Abstract
TD-1792 is a first-in-class glycopeptide-cephalosporin heterodimer that exhibits bactericidal activity against Gram-positive pathogens. We conducted a randomized, double-blind, active-control, phase II trial in patients with complicated skin and skin structure infections caused by suspected or confirmed Gram-positive organisms. Patients 18 to 65 years old were randomized to receive 7 to 14 days of either TD-1792 (2 mg/kg of body weight intravenously [i.v.] every 24 h [q24h]) or vancomycin (1 g i.v. q12h, with dosage regimens adjusted per site-specific procedures). A total of 197 patients were randomized and received at least one dose of study medication. Rates of clinical success at the test-of-cure evaluation were similar in all analysis populations. Among 170 clinically evaluable patients, cure rates were 91.7% and 90.7% in the TD-1792 and vancomycin groups, respectively (95% confidence interval [CI] of −7.9 to 9.7 for the difference). In microbiologically evaluable patients with methicillin-resistant Staphylococcus aureus at baseline (n = 75), cure rates were 94.7% in the TD-1792 group and 91.9% in the vancomycin group. Microbiological eradication of Gram-positive pathogens (n = 126) was achieved in 93.7% and 92.1% of patients in the TD-1792 and vancomycin groups, respectively. Seven patients were discontinued from study medication due to an adverse event (AE): 2 and 5 in the TD-1792 and vancomycin groups, respectively. AEs were of similar types and severities between the two groups, other than pruritus, which was more common in patients who received vancomycin. No patients in the TD-1792 group experienced a serious AE. This study supports further clinical development of TD-1792 in patients with Gram-positive infection.
INTRODUCTION
Dramatic changes are occurring in the epidemiology of infections due to methicillin-resistant Staphylococcus aureus (MRSA) (4). Rates of MRSA infection are increasing worldwide (5, 9, 20). MRSA acquired in the community (CA-MRSA) is now the most common cause of skin and skin structure infections in the United States (12, 17, 25). CA-MRSA (usually clone USA 300) produces not only skin infections but also invasive pulmonary and bloodstream infections (8, 13, 22). Strains of MRSA displaying various degrees of resistance to vancomycin have emerged in clinical practice. In addition, MRSA considered susceptible to vancomycin may be associated with worse clinical outcomes depending on the MICs for the isolate (2, 11, 21, 24). These changes have prompted interest in the development of new drugs to treat infections due to MRSA.
TD-1792 is a heterodimer antibiotic composed of vancomycin covalently tethered through a chemically stable linker to a cephalosporin moiety. Each of these moieties targets distinct sites in bacterial cell wall synthesis, namely, d-Ala-d-Ala-containing peptidoglycan precursors and transpeptidase active sites in penicillin binding proteins (15, 16). Antibacterial activity of TD-1792 is largely unaffected by coexisting resistance mechanisms, including methicillin and oxacillin resistance in staphylococci, penicillin resistance in pneumococci, fluoroquinolone resistance in streptococci, and quinupristin-dalfopristin resistance among several tested species (7). The antibacterial activity of TD-1792 cannot be reproduced by its individual components tested alone or in combination (6). This finding is consistent with a multivalent mode of action. TD-1792 exhibits potent bactericidal activity at low multiples of the MIC against Gram-positive organisms and a prolonged postantibiotic effect against methicillin-susceptible S. aureus (MSSA) and MRSA (3). TD-1792 is inactive against Gram-negative bacteria. The pharmacokinetics of TD-1792 in healthy volunteers are linear, including a half-life of 9 to13 h (27). TD-1792 is moderately protein bound (∼50%) and is principally eliminated in the urine by rats and dogs (23).
This phase II study explored the safety and efficacy of TD-1792 in patients with complicated skin and skin structure infections (cSSSI) caused by suspected or confirmed Gram-positive organisms.
(This study was presented in part at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], Chicago, IL 2007 [poster L-1147].)
MATERIALS AND METHODS
This randomized, double-blind, active-controlled, phase II clinical trial was conducted at 15 sites in the United States. The study design and conduct were consistent with U.S. Food and Drug Administration (FDA) guidance for complicated skin and skin structure infections in effect at the time the study was performed (26). All centers received approval to conduct the study from their institutional review boards, and informed consent was obtained from each patient prior to participation in the study. After screening, eligible patients were randomized utilizing a third-party interactive voice response system.
Patient population.
