observations may also serve to explain the selectivity of sulbactam against A. baumannii, since studies as to whether sulbactam can interact with other PBPs in other Gram-negative bacteria in a similar manner are lacking.
What are the significance of our findings? Do the low IC50s explain the efficacy of the combination? The current understanding of cell wall physiology in A. baumannii and our kinetic experiments performed here do not allow us to make an assumption about the interaction between IC50 measurements and the clinical efficacy of the combination. However, we propose the following arguments. First, in the clinic, ampicillin-sulbactam is given as a 3-g dose (2 g of ampicillin and 1 g of sulbactam). This is a concentration 33% greater than that of any β-lactam administered. We suspect that this combined amount and the low IC50s result in the complete saturation of all the binding sites (at least for PBP1a and PBP3). Second, there may be another cell wall target that binds sulbactam. Further studies are required to unravel the mechanistic basis behind PBP inhibition in A. baumannii.
ACKNOWLEDGMENTS
J.D.B. is supported by the Robert A. Welch Foundation (grant N-0871). Grants from the Veterans Affairs Career Development Program (to K.M.P.-W.), the Veterans Affairs Merit Review Program (to M.J.S., L.B.R., and R.A.B.), the National Institutes of Health (RO1 AI063517-07 to R.A.B. and 5R01AI045626-11 to L.B.R.), Geriatric Research Education and Clinical Care VISN 10 (to R.A.B.), and Pfizer (to R.A.B., M.J.S., L.B.R., and M.E.H.) supported these studies.
Footnotes
Published ahead of print 20 August 2012
REFERENCES
- 1. Amudhan SM, Sekar U, Arunagiri K, Sekar B. 2011. OXA β-lactamase-mediated carbapenem resistance in Acinetobacter baumannii. Indian J. Med. Microbiol. 29:269–274 [DOI] [PubMed] [Google Scholar]
- 2. Baang JH, et al. 2012. Longitudinal epidemiology of multidrug-resistant (MDR) Acinetobacter species in a tertiary care hospital. Am. J. Infect. Control 40:134–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balaji V, Jeremiah SS, Baliga PR. 2011. Polymyxins: antimicrobial susceptibility concerns and therapeutic options. Indian J. Med. Microbiol. 29:230–242 [DOI] [PubMed] [Google Scholar]
- 4. Beceiro A, et al. 2009. In vitro activity and in vivo efficacy of clavulanic acid against Acinetobacter baumannii. Antimicrob. Agents Chemother. 53:4298–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chung DR, et al. 2011. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am. J. Respir. Crit. Care Med. 184:1409–1417 [DOI] [PubMed] [Google Scholar]
- 6. Clark RB. 1996. Imipenem resistance among Acinetobacter baumannii: association with reduced expression of a 33–36 kDa outer membrane protein. J. Antimicrob. Chemother. 38:245–251 [DOI] [PubMed] [Google Scholar]
- 7. Copeland RA. 2000. Enzymes: a practical introduction to structure, mechanism, and data analysis. John Wiley & Sons, Inc., New York, NY [Google Scholar]
- 8. Dallo SF, Weitao T. 2010. Insights into Acinetobacter war-wound infections, biofilms, and control. Adv. Skin Wound Care 23:169–174 [DOI] [PubMed] [Google Scholar]
- 9. Davies TA, et al. 2011. Longitudinal survey of carbapenem resistance and resistance mechanisms in Enterobacteriaceae and non-fermenters from the USA in 2007-09. J. Antimicrob. Chemother. 66:2298–2307 [DOI] [PubMed] [Google Scholar]
- 10. Doyle JS, Buising KL, Thursky KA, Worth LJ, Richards MJ. 2011. Epidemiology of infections acquired in intensive care units. Semin. Respir. Crit. Care Med. 32:115–138 [DOI] [PubMed] [Google Scholar]
- 11. Espinal P, Roca I, Vila J. 2011. Clinical impact and molecular basis of antimicrobial resistance in non-baumannii Acinetobacter. Future Microbiol. 6:495–511 [DOI] [PubMed] [Google Scholar]
- 12. Esterly JS, Richardson CL, Eltoukhy NS, Qi C, Scheetz MH. 2011. Genetic mechanisms of antimicrobial resistance of Acinetobacter baumannii. Ann. Pharmacother. 45:218–228 [DOI] [PubMed] [Google Scholar]
- 13. Fali A, du Plessis M, Wolter N, Klugman KP, von Gottberg A. 2010. Single report of β-lactam resistance in an invasive Haemophilus influenzae isolate from South Africa mediated by mutations in penicillin-binding protein 3, 2003-2008. Int. J. Antimicrob. Agents 36:480–482 [DOI] [PubMed] [Google Scholar]
