Abstract
The aims of this study were to evaluate free levels of fluconazole (FCZ) in the kidneys of healthy and Candida albicans-infected Wistar rats using microdialysis and to establish the relationship between free renal and total plasma levels under both conditions. Microdialysis recovery rates were determined in vitro by dialysis, and retrodialysis recovery rates were determined in vivo by retrodialysis. The recovery rate was around 50%, independent of the method, drug concentration, or condition (in vitro or in vivo) used. FCZ kidney penetration in healthy and infected rats was investigated after the administration of 10 mg/kg of body weight intravenously (i.v.) or 50 mg/kg orally (n = 6/group) and blood and microdialysate sample harvesting at predetermined time points up to 24 and 18 h, respectively. There were no statistical differences between the area under the free concentration-time curve (AUC0–∞) values in plasma and in tissue for either healthy or infected groups for the same dose regimen investigated. The antifungal tissue penetrations were similar for both doses and under all conditions investigated (ranging from 0.77 to 0.84). The unbound fraction of FCZ was concentration independent (86.0% ± 2.0%), allowing the prediction of free renal levels using pharmacokinetic parameters obtained from total plasma fitting. The results showed that free renal and free plasma levels are similar in healthy and systemically C. albicans-infected rats. Therefore, free plasma levels are a good surrogate to estimate free FCZ renal concentrations in systemic candidiasis and can be used to optimize dosing regimens for this drug.
INTRODUCTION
Systemic fungal infections have become a serious public health problem, since they occur more frequently in the immunocompromised population, which has increased considerably in the last 2 decades due to numerous factors (22). Candida species have been identified as the fourth leading cause of fungal infections in immunocompromised patients, with a fatality rate of around 40%. Among patients undergoing bone marrow transplants and intensive chemotherapy, the rate of mortality of this disease is higher than 80% (6).
Fungal infections can take place on mucous membranes or undergo hematogenous dissemination. In the kidney, the viable count steadily increases with the progression of the infection, becoming the most severely infected organ in disseminated candidiasis (5). The antifungal azole fluconazole (FCZ) is the drug of choice for the treatment of candidemia in patients infected with Cryptococcus and Candida spp. except Candida krusei (16).
One of the most surprising aspects of antifungal therapy, however, is the lack of a consensus regarding the establishment of the parameters guiding the dose range. Although pharmacokinetic-pharmacodynamic (PK-PD) indices have recently been established for antifungal agents, following the indices established for antimicrobials, they have limitations as markers of clinical efficacy like the use of the MIC as a pharmacodynamic parameter and the consideration of total or free antifungal concentrations in plasma (1). The AUC/MIC ratio (area under the concentration-time curve over 24 h in the steady state divided by the MIC) is the predictive PK-PD index for FCZ (2).
The unbound fraction of antimicrobials and antifungals in the interstitial fluid is responsible for their pharmacological responses. In order to determine free concentrations, microdialysis (MD) in vivo has found applications in pharmacokinetic studies, especially in the investigation of drug distribution processes in various tissues and organs of animals and humans. The microdialysis technique can also be used to compare the penetrations of drugs into healthy and infected tissues (11), which may differ due to tissue alterations observed at the infection site, such as decreased pH, increased temperature, swelling as a result of plasma extravasation, and leukocyte migration, among others (18).
The plasma pharmacokinetics of FCZ are well established (10). However, the relationship between total and free concentrations of fluconazole in plasma and free concentrations in tissues using the microdialysis technique has been established in only a few studies using rats (13, 14, 24, 25), and no study has been conducted to date to investigate the free tissue level of FCZ by microdialysis in systemic candidiasis.
An evaluation of voriconazole renal penetration using microdialysis conducted by our research group using healthy Wistar rats in comparison with animals infected by Candida albicans and Candida krusei showed that systemic candidiasis did not affect renal penetration by this azole (4).
Considering that the kidneys are the most compromised organs in disseminated candidiasis (5) and that FCZ is extensively used to treat systemic Candida sp. infections (20), this study was conducted to investigate, by microdialysis, FCZ penetration into the kidneys of healthy and Candida albicans-infected Wistar rats and to establish the relationship between total plasma and free renal levels of the drug.