Patients were eligible for the study if they were 18 to 65 years old with a cSSSI diagnosis, including major abscess, infected burns, deep and/or extensive cellulitis, infected ulcer, or wound infection. Patients were required to have purulent drainage and a collection of pus, or at least three of the following signs and symptoms: erythema, fluctuation, heat and/or localized warmth, pain and/or tenderness to palpation, swelling and/or induration, fever (>38°C), and white blood count of >10,000/mm3 or >15% bands. Additional enrollment criteria included the need for at least 7 days of intravenous antibiotic treatment, an accessible site for culture, and a calculated creatinine clearance (Cockcroft-Gault equation) of >80 ml/min, or 50 to 80 ml/min if the serum creatinine value was within the normal range and no risk factors for renal dysfunction (e.g., diabetes and cardiovascular disease) were present.
Exclusion criteria were potentially effective systemic antibiotic therapy for more than 24 h before randomization (unless a pathogen resistant to the prior therapy was recovered or the patient was considered a clinical failure after 3 days of prior therapy), other concurrent systemic antibiotics potentially effective against the pathogen(s) under study, moderate to severe liver disease (Child-Pugh class B or C) or an alanine or aspartate aminotransferase (ALT or AST, respectively) level >5 times the upper normal limit, an expected absolute neutrophil count of <500/mm3, HIV infection with a CD4 cell count of <100/mm3, a QT corrected (QTc) interval of >500 ms, uncomplicated SSSI, infections associated with unremovable prosthetic material, burns involving >20% of the body surface, osteomyelitis, necrotizing fasciitis, diabetic foot ulcers, or ischemic wounds.
Antimicrobial therapy.
Patients were randomized on a 1:1 basis to receive either intravenous TD-1792 at 2 mg/kg of body weight every 24 h or intravenous vancomycin at 1 g every 12 h for 7 to 14 days. The vancomycin dose could be adjusted per site-specific guidelines for weight and renal function; however, a regimen of infusion every 12 h was mandatory. Plasma vancomycin levels considered necessary by the site for patient care were allowed and monitored by a “blinded” pharmacist who was responsible for dosage adjustments. To maintain the study blind with regard to dosing frequency, an additional dummy infusion was administered to patients in the TD-1792 group each day. A switch to oral antibiotic therapy was not allowed. Metronidazole and aztreonam were permitted for patients with polymicrobial infection where Gram-negative or anaerobic microorganisms were proven or suspected.
Study populations.
Four populations were defined for the analysis: (i) all-treated (AT), patients who received at least one dose of study drug; (ii) modified AT (MAT), patients in the AT population who also had a pathogen recovered from pretreatment cultures of the primary infection site and/or blood; (iii) clinically evaluable (CE), patients in the AT population whose adherence to the protocol made it reasonable to infer that his or her clinical response reflected the effect of the study drug; and (iv) microbiologically evaluable (ME), patients in the CE population who had a Gram-positive pathogen recovered from pretreatment cultures of the primary infection site and/or blood.
Clinical and microbiological evaluations.
Clinical assessments were conducted daily through the end-of-therapy (EOT) evaluation, which occurred within the 3 days after the last dose of study medication. The test-of-cure (TOC) visit was carried out 7 to 14 days after the last dose of study medication. The extent of infection, surgical procedures utilized to manage the infection, adverse events (AE), and concomitant medications were recorded at each evaluation. Safety laboratory tests were performed at pretreatment, every third day during the treatment period, at EOT, and at follow-up (FU)/TOC visits. Electrocardiogram (ECG) analysis was performed pretreatment, on day 3, 4, or 5 during the treatment period, and at EOT visits. Gram stain and culture of specimens from the primary lesion were obtained at baseline and were repeated at EOT and/or TOC if significant lesions were still present and the patient was considered to have an ongoing infection. Cultures of deep tissue, including needle aspiration, biopsy, or surgically obtained specimens, were to be performed. Sterile collection of pus or drainage fluid was acceptable; however, a swab of the lesion was not accepted as a specimen collection method. Pathogen identification was confirmed and susceptibility to TD-1972 was tested at a central laboratory (Covance Central Laboratory, Indianapolis, IN).
Pharmacokinetic evaluation.
Pharmacokinetic sampling was performed at selected sites for evaluation of plasma TD-1792. A total of 7 samples were obtained per subject on either day 3, 4, or 5 at the following sampling time points: prior to the infusion (trough), immediately following the infusion, and 30 min and 1, 3, 8, and 23 h after the end of the infusion. No analysis of vancomycin was performed.
Statistical analysis.
