Abstract
Lactococcin 972 (Lcn972) is a cell wall-active bacteriocin that inhibits cell wall biosynthesis in Lactococcus lactis. In this work, the transcriptomes of the Lcn972-resistant (Lcnr) mutant L. lactis D1 and its parent strain were compared to identify factors involved in Lcn972 resistance. Upregulated genes included members of the cell envelope stress (CesSR) regulon, the penicillin-binding protein pbpX gene and gene llmg2447, which may encode a putative extracytoplasmic function (ECF) anti-sigma factor. The gene llmg2447 is located downstream of the nonfunctional ECF gene sigXpseudo. Nisin-controlled expression of llmg2447 led to high Lcn972 resistance in L. lactis, with no cross-resistance to other cell wall-active antimicrobials. Upregulation of llmg2447 in L. lactis D1 (Lcnr) was linked to the integration of insertion element IS981 into the llmg2447 promoter region, replacing the native −35 box and activating the otherwise silent promoter P2447. This is the first example of an orphan ECF anti-sigma factor involved in bacteriocin resistance. This new role in neutralizing cell wall-active compounds (e.g., Lcn972) could have evolved from a putative primary function of Llmg2447 in sensing cell envelope stress.
INTRODUCTION
Integrity of the bacterial cell envelope is crucial for the survival of most bacteria. In Gram-positive bacteria, the main component of the cell wall is the peptidoglycan, a polymer made of alternate β-1→4 N-acetylglucosamine and N-acetylmuramic acid chains covalently cross-linked by short peptides (40). This three-dimensional mesh-like structure surrounds the cytoplasmic membrane and is needed to maintain cell shape and to counteract the inner osmotic pressure. The biosynthesis of peptidoglycan proceeds stepwise at the inner side of the cytoplasmic membrane through the lipid intermediates lipid I and lipid II. The peptidoglycan unit in lipid II is translocated across the membrane, polymerized by transglycosylation, and subsequently cross-linked by transpeptidation to pre-existing peptidoglycan (reviewed in references 1 and 4). The cell wall biosynthesis pathway has been, and still is, a validated target for many antibiotics (36).
Antimicrobial peptides produced by bacteria, the so-called bacteriocins, have been studied largely for their potent antibacterial activity. Their production by bacteria “generally recognized as safe,” such as lactic acid bacteria involved in food fermentations, has been the rationale for their use to inhibit undesirable bacteria in food, nisin being the first bacteriocin authorized as a food preservative (11, 15). Under the pressure of the urgent need for new antimicrobials, bacteriocins have been proposed as new anti-infectives, as several of them are also active against multiresistant pathogens (17, 29).
Nisin and other structurally related lantibiotics (lanthionine-containing bacteriocins) have been shown to inhibit cell wall biosynthesis by using the cell wall intermediate lipid II as a docking molecule for effective pore formation (5, 6). However, recognition of lipid II is not restricted solely to pore-forming lantibiotics. We have recently described lactococcin 972 (Lcn972) as the first nonlantibiotic nonpostranslationally modified bacteriocin that binds to lipid II (26). Lcn972 is a plasmid-encoded 7.5-kDa hydrophilic bacteriocin secreted by Lactococcus lactis IPLA972; it is highly bactericidal to lactococci and active in the nanomolar range (26, 27). The lack of structural homology to any other lipid II-binding molecule suggests that Lcn972 carries a novel lipid II-binding motif and could lead the way to the improvement of existing antibiotics. It has also been demonstrated that Lcn972 triggers a cell envelope stress response through the two-component system CesSR in L. lactis, as do other lipid II-binding molecules, such as bacitracin and vancomycin (28). Besides CesSR, no other cell envelope stress-sensing devices have been characterized so far in lactococci. This is in contrast to other Gram-positive bacteria, such as Bacillus subtilis, in which several two-component systems and alternative sigma factors have been implicated in cell envelope stress-sensing and regulatory mechanisms (21).
We have recently characterized L. lactis mutants with reduced susceptibility to Lcn972 to better understand the mode of action of this bacteriocin. L. lactis strain D1 was firstly isolated upon adaptation to increasing Lcn972 concentrations, and a derivative of it, L. lactis D1-20, was selected after L. lactis D1 was grown for 200 generations in the absence of Lcn972 (34). Remarkably, both mutants had an altered peptidoglycan composition with a high content of muropeptides with tripeptide side chains. Moreover, a large chromosomal deletion was identified, although a clear correlation of this mutation with the loss of susceptibility to Lcn972 could not be established and the genetic basis of the resistance remained elusive (34). In this work, genome-wide transcriptomics was carried out in order to identify the mechanism of L. lactis resistance to Lcn972. From the analysis, a particular gene (llmg2447) was identified as a key factor and the molecular basis of its transcriptional activation was further investigated. On the basis of the genetic context of this gene, the role of llmg2447 as an ancient extracytoplasmic function (ECF) anti-sigma factor is discussed.
MATERIALS AND METHODS
Bacteria, plasmids, and culture conditions.
The bacterial strains and plasmids used in this work are listed in Table 1. Lactococcal strains were routinely grown as standing cultures in M17 (Oxoid, Basingstoke, United Kingdom) supplemented with glucose at 0.5% (GM17) at 30°C or in a chemically defined medium (CDM) (10). Escherichia coli DH10B was used as an intermediate cloning host and was grown in 2×YT medium (35) at 37°C with shaking. When needed, the antibiotic chloramphenicol or tetracycline was used at 5 μg/ml. Ampicillin was used at 100 μg/ml. Antibiotics were purchased from Sigma (St. Louis, MO).