- 14. Fernandez-Cuenca F, et al. 2003. Relationship between β-lactamase production, outer membrane protein and penicillin-binding protein profiles on the activity of carbapenems against clinical isolates of Acinetobacter baumannii. J. Antimicrob. Chemother. 51:565–574 [DOI] [PubMed] [Google Scholar]
- 15. Fisher JF, Mobashery S. 2010. Host-guest chemistry of the peptidoglycan. J. Med. Chem. 53:4813–4829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garnacho-Montero J, Amaya-Villar R. 2010. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Curr. Opin. Infect. Dis. 23:332–339 [DOI] [PubMed] [Google Scholar]
- 17. Gehrlein M, Leying H, Cullmann W, Wendt S, Opferkuch W. 1991. Imipenem resistance in Acinetobacter baumanii is due to altered penicillin-binding proteins. Chemotherapy 37:405–412 [DOI] [PubMed] [Google Scholar]
- 18. Georgopapadakou NH. 1993. Penicillin-binding proteins and bacterial resistance to β-lactams. Antimicrob. Agents Chemother. 37:2045–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Georgopapadakou NH, Liu FY. 1980. Penicillin-binding proteins in bacteria. Antimicrob. Agents Chemother. 18:148–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goffin C, Ghuysen JM. 1998. Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs. Microbiol. Mol. Biol. Rev. 62:1079–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gupta A, et al. 2011. Incidence, risk stratification, antibiogram of pathogens isolated and clinical outcome of ventilator associated pneumonia. Indian J. Crit. Care Med. 15:96–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta A, et al. 2011. Burden of healthcare-associated infections in a paediatric intensive care unit of a developing country: a single centre experience using active surveillance. J. Hosp. Infect. 78:323–326 [DOI] [PubMed] [Google Scholar]
- 23. Han S, et al. 2011. Distinctive attributes of β-lactam target proteins in Acinetobacter baumannii relevant to development of new antibiotics. J. Am. Chem. Soc. 133:20536–20545 [DOI] [PubMed] [Google Scholar]
- 24. Hujer AM, et al. 2005. Structure-activity relationships of different β-lactam antibiotics against a soluble form of Enterococcus faecium PBP5, a type II bacterial transpeptidase. Antimicrob. Agents Chemother. 49:612–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hujer KM, et al. 2006. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob. Agents Chemother. 50:4114–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kiratisin P, Apisarnthanarak A, Kaewdaeng S. 2010. Synergistic activities between carbapenems and other antimicrobial agents against Acinetobacter baumannii including multidrug-resistant and extensively drug-resistant isolates. Int. J. Antimicrob. Agents 36:243–246 [DOI] [PubMed] [Google Scholar]
- 27. Labia R, Morand A, Lelievre V, Mattioni D, Kazmierczak A. 1986. Sulbactam: biochemical factors involved in its synergy with ampicillin. Rev. Infect. Dis. 8(Suppl 5):S496–S502 [DOI] [PubMed] [Google Scholar]
- 28. Limansky AS, Mussi MA, Viale AM. 2002. Loss of a 29-kilodalton outer membrane protein in Acinetobacter baumannii is associated with imipenem resistance. J. Clin. Microbiol. 40:4776–4778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Llarrull LI, Fisher JF, Mobashery S. 2009. Molecular basis and phenotype of methicillin resistance in Staphylococcus aureus and insights into new β-lactams that meet the challenge. Antimicrob. Agents Chemother. 53:4051–4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luo L, et al. 2011. Efflux pump overexpression in conjunction with alternation of outer membrane protein may induce Acinetobacter baumannii resistant to imipenem. Chemotherapy 57:77–84 [DOI] [PubMed] [Google Scholar]
- 31. Marchaim D, et al. 2011. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit Medical Center. Infect. Control Hosp. Epidemiol. 32:861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marra AR, et al. 2011. Nosocomial bloodstream infections in Brazilian hospitals: analysis of 2,563 cases from a prospective nationwide surveillance study. J. Clin. Microbiol. 49:1866–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Masson JM, Kazmierczak A, Labia R. 1983. Interactions of clavulanic acid and sulbactam with penicillin binding proteins. Drugs Exp. Clin. Res. IX:513–518 [Google Scholar]