MATERIALS AND METHODS
Chemicals and reagents.
FCZ was purchased from Cosmetrade (Brazil). Sodium chloride, calcium chloride, potassium chloride, and sodium monobasic phosphate were purchased from Reagen (Brazil). Analytical-grade formic acid, dichloromethane, and ethyl ether were purchased from Quimex (Brazil). Liquid chromatography (LC)-grade methanol and acetonitrile were purchased from Tedia. High-performance liquid chromatography (HPLC) water was obtained with the Milli-Q system (Millipore).
Microdialysis system.
The microdialysis system consisted of a PHD 2000 syringe infusion pump (Harvard). A microliter syringe (1 ml, gas tight) was used to provide the perfusate solution. CMA/20 microdialysis probes (membrane length of 4 mm and cutoff of 20 kDa; CMA/Microdialysis AB, Solna, Sweden) were employed in this study. Ringer's solution contained 148 mM Na+, 2.3 mM Ca2+, 4 mM K+, and 157 mM Cl−.
Influence of perfusion flow rate and drug concentration on in vitro recovery.
The influence of the perfusion flow rate on the relative recovery of FCZ was evaluated by using four distinct flow rates: 1.5, 2, 2.5, and 3 μl/min. For the determination of the influence of the FCZ concentration on recovery rates, the flow rate was fixed at 2 μl/min, and three different FCZ concentrations were used to determine recovery by retrodialysis and dialysis: 0.5, 1.0, and 2.0 μg/ml. The recoveries at higher FCZ concentrations (50, 100, and 500 μg/ml) were also investigated. The relative recovery of FCZ (n = 3) was determined by dialysis and retrodialysis using a 1-μg/ml solution described previously (3, 23).
The relative recovery by dialysis (RRD) was calculated as follows:
| (1) |
where Cdial is the drug concentration in the dialysate and Cext is the drug concentration in the medium surrounding the microdialysis probe.
The relative recovery by retrodialysis (RRRD) was calculated as follows:
| (2) |
where Cperf is the drug concentration in the perfusate solution.
Animals.
The protocols for animal experiments were approved by the Universidade Federal do Rio Grande do Sul Ethics in Research Committee (protocol number 2008187).
Male Wistar rats (250 to 350 g), purchased from the State Foundation for Research and Production in Health (FEPPS, Porto Alegre, Brazil), were used in the experiments. The animals were housed under standard conditions (7). The animals that received fluconazole oral doses were deprived of food 12 h before experimentation and 4 h after dosing. Water was allowed ad libitum.
In vivo recovery.
The in vivo recovery of FCZ was determined by retrodialysis using three anesthetized male Wistar rats according to the surgical procedure described below. Recovery was determined by using Ringer's solutions containing FCZ at 1 μg/ml (2.0 μl/min). Microdialysate samples were collected from each probe in 30-min intervals up to 2 h. Drug concentrations in the dialysate sample (Cdial) and in the perfusate solution (Cperf) were determined by HPLC. The in vivo apparent recovery by retrodialysis was calculated by using equation 2.
FCZ kidney penetration experimental design.
The rats were randomly distributed into four groups of six animals each: two healthy groups received either 10 mg/kg of body weight intravenously (i.v.) or a 50-mg/kg oral gavage of FCZ; the other two groups were infected with C. albicans ATCC 18804 and received either one of the two doses by the same routes. The FCZ solution for both administrations was prepared in a 0.9% NaCl solution containing 1% dimethyl sulfoxide (DMSO).
Induction of infection.
The infection protocol for Wistar rats employed in this study was previously described by Araujo et al. (4). Briefly, nonimmunocompromised animals were infected with C. albicans (ATCC 188041) 2 days prior to the pharmacokinetic experiments by i.v. injection through the lateral tail vein of 0.1 ml of the inoculum containing 2.5 × 106 CFU/ml of the fungal strain, prepared in 0.9% sterile saline.