Table 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Descriptiona | Reference |
|---|---|---|
| L. lactis | ||
| MG1363 | Plasmid-free derivative of L. lactis NCDO712 | 16 |
| MG1614 | Strr Rifr derivative of L. lactis MG1363, Lcn972s | 16 |
| D1 | MG1614, highly Lcn972-resistant mutant | 34 |
| D1-20 | MG1614, low Lcn972 resistance mutant | 34 |
| NZ9000 | MG1363, pepN::nisRK; host for nisin-inducible gene expression | 12 |
| IL1403 | Plasmid free, complete ECF sigX gene | 2 |
| E. coli DH10B | Plasmid free, cloning host | Invitrogen |
| Plasmids | ||
| pCR2.1 | Cloning of PCR products, Apr | Invitrogen |
| pNZ8020 | Nisin-inducible promoter PnisA, Cmr | 12 |
| pPTPL | lacZ reporter plasmid, Tetr | 8 |
| pBL51 | pNZ8020::llmg2447 | This work |
| pBL51Cla | pBL51ΔClaI site, frameshift in llmg2447 | This work |
| pBL56 | pCR2.1::wild-type llmg2447 promoter (P2447) | This work |
| pBL57 | pCR2.1::hybrid IS981-llmg2447 promoter (PIS981::2447) | This work |
| pBL58 | pPTPL::wild-type llmg2447 promoter (P2447) | This work |
| pBL59 | pPTPL::hybrid IS981-llmg2447 promoter (PIS981::2447) | This work |
Str, streptomycin; Rif, rifampin; Em, erythromycin; Ap, ampicillin; Tet, tetracycline; superscript r, resistant; superscript s, susceptible.
Transcriptome analysis.
Genome-wide transcriptional experiments were performed using DNA microarrays covering 2,308 of the 2,435 predicted open reading frames (ORFs) in the genome of L. lactis MG1363. DNA microarray experiments were carried out essentially by following the methods for cell disruption, RNA isolation, RNA quality control, cDNA synthesis, indirect labeling, hybridization, and scanning described previously (39). RNA was extracted from four biological replicates of exponentially growing L. lactis MG1614 and D1 cultures in GM17 at 30°C (optical density at 600 nm [OD600] of 0.4 at harvesting). Data were processed as described previously (28).
Overexpression of L. lactis llmg2447.
The gene llmg2447 was amplified by PCR using 50 ng of genomic DNA of L. lactis MG1363, primers X4 and X5 (Table 2), and the high-fidelity Pwo polymerase (Roche Applied Science, Penzberg, Germany) in accordance with the manufacturer's recommendations and an annealing temperature of 50°C. The PCR product was purified using the Illustra GFX PCR DNA and Gel Band Purification kit (GE Healthcare, Buckinghamshire, United Kingdom) restricted with PstI and EcoRI and subsequently cloned into pNZ8020 under the control of the nisin-inducible promoter PnisA, yielding plasmid pBL51 in L. lactis NZ9000. A truncated version of llmg2447 was created by digestion of pBL51 at the unique internal ClaI site, filling of the cohesive ends with the DNA polymerase I Klenow fragment (TaKaRa, Otsu, Japan), and blunt-end ligation to give plasmid pBL51Cla. For induction, purified nisin (kindly supplied by Aplin & Barrett Ltd., Dorset, England) was added to melted GM17 before plating at 0.05, 0.1, and 0.2 ng/ml, as indicated.
Table 2.
Primers used in this work
| Primer | 5′–3′ sequence (underlined restriction site) | Position |
|---|---|---|
| X1 | TTTTGTCTGAATTCCAAAACTCTT (EcoRI) | 5′ llmg2447 promoter |
| X2 | CCGGATCCATTTCCTCCCCTCTC (BamHI) | 3′ llmg2447 promoter |
| X3 | TGTTCGATACATAAAAGCCCG | 3′ llmg2447 promoter |
| X4 | ATCTGCAGTGGCTCCTCTAATC (PstI) | 5′ llmg2447 gene |
| X5 | GCAAAGAATTCAATGACCGCCT (EcoRI) | 3′ llmg2447 gene |
| X6 | ACGAATTCATCAAAGTTTAGGGTATC (EcoRI) | 5′ IS981::2447 promoter |
Antimicrobial susceptibility assays.
Inhibitory activities were determined by the spot-on-the-lawn test (34). Briefly, melted GM17 agar (supplemented or not supplemented with nisin) was inoculated with overnight lactococcal cultures. Once solidified, 5-μl drops of 2-fold serial dilutions of Lcn972 purified as described previously (28) (3,200 to 0 arbitrary units [AU]/ml); nisin (200 to 0 μg/ml); and bacitracin, penicillin G, and vancomycin (64 to 0 μg/ml) were spotted onto the plates. The MIC was defined as the lowest concentration that gave a clear inhibition halo after overnight incubation.
Promoter cloning and lacZ fusions.
The wild-type P2447 and the hybrid PIS981::2447 promoter regions were amplified by PCR from genomic DNA (50 ng) of L. lactis MG1363 and L. lactis D1, respectively, with PuReTaq Ready-To-Go PCR beads (GE Healthcare, Buckinghamshire, United Kingdom). The common reverse primer X2 was used in combination with X1 or X6 to amplify P2447 and PIS981::2447, respectively (Table 2). The wild-type and hybrid promoter fragments were cloned into pCR2.1 (Invitrogen, Barcelona, Spain) in E. coli DH10B, resulting in plasmids pBL56 and pBL57, respectively, and confirmed by DNA sequencing. Both promoter fragments were excised with BamHI and EcoRI and subcloned into reporter plasmid pPTPL upstream of the promoterless E. coli lacZ gene. The ligation mixtures were used to transform L. lactis NZ9000, yielding reporter plasmids pBL58 (P2447-lacZ) and pBL59 (PIS981::2447-lacZ) (Table 1). These plasmids were also introduced into L. lactis IL1403.
Enzymatic activity assays.