- 34. Michalopoulos A, Falagas ME. 2010. Treatment of Acinetobacter infections. Expert Opin. Pharmacother. 11:779–788 [DOI] [PubMed] [Google Scholar]
- 35. Mushtaq S, Warner M, Livermore D. 2010. Activity of the siderophore monobactam BAL30072 against multiresistant non-fermenters. J. Antimicrob. Chemother. 65:266–270 [DOI] [PubMed] [Google Scholar]
- 36. Neonakis IK, Spandidos DA, Petinaki E. 2011. Confronting multidrug-resistant Acinetobacter baumannii: a review. Int. J. Antimicrob. Agents 37:102–109 [DOI] [PubMed] [Google Scholar]
- 37. Obara M, Nakae T. 1991. Mechanisms of resistance to β-lactam antibiotics in Acinetobacter calcoaceticus. J. Antimicrob. Chemother. 28:791–800 [DOI] [PubMed] [Google Scholar]
- 38. Pongpech P, et al. 2010. Antibacterial activity of carbapenem-based combinations against multidrug-resistant Acinetobacter baumannii. J. Med. Assoc. Thailand 93:161–171 [PubMed] [Google Scholar]
- 39. Quale J, Bratu S, Landman D, Heddurshetti R. 2003. Molecular epidemiology and mechanisms of carbapenem resistance in Acinetobacter baumannii endemic in New York City. Clin. Infect. Dis. 37:214–220 [DOI] [PubMed] [Google Scholar]
- 40. Ramirez MS, Adams MD, Bonomo RA, Centron D, Tolmasky ME. 2011. Genomic analysis of Acinetobacter baumannii A118 by comparison of optical maps: identification of structures related to its susceptibility phenotype. Antimicrob. Agents Chemother. 55:1520–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sanchez A, Gattarello S, Rello J. 2011. New treatment options for infections caused by multiresistant strains of Pseudomonas aeruginosa and other nonfermenting Gram-negative bacilli. Semin. Respir. Crit. Care Med. 32:151–158 [DOI] [PubMed] [Google Scholar]
- 42. San Millan A, et al. 2011. Contribution of ROB-1 and PBP3 mutations to the resistance phenotype of a β-lactamase-positive amoxicillin/clavulanic acid-resistant Haemophilus influenzae carrying plasmid pB1000 in Italy. J. Antimicrob. Chemother. 66:96–99 [DOI] [PubMed] [Google Scholar]
- 43. Sheng WH, et al. 2011. Comparative in vitro antimicrobial susceptibilities and synergistic activities of antimicrobial combinations against carbapenem-resistant Acinetobacter species: Acinetobacter baumannii versus Acinetobacter genospecies 3 and 13TU. Diagn. Microbiol. Infect. Dis. 70:380–386 [DOI] [PubMed] [Google Scholar]
- 44. Spratt BG. 1977. Properties of the penicillin-binding proteins of Escherichia coli K12. Eur. J. Biochem. 72:341–352 [DOI] [PubMed] [Google Scholar]
- 45. Suh B, Shapiro T, Jones R, Satishchandran V, Truant AL. 1995. In vitro activity of β-lactamase inhibitors against clinical isolates of Acinetobacter species. Diagn. Microbiol. Infect. Dis. 21:111–114 [DOI] [PubMed] [Google Scholar]
- 46. Towner KJ. 2009. Acinetobacter: an old friend, but a new enemy. J. Hosp. Infect. 73:355–363 [DOI] [PubMed] [Google Scholar]
- 47. Urban C, et al. 1993. Effect of sulbactam on infections caused by imipenem-resistant Acinetobacter calcoaceticus biotype anitratus. J. Infect. Dis. 167:448–451 [DOI] [PubMed] [Google Scholar]
- 48. Urban C, Go E, Mariano N, Rahal JJ. 1995. Interaction of sulbactam, clavulanic acid and tazobactam with penicillin-binding proteins of imipenem-resistant and imipenem-susceptible Acinetobacter baumannii. FEMS Microbiol. Lett. 125:193–198 [Google Scholar]
- 49. Zapun A, Contreras-Martel C, Vernet T. 2008. Penicillin-binding proteins and β-lactam resistance. FEMS Microbiol. Rev. 32:361–385 [DOI] [PubMed] [Google Scholar]
- 50. Zavascki AP, Carvalhaes CG, Picao RC, Gales AC. 2010. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev. Anti Infect. Ther. 8:71–93 [DOI] [PubMed] [Google Scholar]
- 51. Zhao G, Meier TI, Kahl SD, Gee KR, Blaszczak LC. 1999. Bocillin FL, a sensitive and commercially available reagent for detection of penicillin-binding proteins. Antimicrob. Agents Chemother. 43:1124–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]