Pharmacokinetic studies.
On the day of the experiments, urethane-anesthetized infected animals (1.25 g/kg intraperitoneally [i.p.]) had a sampling cannula surgically inserted into the carotid artery. The microdialysis probe was inserted into the kidney cortex (3). The probes were allowed to equilibrate for 1 h before FCZ dosing by the i.v. or oral route. At predetermined times before i.v. dosing (time zero), after i.v. dosing (0.08, 0.25, 0.5, 0.75, 1, 2, 4, 8, 12, 18, and 24 h), and after oral dosing (0.25, 0.5, 0.75, 1, 1.5, 2, 4, 8, 12, 18, and 24 h), approximately 200 μl of blood was withdrawn into heparinized tubes. Plasma was separated by centrifugation (6,800 × g at 21°C ± 1°C for 15 min) and stored at −20°C until analysis. Microdialysis samples were collected over 18 h at 30-min intervals and were assayed directly after the experiment, without processing.
The unbound concentrations of FCZ in the kidney were calculated from the measured microdialysate concentrations by using the relative recovery rate determined by retrodialysis in vivo.
The plasma and tissue concentration-versus-time profiles were analyzed individually for each animal by using the noncompartmental approach to determine the following pharmacokinetic parameters: peak plasma concentration (Cmax), time of maximum concentration (tmax), elimination rate constant (ke), area under the concentration-time curve from 0 h to infinity (AUC0–∞), clearance (CLtot), half-time (t1/2), volume of distribution (V), and absolute bioavailability (Fabs) (21). The ratio of AUC0–∞, tissue/AUC0–∞, plasma was calculated as a measure of drug penetration into the kidney.
In order to establish the relationship between total plasma and free tissue levels, the individual profiles were fitted by using the SCIENTIST v.2.0.1 nonlinear regression program (MicroMath). One-, two-, and three-compartment models with or without weighting schemes were evaluated. The best model to fit the data was chosen based on the random distribution of the residual plot, the correlation coefficient, and the model selection criterion (MSC) given by the software.
The individual FCZ plasma profiles obtained after intravenous and oral administrations were best described by the two-compartmental open model (equation 3) and the one-compartment open model with first-order elimination and first-order absorption (equation 4), respectively:
| (3) |
where C is the total plasma concentration; a and b are the intercepts for the distribution and elimination phases, respectively; α and β are the rate constants representing distribution and elimination, respectively; and t is time, and
| (4) |
where D is the dose administered, Fabs is the absolute bioavailability, V is the volume of distribution, and ka is the first-order absorption rate constant. The best individual fit after i.v. and oral dosing was obtained by using 1/concentration as a weighting scheme.
The pharmacokinetic constants obtained from plasma fitting were used to predict free kidney concentrations using equations 5 and 6 after intravenous and oral dosing, respectively:
| (5) |
| (6) |
where Ct is the free tissue level of the drug and fu is the fraction unbound in plasma.
Pharmacokinetic parameters in plasma were also determined after compartmental modeling (21).
Protein binding.
Different FCZ concentrations (10, 50, 100, and 1,000 μg/ml) were used to evaluate in vitro rat plasma protein binding by employing microdialysis. The experiments were carried out with a pool of plasma from different animals. Microdialysis probes (n = 3) previously calibrated in vitro were inserted into plasma samples at different concentrations and allowed to equilibrate at 37°C ± 1°C for 1 h before sampling. Retrodialysis was carried out at a flow rate of 2.0 μl/min using Ringer's solution. Three samples were collected at 30-min intervals. An aliquot for the analysis of the total plasma concentration was removed before microdialysis sampling. Protein binding was determined as the ratio between the difference in the total and unbound concentrations and the total concentration, after unbound concentrations were corrected for by the in vivo relative recovery rate.
Quantification of FCZ in plasma and microdialysate samples.
The quantification of FCZ in rat plasma and microdialysate samples was performed by liquid chromatography with tandem mass spectrometry (LC-MS/MS) and liquid chromatography with UV detection (LC-UV), respectively.