Lactococcal strains were initially screened for β-galactosidase activity on GM17 agar plates supplemented with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; Apollo Scientific, Stockport, United Kingdom) at 80 μg/ml. Unless indicated otherwise, enzyme activity was quantified in L. lactis in the late exponential growth phase (OD600 of 2.0). Cells from 1 ml of culture were washed and resuspended in 1 ml of Z buffer (100 mM sodium phosphate buffer [pH 6.8], 10 mM KCl, 1 mM MgSO4) and permeabilized with 0.06 mg/ml hexadecyltrimethylammonium bromide (Sigma-Aldrich, St. Louis, MO) for 10 min at room temperature. The enzymatic reaction was conducted in microtiter plates using 170 μl of permeabilized cells (or dilutions thereof) in Z buffer and o-nitrophenyl-β-d-galactopyranoside (Sigma-Aldrich, St. Louis, MO) at a 0.5 mM final concentration in a total volume of 200 μl. The reaction mixture was incubated at 37°C, and absorbance at 420 nm (A420) was measured for 20 min in a Benchmark Plus microplate spectrophotometer (Bio-Rad). Specific activity of permeabilized cells was calculated as ΔmA420/min/OD600 unit. Assays were carried out at least in triplicate and with two independent cultures. When indicated, Lcn972 was added to the cultures at 10, 20, and 40 AU/ml and incubated for 30 min at 30°C prior to the enzymatic activity assay. Induction of the wild-type promoter P2447 in L. lactis IL1403 was screened in microtiter plates with cultures growing in CDM-glucose in the presence of subinhibitory concentrations of Lcn972, bacitracin, nisin, and vancomycin.
Bioinformatic tools.
Nucleotide and protein BLAST (http://blast.ncbi.nlm.nih.gov/) searches were used to find homologous genes or proteins, respectively. Structural predictions and motif searches were performed with InterProScan (http://www.ebi.ac.uk/InterProScan/) and Pfam (http://pfam.sanger.ac.uk/). σ70 promoter sequences were identified using Bprom (Softberry, Inc., Mount Kisco, NY). Protein topology was predicted with TMHMM Server v2.0 (http://www.cbs.dtu.dk/services/TMHMM-2.0/) and SOSUI (http://bp.nuap.nagoya-u.ac.jp/sosui/). The Microbial Signal Transduction database (Agile Genomics, LLC, Mount Pleasant, SC) was consulted to identify ECF sigma factors in L. lactis.
Microarray data accession numbers.
The DNA microarray data are available at the Gene Expression Omnibus repository under accession number GSE38349.
RESULTS
Transcriptional analysis of Lcn972-resistant L. lactis D1.
The genome-wide transcriptomic profile of L. lactis D1 was compared to that of its parent, L. lactis MG1614, to identify mutations involved in loss of susceptibility to the bacteriocin Lcn972 (Table 3). Several genes were differentially expressed. Downregulation of genes already identified as playing a role in tolerance to Lcn972 (celB, ptcC) was seen (9). Furthermore, a number of genes putatively involved in carbohydrate metabolism were seen as “downregulated” because they are absent from L. lactis D1 as a consequence of the large deletion, encompassing genes llmg0736 to llmg0751, previously identified in this strain (34). Induction of genes of the cell envelope stress response triggered by Lcn972 in L. lactis (llmg2164-llmg2163) and of other genes known to be induced in response to Lcn972 and osmotic shock (busAA, busAB) was observed (28). Thus, the primary response to Lcn972 was still activated in L. lactis D1 although Lcn972 was not present in the cultures analyzed. The highest induction level observed (35-fold) was that of llmg2447. This gene has not been identified previously as part of the primary response of L. lactis MG1614 to Lcn972 or as a member of the CesSR regulon. Therefore, we proceeded to assess its role as a factor in bacteriocin or cell wall-active antimicrobial resistance.
Table 3.
Differential gene expression in Lcn972-resistant L. lactis D1 and its parent, L. lactis MG1614
| Category, locus tag, and gene | Ratioa | Bayesian Pb | Functionc |
|---|---|---|---|
| Carbohydrate/amino acid transport and metabolism | |||
| llmg0187, celB | −3.99 | 1.12E-04 | Cellobiose-specific PTS system IIC component |
| llmg0437, ptcB | −2.57 | 1.06E-08 | Cellobiose-specific PTS system IIB component |
| llmg0454 | −3.38 | 9.67E-11 | β-Glucoside-specific PTS system IIABC component |
| llmg0458 | −5.60 | 1.47E-11 | Putative glucosyltransferase |
| llmg0739, malE | −6.35 | 3.91E-13 | Maltose ABC transporter substrate binding protein |
| llmg0740, dexC | −3.91 | 4.71E-12 | Neopullulanase |
| llmg0741, dexA | −3.75 | 1.61E-04 | Oligo-1,6-α-glucosidase |
| llmg0743, amyY | −2.55 | 2.84E-05 | α-Amylase |
| llmg0745, mapA | −6.35 | 4.58E-09 | Maltose phosphorylase |
| llmg0749, dxsB | −6.05 | 4.94E-14 | 1-Deoxy-d-xylulose-5-phosphate synthase |
| llmg0751, ascB | −12.72 | 5.21E-14 | 6-Phospho-β-glucosidase |
| llmg1048, busAA | 2.68 | 4.54E-14 | Glycine betaine/proline ABC transporter |
| llmg1049, busAB | 3.00 | 1.07E-12 | Glycine betaine-binding periplasmic protein |
| llmg1164 | 2.77 | 5.74E-04 | Putative sugar ABC transporter permease |
| llmg1869, apu | 8.42 | 3.33E-16 | Amylopullulanase |
| llmg1871, glgP | 6.91 | 2.22E-16 | Glycogen phosphorylase |
| Signal transduction/regulatory mechanisms | |||
| llmg0746, malR | −25.19 | 3.33E-16 | Maltose operon transcriptional repressor |
| llmg0747, llrF | −10.33 | 5.66E-15 | Two-component system regulator LlrF |
| llmg0748, kinF | −5.83 | 3.03E-12 | Sensor protein kinase KinF |
| llmg2163 | 2.24 | 2.73E-11 | Hypothetical protein, PspC domain |
| llmg2164 | 2.86 | 1.15E-12 | Hypothetical protein |
| llmgpseudo76, sigX | 13.15 | 2.89E-15 | ECF sigma factor |
| llmg2447 | 37.56 | 1.47E-14 | Putative anti-ECF sigma factor |
| Miscellaneous | |||
| llmg0619 | 2.58 | 7.14E-13 | Hypothetical protein |
| llmg0669 | −5.35 | 8.07E-10 | Hypothetical protein |
| llmg0752, pip | −3.08 | 1.20E-09 | Phage infection protein |
| llmg1271, gidC | −2.66 | 4.94E-09 | tRNA (uracil-5-)-methyltransferase Gid |
| llmg1679, pbpX | 6.21 | 1.73E-14 | Penicillin-binding protein |
| llmg2353, rplQ | −31.81 | 4.44E-16 | Ribosomal protein L17 |
| llmg2409, polC | 2.86 | 5.23E-13 | DNA polymerase III PolC |
Values for genes whose expression changes >2.5-fold are shown. Negative values indicate downregulation.