This study was designed to assess the safety and efficacy of TD-1792 in patients with cSSSI. The primary efficacy endpoint was clinical response determined by the investigator at the TOC visit. The primary efficacy analysis tested the hypothesis of TD-1792's clinical noninferiority to vancomycin. Based on assumptions made in designing the study (enrollment of 100 patients per arm, 80% of patients clinically evaluable, and 90% cure rates in both arms) and employing a noninferiority margin of 15%, a one-sided test for noninferiority with a 0.025 significance level would have 88% power.
Clinical response at EOT and at TOC (primary endpoint) was defined as one of the following: (i) cure, i.e., resolution of signs and symptoms of cSSSI to the point that no further antibiotic therapy was required; (ii) failure, i.e., inadequate response to study therapy; and (iii) missing, i.e., no determination reported. Significant surgical intervention (e.g., more than routine bedside procedures) after initiation of study medication and on more than two occasions during the study constituted evidence of clinical failure. Failures at EOT were carried forward to TOC.
Secondary endpoints included clinical cure and microbiological eradication rates in the ME population. In this patient population, the baseline pathogen was considered eradicated at EOT or TOC if the pathogen was not detected by culture or if the subject's clinical response was a cure and there was no lesion available for culture. Confidence intervals (CI) for the difference between treatment groups were calculated using Agresti-Caffo adjustment (1).
After the completion of this trial, the FDA issued a new guidance for acute bacterial skin and skin structure infections (ABSSSI) (26). Therefore, a post hoc analysis of efficacy applying the new FDA guidance was also performed for patients with lesion sizes of ≥75 cm2 and Gram-positive infection only. For this analysis (early clinical endpoint), clinical success was defined as resolution (absence) of fever (i.e., <37.7°C) and no progression in lesion size at 72 h.
RESULTS
Study population and baseline characteristics.
A total of 197 patients (98 in the TD-1792 group and 99 in the vancomycin group) were randomized and received study medication (Table 1). There were 86 to 87% clinically evaluable patients in each treatment group, and 64% were microbiologically evaluable.
Table 1.
Study populations and baseline patient characteristics in the all-treated population
| Parameter | Valuee for treatment group |
||
|---|---|---|---|
| TD-1792 | Vancomycin | Total | |
| Study populations | |||
| All-patients randomized | 101 | 102 | 203 |
| All-treated | 98 (100%) | 99 (100%) | 197 (100%) |
| Modified all-treated | 76 (78%) | 76 (77%) | 152 (77%) |
| Clinically evaluable | 84 (86%) | 86 (87%) | 170 (86%) |
| Microbiologically evaluable | 63 (64%) | 63 (64%) | 126 (64%) |
| Patient demographics | |||
| Mean age in years (±SD) | 40 (11.2) | 40 (11.3) | 40 (11.2) |
| Male | 61 (62%) | 59 (60%) | 120 (61%) |
| White | 76 (78%) | 77 (78%) | 153 (78%) |
| Mean body mass index (kg/m2) (±SD) | 31 (8.4) | 29 (8.4) | 30 (8.4) |
| Body mass index ≥ 30 | 42 (43%) | 32 (32%) | 74 (38%) |
| Creatinine clearance | |||
| >50–80 ml/min | 18 (19%) | 18 (19%) | 36 (19%) |
| >80 ml/min | 78 (80%) | 77 (79%) | 155 (80%) |
| Most common predisposing conditions | |||
| Trauma | 12 (12%) | 20 (20%) | 32 (16%) |
| Diabetes | 14 (14%) | 11 (11%) | 25 (13%) |
| Prior surgery | 7 (7%) | 1 (1%) | 8 (4%) |
| Bite | 6 (6%) | 2 (2%) | 8 (4%) |
| Chronic skin diseases | 4 (4%) | 2 (2%) | 6 (3%) |
| Types of infection | |||
| Abscess | 56 (57%) | 60 (61%) | 116 (59%) |
| Cellulitis | 30 (31%) | 25 (25%) | 55 (28%) |
| Wound infection | 11 (11%) | 13 (13%) | 24 (12%) |
| Infected burn | 0 (0%) | 1 (1%) | 1 (<1%) |
| Infected ulcer | 1 (1%) | 0 (0%) | 1 (<1%) |
| Primary site of infection | |||
| Lower extremities | 37 (38%) | 42 (42%) | 79 (40%) |
| Upper extremities | 25 (26%) | 30 (30%) | 55 (28%) |
| Back torso | 19 (19%) | 11 (11%) | 30 (15%) |
| Front torso | 11 (11%) | 9 (9%) | 20 (10%) |
| Head and neck | 6 (6%) | 7 (7%) | 13 (7%) |
| Median area of lesion, in cm2 (±IQR)a | 120 (60, 320) | 165 (56, 360) | 132 (60, 330) |
| Median area of abscess, in cm2 (±IQR) | 120 (67, 230) | 136 (52, 328) | 121 (60, 255) |
| Patients with abscess area ≥75 cm2b | 39/56 (70%) | 40/60 (67%) | 79/116 (68%) |
| Permitted concomitant antibacterials | 30 (31%) | 37 (37%) | 67 (34%) |
| Aztreonam | 22 (22%) | 35 (35%) | 57 (29%) |
| Metronidazole | 25 (26%) | 23 (23%) | 48 (24%) |
| At least one surgical procedure at the infection sitec | 29 (30%) | 27 (27%) | 56 (28%) |
| Prior antimicrobial therapyd | 68 (69%) | 78 (79%) | 146 (74%) |
| >24 h of prior antimicrobial therapy | 18 (18%) | 30 (30%) | 48 (24%) |
IQR, interquartile range.