A method for the quantification of FCZ in plasma was validated according to U.S. Food and Drug Administration (FDA) guidelines (8; F. J. Azeredo, F. T. Uchoa, and T. Dalla Costa, submitted for publication). Briefly, 20 μl of 0.1 M NaOH was added to plasma aliquots (90 μl) and extracted with 1.0 ml of ethyl ether-dichloromethane (70:30, vol/vol). After centrifugations (18,000 × g for 15 min), the supernatant was dried under a vacuum at 50°C, and the residue was reconstituted in 100 μl of the mobile phase. Following a second centrifugation (18,000 × g for 5 min), 20-μl supernatant aliquots were injected into an LC instrument (Shimadzu, Kyoto, Japan) in tandem with a Micromass Quattro LC mass spectrometer system (Waters, Milford, MA). Chromatography was performed by using a Shimadzu Shim-pack column (150-mm by 4.6-mm internal diameter [i.d.]) protected by a guard column packed with same material. The mobile phase (methanol-water-formic acid mixture [90:10:0.1, vol/vol/vol]) was pumped at 0.3 ml/min. Detection was performed in the positive electrospray ionization (ES+) mode by monitoring the parental fragment transitions at 307.1 > 219.6 m/z for FCZ. The analyses were conducted by using Mass-Lynx software (version 3.5). The method was sensitive and accurate within the concentration range of 10 to 2,000 ng/ml.
For the quantification of FCZ in microdialysate samples, a previously reported LC-UV method was adapted (12). A Waters HPLC system equipped with a 600 pump controller, a 717 Plus automatic injector, a 2487 dual λ absorbance detector, and Millennium 321 software was used. Chromatography was performed on a Waters NovaPak C18 HPLC column (3.9-mm by 150-mm i.d.) preceded by a guard column (3.9 mm by 20 mm, with a 4-mm particle size) packed with the same material. The mobile phase, 10 mM sodium phosphate buffer-acetonitrile (70:30, vol/vol), adjusted to pH 5.7 with phosphoric acid, was pumped at a flow rate of 1.0 ml/min. FCZ detection was performed at 210 nm, and 40-μl sample aliquots were injected. A linear calibration curve was obtained in the range of 0.1 to 50 μg/ml by using the drug peak area. Prior to use, the method was validated according to U.S. FDA guidelines (9).
Statistical analysis.
The differences between pharmacokinetic parameters determined among different groups or microdialysis recovery rates determined under different conditions in vitro or in vivo were evaluated by analysis of variance (ANOVA) or Student's t test, as appropriate (α = 0.05).
RESULTS AND DISCUSSION
Candida albicans is the main etiological agent responsible for invasive fungal infections, being the most used Candida sp. in in vivo models of candidiasis due to its high virulence in immunocompetent animals (10).
Antifungal tissue penetration is fundamental for the better design of dosing regimens (2). An inadequate tissue penetration of these agents can lead to therapeutic failure and resistance. Pharmacokinetic evaluations of antifungal agents should therefore be based on pharmacologically active free tissue concentrations rather than on total plasma concentrations (15). Changes in the free drug equilibrium between plasma and the interstitial space can be expected in Candida sp.-infected tissues due to the secretion of phospholipases and proteinases, which change the physiological conditions of the infected organ and induce an inflammatory response by macrophages (18).
Studies employing microdialysis to determine free tissue concentrations of antifungal agents are rare in the literature. There have been articles that reported FCZ brain penetration in rats (17) and subcutaneous tissue distribution in humans (19). In the present study, microdialysis was employed to measure FCZ penetration into the kidneys of healthy and systemically C. albicans-infected Wistar rats and to correlate these values with total plasma concentrations of the drug.
The relative recovery rates of FCZ determined in vitro by the RRD were 50.1% ± 4.3%, 53.4% ± 3.1%, 43.9% ± 3.3%, and 40.3% ± 3.5%, and those determined by the RRRD were 51.9% ± 3.3%, 54.2% ± 1.2%, 45.8% ± 5.3%, and 43.7% ± 3.3% for the 1.5-, 2.0-, 2.5-, and 3.0-μl/min flow rates, respectively. For the same flow rate, there was no statistical difference between the recovery rates determined by both methods, indicating that FCZ does not stick to the microdialysis probes and probe tubing.