Determined by Cyber-T test (24).
According to GenBank accession number AM406671.1.
In silico analysis of llmg2447.
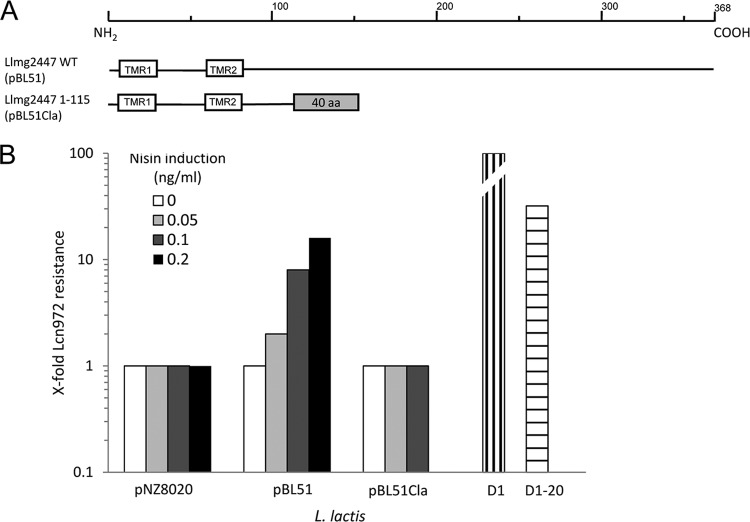
The gene llmg2447 encodes a 368-amino-acid (aa) protein in L. lactis MG1363 (GenBank accession no. AM406671.1). Topology predictions distinguished two N-terminally located transmembrane regions (Fig. 1A) and a 285-aa extracellular C-terminal domain. BLASTp searches identified proteins encoded by other available L. lactis genomes with a high degree of identity (85 to 91%), namely, those encoded by yweB in L. lactis IL1403, KF147, and CV56, and LACR_2469 in L. lactis SK11 (GenBank accession no. NP_268324, YP_003354804, ADZ64814, and YP_812019, respectively). No recognizable conserved domains could be identified in order to ascribe a putative function to Llmg2447. However, llmg2447 and its L. lactis homologues are preceded by a gene that codes for an ECF sigma factor (Pfam PF07638) annotated as sigX in L. lactis IL1403 (GenBank accession no. AAK06264) (see Fig. 2). An ECF sigma factor gene is usually transcribed together with a gene that codes for an anti-sigma factor, a membrane protein involved in regulation of the activity of the cognate ECF sigma factor (19). Considering this genetic context, we postulate that llmg2447 specifies an anti-sigma factor. In the case of L. lactis MG1363, the gene of the cognate ECF sigma factor is a pseudogene by virtue of a single mutation in the Lys5 codon (AAA to TAA), which introduces a premature stop codon into sigX (41). The same mutation was confirmed by DNA sequencing to be present in L. lactis MG1614 and in the Lcnr L. lactis strains D1 and D1-20 (data not shown).
Fig 1.
Overexpression of llmg2447 reduces susceptibility to Lcn972. (A) Domain architecture of Llmg2447 (368 aa) and its truncated version Llmg2447_1-115, which are encoded by pBL51 and pBL51Cla, respectively. TMR, transmembrane region as predicted by TMHMM Server v2.0 and SOSUI (see the text). C-terminal residues that are encoded by vector sequences are shown in the shaded box. WT, wild type. (B) Fold increase in resistance to Lcn972, relative to that under noninducing conditions (0 ng/ml nisin), of L. lactis carrying the empty vector (pNZ8020) or llmg2447-expressing vector pBL51 or pBL51Cla after induction with several concentrations of nisin (inset). Resistance of the Lcn972-resistant mutants L. lactis D1 and D1-20 is given relative to that of their parent, L. lactis MG1614. The increase in L. lactis D1 resistance was 400-fold and is indicated by the broken column. Results of a representative experiment are shown.
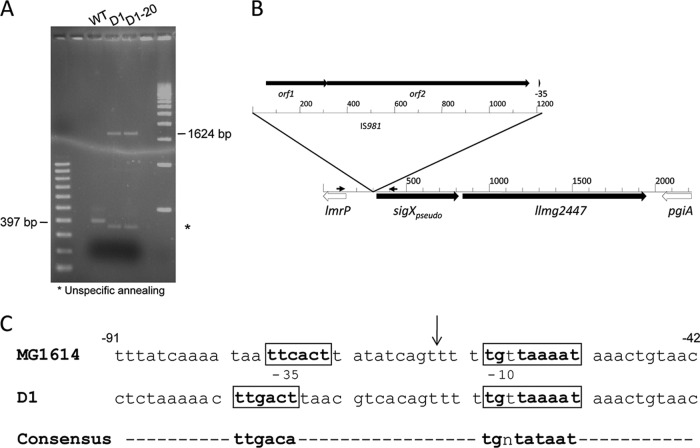
Fig 2.
Insertion of IS981 into the llmg2447 promoter region (P2447) in Lcn972-resistant (Lcnr) L. lactis. (A) PCR amplification of the lmrP-sigXpseudo intergenic region of L. lactis MG1614 (wild type [WT]) and Lcnr L. lactis strains D1 and D1-20. Primer positions are shown by small black arrows in panel B. (B) Schematic view of the insertion of IS981 in L. lactis D1. The rulers indicate nucleotides numbers. (C) Nucleotide sequence of wild-type P2447 in L. lactis MG1614 and that of the hybrid promoter in L. lactis D1. The same DNA sequence is present in L. lactis MG1363 and D1-20, respectively. Base numbering relative to the translation start site is given. The vertical arrow indicates the position of the IS981 insertion. Putative −35 and −10 regions are boxed and in bold.
Overexpression of llmg2447 reduces susceptibility to Lcn972 but does not protect against other antimicrobials.
To ascertain the role of llmg2447 in resistance to Lcn972, the gene was cloned under the control of nisin-inducible promoter PnisA into pBL51. A truncated version of llmg2447 was generated by filling in the unique internal ClaI site in llmg2447, specifying the protein fragment Llmg2447_1-115. This protein retains the two putative N-terminal transmembrane regions TMR1 and TMR2 of Llmg2447; it contains 40 unrelated amino acid residues derived from the vector in its C terminus (pBL51ClaI; Fig. 1).
Upon induction with increasing concentrations of nisin, L. lactis NZ9000/pBL51 became less susceptible to Lcn972 in a concentration-dependent fashion (Fig. 1B), reaching a plateau of 16-fold increased resistance. This is below the resistance level of L. lactis D1 and D1-20 (Fig. 1B). It was not possible to use higher concentrations of the inducer (>0.5 ng/ml) because growth of L. lactis NZ9000/pBL51 was seriously compromised. This may be due to the intrinsic constraints for production of membrane proteins in L. lactis (31). On the other hand, overexpression of llmg2447 did not change the profile of L. lactis susceptibility to other cell wall-active antibiotics such as bacitracin, penicillin G, and vancomycin; only the resistance to nisin was increased 2-fold (data not shown). Thus, the protein Llmg2447 appears to be rather specific for Lcn972.
Overall, these results validate the transcriptomic data and confirm the role of llmg2447 in counteracting the inhibitory activity of Lcn972. Interestingly, the truncated Llmg2447_1-115 protein encoded by pBL1Cla was unable to confer lower susceptibility (Fig. 1), pointing to a possible interference of the extracellular C-terminal domain with Lcn972 activity. However, expression of the truncated llmg2447 gene was more deleterious than expression of the complete version, and the clones could be induced only with low nisin concentrations (<0.2 ng/ml) (Fig. 1). Consequently, less protein would be synthesized and that might not be enough to confer Lcn972 resistance. Moreover, the stability of the truncated protein could also be compromised.
Mobilization of the insertion sequence IS981 into the llmg2447 locus in Lcnr L. lactis.
We looked for mutations within the llmg2447 locus that could explain the high level of llmg2447 expression detected by the transcriptome analysis of L. lactis D1. Only one putative σ70 promoter, P2447, could be identified in the lmrP-sigXpseudo intergenic region of L. lactis MG1363 (Fig. 2). This region was amplified by PCR from the L. lactis MG1614 chromosome with primers X1 and X3 (Table 2), and the expected 397-bp PCR product was detected. In contrast, L. lactis D1 yielded a 1.6-kbp fragment (Fig. 2). The same enlarged PCR product was detected in L. lactis D1-20.
Sequencing of the PCR products confirmed that the promoter region in L. lactis MG1614 was indistinguishable from that in MG1363. Moreover, the two Lcnr mutants were also identical to each other. Subsequent BLASTn/BLASTp searches revealed that both Lcnr strains carry a copy of the IS981 element inserted into the llmg2447 locus. The 1,223-bp insertion was 99% identical at the nucleotide level to IS981 (GenBank accession no. M33933) and contained the two ORFs, orf1 and orf2, that encode the transposase functions, as described previously (32). Insertion had taken place 63 nucleotides (nt) upstream of the AUG start codon of sigXpseudo and resulted in a 4-nt CAGT duplication at either side of the insertion (Fig. 2B).
Closer inspection revealed that IS981 had been inserted between the putative −35 and −10 regions of P2447, replacing the former with a new −35 region that is present at the 3′ end of the insertion element (Fig. 2C). This new hybrid promoter, PIS981::2447, could be responsible for the higher llmg2447 expression level in the Lcn972-resistant strains. The newly introduced −35 region is located at a proper distance (7) of 18 nt from the extended −10 region (the original distance between the −35 and −10 boxes is only 15 bp) and has only one mismatch with the canonical sequence TTGACA, in contrast to the two mismatches present in the −35 box of P2447 (Fig. 2C).
Activation of llmg2447 in L. lactis D1 is mediated by IS981.