The influence of the drug concentration on the microdialysis recoveries of drug was evaluated by using a 2.0-μl/min flow rate. The average recovery rates obtained by dialysis were 52.1% ± 3.9%, 53.1% ± 2.9%, and 52.0% ± 1.7%, and those obtained by retrodialysis were 51.5% ± 2.6%, 52.9% ± 2.6%, and 49.8% ± 1.9% for FCZ concentrations of 0.5, 1, and 2 μg/ml, respectively. For the higher concentrations investigated (10, 100, and 500 μg/ml), no statistical differences in the recovery rates were observed by dialysis or retrodialysis in relation to the recovery rates determined for lower concentrations.
The in vivo RRRD was determined to be 49.7% ± 2.3%, slightly lower than the recovery rate determined in vitro by retrodialysis at the same flow rate but not statistically different. The in vivo mean recovery rate was used to back-calculate FCZ tissue levels in the animal experiments.
The results showed that FCZ recovery is concentration independent and inversely proportional to the flow rate, in agreement with previously reported data (20). Independent of the method employed, whether dialysis or retrodialysis, the FCZ recovery rate was around 50% in vitro as well as in vivo. Different from voriconazole, which shows a log P of 1.8, which is important for the treatment of yeast infections but causes drug binding to microdialysis tubing, making it difficult to conduct this kind of experiment (3), FCZ shows a log P of 0.5, which ensures that the drug can freely move inside the microdialysis probe tubing, not interfering with the conduction of the experiment.
FCZ protein binding determined over the plasma concentration range observed in animals (10 to 1,000 μg/ml), 14.0% ± 2.0%, was constant and independent of the concentration investigated. This average value was used for the prediction of free plasma levels based on the total concentrations determined and also for the prediction of tissue free levels.
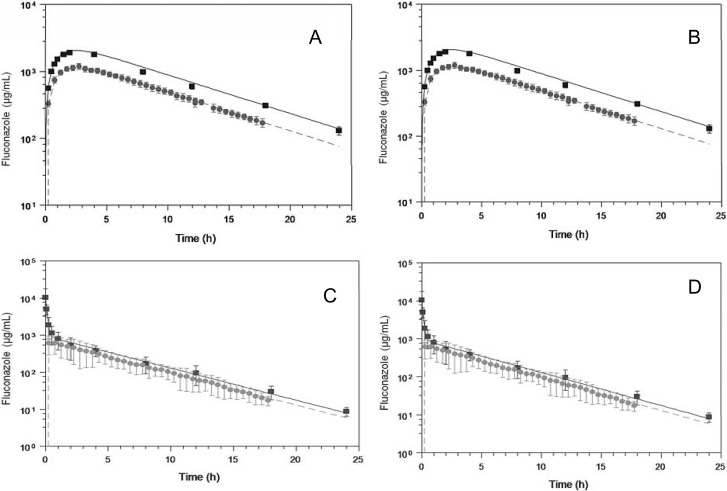
FCZ total plasma and free kidney concentration-time profiles after the administration of 10-mg/kg i.v. and 50-mg/kg oral doses to healthy and systemically C. albicans-infected Wistar rats are shown in Fig. 1. Total plasma concentrations after intravenous and oral dosing were fitted to a two-compartment open model and a one-compartment model with first-order absorption and first-order elimination, respectively.
Fig 1.
Mean FCZ total plasma (■) and free kidney (●) concentration-time profiles in healthy (A and C) and Candida albicans-infected (B and D) Wistar rats after 50-mg/kg oral (A and B) or 10-mg/kg i.v. bolus (C and D) dosing. Solid lines represent total plasma levels fitted by using equations 3 and 4 after i.v. or oral dosing, respectively. Dashed lines represent free kidney levels predicted by using equations 5 and 6 for i.v. and oral dosing, respectively (see the text for details) (means ± standard deviations are shown; n = 6/group).