To confirm and compare the activity of both the wild-type and hybrid PIS981::2447 promoters, they were cloned upstream of the promoterless lacZ gene in plasmid pPTPL, generating pBL58 (P2447-lacZ) and pBL59 (PIS981::2447-lacZ). Visual inspection of the GM17 plates supplemented with X-Gal indicated that the activity of the PIS981::2447 promoter was higher than that of P2447 (Fig. 3). In fact, β-galactosidase activity was detected only with permeabilized cells of L. lactis cells containing the hybrid promoter. Attempts to detect P2447 activity at different growth stages or after treatment of the cells with Lcn972 were unsuccessful (Fig. 3). Under the conditions tested, this promoter is inactive or might require active ECF SigX. To test the latter possibility, we introduced plasmid pBL58 (P2447-lacZ) into L. lactis IL1403, a strain that produces functional ECF SigX (2, 43). However, no β-galactosidase activity was detected during the growth of the strain, even in the presence of Lcn972, bacitracin, nisin, or vancomycin (data not shown).
Fig 3.
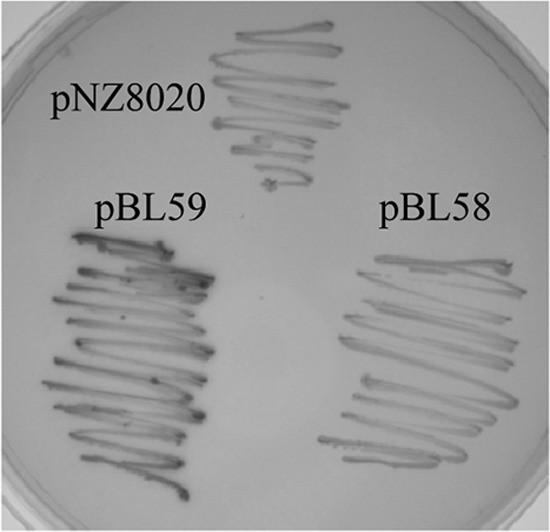
β-Galactosidase activities of reporter plasmids. L. lactis NZ9000 carrying pNZ8020 (empty vector), pBL58 (P2447-lacZ), or pBL59 (PIS981::2447-lacZ) was plated onto a GM17 plate containing 80 μg/ml X-Gal. The mean β-galactosidase specific activity ± the standard deviation in six enzymatic activity assays with two independent cultures of exponentially growing L. lactis NZ9000 cells carrying these plasmids was calculated. Cells carrying pNZ8020, pBL58 (P2447), and pBL58 (P2447) with Lcn972 added at 10, 20, and 40 AU/ml produced no activity, while those carrying pBL59 (PIS981::2447-lacZ) produced 294.2 ± 47.2 ΔmA420/min/OD600 unit.
DISCUSSION
Resistance to bacteriocins may compromise their use as biopreservatives in food or as future anti-infectives. Studies to understand the molecular mechanisms underlying bacteriocin resistance development have been undertaken recently. Genome-wide transcriptomics has been used to analyze resistance to the lantibiotic nisin (22, 25) and the class IIa bacteriocins sakacin P and pediocin PA-1 (30, 38). These reports revealed a rather complex transcriptional response in which more than 100 genes were differentially expressed in the resistant strains relative to their parents. The complexity of these phenotypes is likely due to the dual modes of action of these bacteriocins, i.e., inhibition of cell wall biosynthesis and pore formation by nisin or inactivation of the mannose phosphotransferase (PTS) transporter by the pore-forming class IIa bacteriocins (14, 42).
A general overview of the transcriptomic results presented here indicates that in the case of Lcn972, which inhibits cell wall biosynthesis without perturbing the cytoplasmic membrane, loss of susceptibility involves a discrete number of genes, particularly when those included in the chromosomal deletion (34) are disregarded. This deletion in the Lcn972-resistant strains encompassed maltose catabolic genes (malEFG, mapA, malR) and several genes involved in the degradation of polysaccharides. The upregulation of genes with similar functions in the resistant mutant, such as those for amylopullulanase (apu) and glycogen phosphorylase (glgP), is presumably done as a response to the absence of the deleted genes rather than to contribute directly to Lcn972 resistance. On the other hand, overexpression of pbpX, which codes for the transpeptidase PBP2X, is most likely the reason for the higher content of tripeptide muropeptides in the peptidoglycan of L. lactis D1, which has already been linked to resistance to Lcn972 (34).
Overall, of all of the genes with altered expression in L. lactis D1, llmg2447 appears to play a key role in Lcn972 resistance. In fact, resistance levels increased in a concentration-dependent manner relative to llmg2447 expression up to 16-fold (see Fig. 1). Yet, nisin-induced expression of llmg2447 never led to a level as high as that of the mutant L. lactis D1, which is likely explained by an extra protective effect of other gene products already associated with resistance to Lcn972, such as those of llmg2164, llmg2163, and celB (9, 33).
Activation of llmg2447 in L. lactis D1 is caused by the insertion of IS981 into the promoter region of llmg2447 during adaptation of the strain to Lcn972. It is worth noting that this insertion event appears to have been stabilized in the population because it is still present in L. lactis D1-20, i.e., after subculturing for 200 generations in the absence of selective pressure. The role of IS981 in the adaptive evolution of L. lactis under stressful conditions is well documented (13). It has been involved, for example, in the activation of an alternative lactate dehydrogenase gene (ldhB) in L. lactis ldhA mutants (3). As in the case of llmg2447, transcriptional activation of ldhB is mediated by the site- and orientation-specific insertion of IS981, so that a new hybrid and constitutive promoter is created.
Despite the role of llmg2447 in Lcn972 resistance, this gene was not previously detected in the response of L. lactis to Lcn972 (28). This can be explained now by the fact that the wild-type promoter P2447 is inactive, even when L. lactis is challenged with Lcn972. Moreover, disruption of llmg2447 in L. lactis MG1363 did not change the strain's susceptibility to Lcn972 (our own unpublished results). Therefore, under standard laboratory conditions, llmg2447 appears to be silent or may require a transcriptional activator or particular sigma factor. In this context, we postulate that llmg2447 is part of an ancestral cell envelope stress-sensing regulatory device made up of an ECF sigma factor (sigX in L. lactis IL1403, sigXpseudo in L. lactis MG1363) and its cognate anti-sigma factor, yweB (in strain IL1403) or llmg2447 (in strain MG1363). Generally, the anti-sigma factor is a membrane protein (i.e., Llmg2447) that interacts directly with the ECF sigma factor, keeping it inactive under physiological conditions. Upon stress sensed by the anti-sigma factor, the ECF sigma factor is released and is able to direct the transcription of specific genes, that of its own gene and that of the anti-sigma factor gene (19, 37). Absence of activity of the llmg2447 promoter could thus be due to the lack of a functional cognate sigma factor in L. lactis MG1363. However, when the promoter was introduced into L. lactis IL1403, a strain with a theoretically functional sigX gene, it was still inactive, even in the presence of Lcn972 and other cell wall-active antimicrobials as stimuli. Previous reports have shown that two promoters, one SigA dependent and the other SigX dependent, are involved in sigX transcription in L. lactis IL1403 (43). Lack of activation of P2447 in L. lactis IL1403 could be due to the absence of the appropriate promoter signatures recognized by IL1403 SigX or to the inability of the stimuli (i.e., Lcn972, bacitracin, nisin, and vancomycin) to induce the system in L. lactis IL1403. ECF sigma factors usually require external stimuli to activate their own transcription (19, 21).
L. lactis sigX is a member of the ECF110 group established by Starón et al. (37) together with those of Streptococcus agalactiae, S. suis, and S. equi. However, there are no clues to the function of the only ECF sigma factor in lactococci. Considering the role of llmg2447 in protecting against the cell wall-active bacteriocin Lcn972, it is tempting to speculate that L. lactis ECF SigX is part of a cell envelope-sensing regulatory system. Indeed, ECF sigma factors are key elements in the responses of B. subtilis, E. coli, and Enterococcus faecalis to cell wall-active antimicrobials such as lysozyme (18, 20, 23).
In conclusion, the putative membrane protein encoded by llmg2447 has been identified as a main factor in L. lactis resistance to the cell wall-active bacteriocin Lcn972, although the way it protects L. lactis remains to be elucidated. The genetic context of llmg2447 supports the notion that it may have been part of an ancestral ECF sigma factor regulatory system. While this regulative element may not be physiologically relevant in L. lactis, the presence of homologous systems in important pathogens such as S. agalactiae, a bacterium often involved in mastitis and other infectious diseases, deserves further attention.
ACKNOWLEDGMENTS
This work was funded by grants BIO2007-65061 and BIO2010-17414 from the Ministerio de Economía y Competitividad (Spain). C.R. is the recipient of a predoctoral JAE-CSIC fellowship. O.P.K. was supported by the Kluyver Center for Genomics of Industrial Fermentation.
We thank A. de Jong (Rijksuniversiteit Groningen, Groningen, The Netherlands) for his help with the handling of microarray data.
Footnotes
Published ahead of print 13 August 2012
REFERENCES
- 1. Barreteau H, et al. 2008. Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol. Rev. 32:168–207 [DOI] [PubMed] [Google Scholar]
- 2. Bolotin A, et al. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bongers RS, et al. 2003. IS981-mediated adaptive evolution recovers lactate production by ldhB transcription activation in a lactate dehydrogenase-deficient strain of Lactococcus lactis. J. Bacteriol. 185:4499–4507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouhss A, Trunkfield AE, Bugg TD, Mengin-Lecreulx D. 2008. The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol. Rev. 32:208–233 [DOI] [PubMed] [Google Scholar]
- 5. Breukink E, et al. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361–2364 [DOI] [PubMed] [Google Scholar]
- 6. Brötz H, et al. 1998. Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics. Mol. Microbiol. 30:317–327 [DOI] [PubMed] [Google Scholar]
- 7. Browning DF, Busby SJW. 2004. The regulation of bacterial transcription initiation. Nat. Rev. Microbiol. 2:57–65 [DOI] [PubMed] [Google Scholar]
- 8. Burgess C, O'Connell-Motherway M, Sybesma W, Hugenholtz J, van Sinderen D. 2004. Riboflavin production in Lactococcus lactis: potential for in situ production of vitamin-enriched foods. Appl. Environ. Microbiol. 70:5769–5777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campelo AB, et al. 2011. The Lcn972 bacteriocin-encoding plasmid pBL1 impairs cellobiose metabolism in Lactococcus lactis. Appl. Environ. Microbiol. 77:7576–7585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castro R, et al. 2009. Characterization of the individual glucose uptake systems of Lactococcus lactis: mannose-PTS, cellobiose-PTS and the novel GlcU permease. Mol. Microbiol. 71:795–806 [DOI] [PubMed] [Google Scholar]
- 11. Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777–788 [DOI] [PubMed] [Google Scholar]
- 12. de Ruyter PG, Kuipers OP, de Vos WM. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Visser JA, Akkermans AD, Hoekstra RF, de Vos WM. 2004. Insertion-sequence-mediated mutations isolated during adaptation to growth and starvation in Lactococcus lactis. Genetics 168:1145–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diep DB, Skaugen M, Salehian Z, Holo H, Nes IF. 2007. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc. Natl. Acad. Sci. U. S. A. 104:2384–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gálvez A, Abriouel H, López RL, Ben Omar N. 2007. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 120:51–70 [DOI] [PubMed] [Google Scholar]