Table 1 shows the pharmacokinetic parameters determined by individually analyzing plasma profiles after i.v. dosing (Fig. 1A and B). The parameters determined by compartmental analysis were not statistically different (P > 0.05) from those determined by the model-independent analysis. The time to peak concentration (tmax) was 0.75 h in kidney, demonstrating that in rats, FCZ penetration into tissues occurs rapidly. The t1/2 and the AUC0–∞ were not statistically different (P > 0.05) across plasma and kidney, indicating good agreement between the free kidney levels determined by microdialysis and the predicted levels based on plasma parameters and protein binding after i.v. dosing. There were no statistical differences between the plasma and tissue pharmacokinetic parameters estimated for healthy and Candida-infected animals. Fluconazole showed high renal penetration values, 0.87 and 0.89 in healthy and infected Wistar rats, respectively.
Table 1.
Pharmacokinetic parameters estimated after a single i.v. bolus of a 10-mg/kg dose of FCZ administered to healthy and C. albicans-infected rats (n = 6/group)
| Pharmacokinetic parametera | Mean value for group ± SD |
|||||
|---|---|---|---|---|---|---|
| Healthy animals |
Infected animals |
|||||
| Plasma, model independent | Plasma, 2-compartment model | Kidney, model independent | Plasma, model independent | Plasma, 2-compartment model | Kidney, model independent | |
| a (μg/ml) | 956.7 ± 78.7 | 1,022.9 ± 166.3 | ||||
| b (μg/ml) | 635.5 ± 88.5 | 759.3 ± 131.8 | ||||
| α (h−1) | 7.03 ± 1.51 | 6.70 ± 1.64 | ||||
| β (h−1) | 0.101 ± 0.004 | 0.103 ± 0.004 | ||||
| t1/2 β (h) | 6.9 ± 0.3 | 6.7 ± 0.2 | ||||
| ke (h−1) | 0.104 ± 0.017 | 0.110 ± 0.007 | 0.101 ± 0.007 | 0.108 ± 0.010 | ||
| t1/2 (h) | 6.8 ± 1.1 | 6.3 ± 0.4 | 6.9 ± 0.4 | 6.5 ± 0.6 | ||
| AUC0–∞ (μg · h/ml) | 6,621 ± 1,592 | 6,446 ± 848.2 | 5,723 ± 1,241 | 7,900 ± 2,007 | 7,512 ± 1,349 | 7,054 ± 1,348 |
| CLtot (ml/min · kg) | 1.6 ± 0.4 | 1.6 ± 0.2 | 1.4 ± 0.5 | 1.4 ± 0.2 | ||
| Vss (liters/kg) | 8.5 ± 2.9 | 7.7 ± 0.9 | 6.8 ± 2.7 | 5.7 ± 1.7 | ||
| Kidney penetrationb | 0.87 | 0.89 | ||||
Vss, volume of distribution at steady state.
Mean AUC0–∞, tissue/mean AUC0–∞, total plasma (n = 6/group). Comparisons were done by ANOVA (α = 0.05).
Pharmacokinetic parameters estimated by individually fitting plasma data to a one-compartment model and by a model-independent analysis of plasma and tissue data after oral FCZ dosing in healthy and C. albicans-infected Wistar rats are summarized in Table 2. The parameters determined by compartmental analysis were not statistically different (α < 0.05) from those determined by the model-independent analysis. The observed plasma Cmax and tmax values were 2,700 ± 538 μg/ml and 2 h, respectively, in healthy animals and 3,357 ± 638 μg/ml and 2 h, respectively, in infected ones. FCZ showed high bioavailability values, ranging from 77% in infected to 84% in healthy Wistar rats, in accordance with the values reported previously by Humphrey et al. (10). The t1/2 values were not statistically different (P > 0.05) between plasma and kidney. The AUC0–∞ values across plasma and kidney were not statistically different, resulting in high FCZ renal penetration after oral dosing (0.77 and 0.78 in healthy and Candida-infected animals, respectively). Good agreement between the free kidney levels determined by microdialysis and the predicted levels based on plasma parameters and protein binding, using an appropriate equation (equation 6), was obtained (Fig. 1C and D).