- 16. Gasson MJ. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hancock RE, Sahl HG. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551–1557 [DOI] [PubMed] [Google Scholar]
- 18. Hayden JD, Ades SE. 2008. The extracytoplasmic stress factor, sigmaE, is required to maintain cell envelope integrity in Escherichia coli. PLoS One 3:e1573 doi:10.1371/journal.pone.0001573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Helmann JD. 2002. The extracytoplasmic function (ECF) sigma factors. Adv. Microb. Physiol. 46:47–110 [DOI] [PubMed] [Google Scholar]
- 20. Ho TD, Hastie JL, Intile PJ, Ellermeier CD. 2011. The Bacillus subtilis extracytoplasmic function σ factor σ(V) is induced by lysozyme and provides resistance to lysozyme. J. Bacteriol. 193:6215–6222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jordan S, Hutchings MI, Mascher T. 2008. Cell envelope stress response in Gram-positive bacteria. FEMS Microbiol. Rev. 32:107–146 [DOI] [PubMed] [Google Scholar]
- 22. Kramer NE, van Hijum SA, Knol J, Kok J, Kuipers OP. 2006. Transcriptome analysis reveals mechanisms by which Lactococcus lactis acquires nisin resistance. Antimicrob. Agents Chemother. 50:1753–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le Jeune A, et al. 2010. The extracytoplasmic function sigma factor SigV plays a key role in the original model of lysozyme resistance and virulence of Enterococcus faecalis. PLoS One 5:e9658 doi:10.1371/journal.pone.0009658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Long AD, et al. 2001. Improved statistical inference from DNA microarray data using analysis of variance and a Bayesian statistical framework. Analysis of global gene expression in Escherichia coli K12. J. Biol. Chem. 276:19937–19944 [DOI] [PubMed] [Google Scholar]
- 25. Majchrzykiewicz JA, Kuipers OP, Bijlsma JJ. 2010. Generic and specific adaptive responses of Streptococcus pneumoniae to challenge with three distinct antimicrobial peptides, bacitracin, LL-37, and nisin. Antimicrob. Agents Chemother. 54:440–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martínez B, et al. 2008. Specific interaction of the unmodified bacteriocin lactococcin 972 with the cell wall precursor lipid II. Appl. Environ. Microbiol. 74:4666–4670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martínez B, Suárez JE, Rodríguez A. 1996. Lactococcin 972: a homodimeric lactococcal bacteriocin whose primary target is not the plasma membrane. Microbiology 142:2393–2398 [DOI] [PubMed] [Google Scholar]
- 28. Martínez B, Zomer AL, Rodríguez A, Kok J, Kuipers OP. 2007. Cell envelope stress induced by the bacteriocin Lcn972 is sensed by the lactococcal two-component system CesSR. Mol. Microbiol. 64:473–486 [DOI] [PubMed] [Google Scholar]
- 29. Montalbán-López M, Sánchez-Hidalgo M, Valdivia E, Martínez-Bueno M, Maqueda M. 2011. Are bacteriocins underexploited? Novel applications for old antimicrobials. Curr. Pharm. Biotechnol. 12:1205–1220 [DOI] [PubMed] [Google Scholar]
- 30. Opsata M, Nes IF, Holo H. 2010. Class IIa bacteriocin resistance in Enterococcus faecalis V583: the mannose PTS operon mediates global transcriptional responses. BMC Microbiol. 10:224 doi:10.1186/1471-2180-10-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pinto JP, Kuipers OP, Marreddy RK, Poolman B, Kok J. 2011. Efficient overproduction of membrane proteins in Lactococcus lactis requires the cell envelope stress sensor/regulator couple CesSR. PLoS One 6:e21873 doi:10.1371/journal.pone.0021873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Polzin KM, McKay LL. 1991. Identification, DNA sequence, and distribution of IS981, a new, high-copy-number insertion sequence in lactococci. Appl. Environ. Microbiol. 57:734–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roces C, et al. 2009. Contribution of the CesR-regulated genes llmg0169 and llmg2164-2163 to Lactococcus lactis fitness. Int. J. Food Microbiol. 133:279–285 [DOI] [PubMed] [Google Scholar]
- 34. Roces C, et al. 2012. Isolation of Lactococcus lactis mutants simultaneously resistant to the cell wall-active bacteriocin Lcn972, lysozyme, nisin and bacteriophage c2. Appl. Environ. Microbiol. 78:4157–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sambrook J, Maniatis T, Fritsch EF. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 36. Schneider T, Sahl HG. 2010. An oldie but a goodie—cell wall biosynthesis as antibiotic target pathway. Int. J. Med. Microbiol. 300:161–169 [DOI] [PubMed] [Google Scholar]
- 37. Starón A, et al. 2009. The third pillar of bacterial signal transduction: classification of the extracytoplasmic function (ECF) sigma factor protein family. Mol. Microbiol. 74:557–581 [DOI] [PubMed] [Google Scholar]
- 38. Tessema GT, Moretro T, Snipen L, Axelsson L, Naterstad K. 2011. Global transcriptional analysis of spontaneous sakacin P-resistant mutant strains of Listeria monocytogenes during growth on different sugars. PLoS One 6:e16192 doi:10.1371/journal.pone.0016192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Hijum SA, et al. 2005. A generally applicable validation scheme for the assessment of factors involved in reproducibility and quality of DNA-microarray data. BMC Genomics 6:77 doi:10.1186/1471-2164-6-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vollmer W, Blanot D, de Pedro MA. 2008. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 32:149–167 [DOI] [PubMed] [Google Scholar]
- 41. Wegmann U, et al. 2007. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J. Bacteriol. 189:3256–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wiedemann I, et al. 2001. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 276:1772–1779 [DOI] [PubMed] [Google Scholar]
- 43. Wydau S, Guillouard I, Dervyn R, Guedon E, Maguin E. 2006. Characterization of SigX, an alternative sigma-factor of Lactococcus lactis, abstr 1.1–64. In Parente E, Cocolin L, Ercolini D, Vannini L. (ed), The 20th International ICFMH Symposium FoodMicro 2006 Alma Mater Studiorum, University of Bologna, Italy [Google Scholar]