Table 2.
Pharmacokinetic parameters estimated after an oral 50-mg/kg dose of FCZ in healthy and C. albicans-infected rats (n = 6/group)
| Pharmacokinetic parameter | Mean value for group ± SD |
|||||
|---|---|---|---|---|---|---|
| Healthy rats |
Infected rats |
|||||
| Plasma, model independent | Plasma, 1-compartment model | Kidney, model independent | Plasma, model independent | Plasma, 1-compartment model | Kidney, model independent | |
| ke (h−1) | 0.102 ± 0.007 | 0.103 ± 0.005 | 0.118 ± 0.011 | 0.113 ± 0.012 | 0.110 ± 0.005 | 0.112 ± 0.017 |
| t1/2 (h) | 6.8 ± 0.7 | 6.7 ± 0.6 | 5.4 ± 0.7 | 6.2 ± 0.6 | 6.3 ± 0.3 | 6.3 ± 1.2 |
| AUC0–∞ (μg · h/ml) | 27,887 ± 4,529 | 21,368 ± 1,524 | 30,214 ± 1,582 | 23,532 ± 1,743 | ||
| CLtot (ml/min · kg) | 1.83 ± 0.31 | 1.58 ± 0.39 | 1.66 ± 0.08 | 1.33 ± 0.22 | ||
| V (liters/kg) | 13.11 ± 2.169 | 15.15 ± 3.19 | 9.39 ± 1.63 | 11.98 ± 1.79 | ||
| ka (h−1) | 0.27 ± 0.05 | 0.34 ± 0.04 | ||||
| Kidney penetrationa | 0.77 | 0.78 | ||||
Mean AUC0–∞, tissue/mean AUC0–∞, total plasma (n = 6/group). Comparisons were done by ANOVA (α = 0.05).
The results obtained for FCZ in this paper are similar to those previously reported for voriconazole (4), indicating that disseminated candidiasis does not interfere with the kidney penetration of these azoles.
In conclusion, FCZ easily penetrates the kidney, and free tissue levels can be predicted based on total concentrations and protein binding in plasma. Furthermore, disseminated Candida sp. infection does not interfere with FCZ kidney penetration, indicating that free plasma concentrations are a good surrogate for active levels in both healthy and infected kidneys and can be used to optimize FCZ dosing regimens to treat disseminated candidiasis.
ACKNOWLEDGMENTS
We thank Alexandre Fuentefria for the donation of the ATCC strain of Candida albicans used in this study.
We thank CNPq-Brazil (process 142351/2009-1) for scholarship and financial support.
Footnotes
Published ahead of print 4 September 2012
REFERENCES
- 1. Andes D. 2006. Pharmacokinetics and pharmacodynamics of antifungals. Infect. Dis. Clin. North Am. 20:679–697 [DOI] [PubMed] [Google Scholar]
- 2. Andes D. 2003. In vivo pharmacodynamics of antifungal drugs in treatment of candidiasis. Antimicrob. Agents Chemother. 47:1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Araujo BV, Silva CF, Haas SE, Dalla Costa T. 2008. Microdialysis as a tool to determine the free kidney levels of voriconazole in rodents: a model to study the technique feasibility for a moderately lipophilic drug. J. Pharm. Biomed. Anal. 47:876–881 [DOI] [PubMed] [Google Scholar]
- 4. Araujo BV, Silva CF, Haas SE, Dalla Costa T. 2009. Free renal levels of voriconazole determined by microdialysis in healthy and Candida sp.-infected Wistar rats. Int. J. Antimicrob. Agents 33:154–159 [DOI] [PubMed] [Google Scholar]
- 5. Balk MW, Crumrine MH, Fischer GW. 1978. Evaluation of miconazole therapy in experimental disseminated candidiasis in laboratory rats. Antimicrob. Agents Chemother. 13:321–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bustamante CI. 2005. Treatment of Candida infection: a view from the trenches! Curr. Opin. Infect. Dis. 18:490–495 [DOI] [PubMed] [Google Scholar]
- 7. CCAC 2003. Guidelines. Canadian Council on Animal Care, Ottawa, Ontario, Canada: http://ccac.ca/Documents/Standards/Guidelines/Facilities.pdf [Google Scholar]
- 8. FDA 2001. Guidance for industry—bioanalytical method validation. FDA, Washington, DC: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM128049.pdf Accessed September 2012 [Google Scholar]
- 9. Graybill J, Montalbo E, Patterson T. 1998. Fluconazole versus Candida albicans: a complex relationship. Antimicrob. Agents Chemother. 42:2938–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Humphrey MJ, Jevons S, Tarbit SH. 1985. Pharmacokinetic evaluation of UK-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob. Agents and Chemother. 28:648–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joukhadar C, Derendorf H, Muller M. 2001. Microdialysis—a novel tool for clinical studies of anti-infective agents. Eur. J. Clin. Pharmacol. 57:211–219 [DOI] [PubMed] [Google Scholar]
- 12. Kim SS, et al. 2007. An optimized analytical method of fluconazole in human plasma by high-performance liquid chromatography with ultraviolet detection and its application to a bioequivalence study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 852:174–179 [DOI] [PubMed] [Google Scholar]
- 13. Lee CH, Yeh PH, Tsai TH. 2002. Hepatobiliary excretion of fluconazole and its interaction with cyclosporin A in rat blood and bile using microdialysis. Int. J. Pharm. 241:367–373 [DOI] [PubMed] [Google Scholar]
- 14. Mauric O, et al. 2011. The ability of fluconazole to penetrate into ventilated, healthy and inflamed lung tissue in a model of severe sepsis in rats. Pharmacology 87:130–134 [DOI] [PubMed] [Google Scholar]
- 15. Muller M, De la Pena A, Derendorf H. 2004. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrob. Agents Chemother. 48:1441–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pappas PG, et al. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38:161–189 [DOI] [PubMed] [Google Scholar]
- 17. Pfaller MA, Pappas PG, Wingard JR. 2006. Invasive fungal pathogens: current epidemiological trends. Clin. Infect. Dis. 43(Suppl 1):S3–S14 [Google Scholar]
- 18. Romani L, Bistoni F, Puccetti P. 2003. Adaptation of Candida albicans to the host environment: the role of morphogenesis in virulence and survival in mammalian hosts. Curr. Opin. Microbiol. 6:338–343 [DOI] [PubMed] [Google Scholar]
- 19. Sasongko L, Williams KM, Day RO, McLachlan AJ. 2003. Human subcutaneous tissue distribution of fluconazole: comparison of microdialysis and suction blister techniques. Br. J. Clin. Pharmacol. 56:551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sauermann R, et al. 2005. Pharmacokinetics and pharmacodynamics of cefpirome in subcutaneous adipose tissue of septic patients. Antimicrob. Agents Chemother. 49:650–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shargel L, Wu-Pong S, Yu ABC. 2005. Applied biopharmaceutics & pharmacokinetics, p 52–184 McGraw-Hill, New York, NY [Google Scholar]
- 22. Sheehan DJ, Hitchcock CA, Sibley CM. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tasso L, Bettoni C, Oliveira L, Dalla Costa T. 2008. Evaluation of gatifloxacin penetration into skeletal muscle and lung by microdialysis in rats. Int. J. Pharm. 358:96–101 [DOI] [PubMed] [Google Scholar]
- 24. Xavier F, et al. 2003. On-line determination of fluconazole in blood and dermal rat microdialysates by microbore high-performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 787:323–331 [DOI] [PubMed] [Google Scholar]
- 25. Yang H, Wang Q, Elmquist WF. 1996. Fluconazole distribution to the brain: a crossover study in freely-moving rats using in vivo microdialysis. Pharm. Res. 13:1570–1575 [DOI] [PubMed] [Google Scholar]