Percentages based on the number of patients with abscesses.
Between first dose and follow-up visit.
Only prior antimicrobials with a start date prior to the first dosing date.
Values are number (percentage) unless otherwise noted.
Baseline demographics and clinical characteristics were similar between the two treatment groups. The majority of patients were male (61%) and white (78%). The average age was 40 years. Obesity was common in both treatment groups. Most patients (80%) had a creatinine clearance of >80 ml/min. The most common infection types were abscess (59%) followed by cellulitis (28%). The overall median lesion sizes (surface areas) were 120 cm2 in the TD-1792 group and 165 cm2 in the vancomycin group (P = 0.37, Wilcoxon rank sum). Abscesses had median surface areas of 120 cm2 in the TD-1792 group and 136 cm2 in the vancomycin group (P = 0.67).
Infections were most commonly located on the extremities. The most common predisposing factors included trauma and diabetes. The majority of patients (74%) had received antimicrobial therapy in the 7 days prior to study enrollment. This included 18% of patients in the TD-1792 group and 30% in the vancomycin group who had received >24 h of prior antimicrobial therapy.
Baseline pathogens and in vitro susceptibilities.
Table 2 displays the most common Gram-positive pathogens isolated at baseline. Staphylococcus aureus was the most common pathogen isolated (85%) from the primary infection site in the ME patients. The majority of S. aureus strains were MRSA (60%). Other Gram-positive pathogens included MSSA (24%), any streptococcus (15%), and any enterococcus (3%). A total of 9 patients (7%) had Gram-positive pathogens isolated from blood cultures. A small proportion of patients (5%) also had Gram-negative pathogens cultured from the primary infection site.
Table 2.
Most common Gram-positive pathogens isolated in microbiologically evaluable patients
| Pathogen | Value (%) for treatment group |
||
|---|---|---|---|
| TD-1792 (n = 63) | Vancomycin (n = 63) | Total (n = 126) | |
| From infection site | 63 (100) | 61 (97) | 124 (98) |
| Gram-positive pathogens only | 61 (97) | 57 (93) | 118 (95) |
| Any Staphylococcus aureusa | 52 (83) | 54 (89) | 106 (85) |
| MRSA | 38 (60) | 36 (59) | 74 (60) |
| MSSA | 12 (19) | 18 (30) | 30 (24) |
| Streptococcus agalactiae | 3 (5) | 3 (5) | 6 (5) |
| Streptococcus mitis | 1 (2) | 3 (5) | 4 (3) |
| Streptococcus pyogenes | 0 | 2 (3) | 2 (2) |
| Any Streptococcus | 9 (14) | 10 (16) | 19 (15) |
| Any Enterococcus | 2 (3) | 2 (3) | 4 (3) |
| From blood | 3 (5) | 5 (8) | 8 (6) |
| Staphylococcus aureus | 3 (5) | 4 (7) | 7 (6) |
| Streptococcus intermedius | 0 | 1 (2) | 1 (<1) |
Susceptibility to oxacillin was not determined in two isolates.
In the group receiving TD-1792, the MICs were determined for 36 strains of MRSA and 11 strains of MSSA. In the group receiving vancomycin, the MICs were determined for 34 strains of MRSA and 14 strains of MSSA. Regardless of treatment group, the MIC90s of TD-1792 were 0.015 μg/ml (range, 0.008 to 0.015 μg/ml) for MRSA and 0.008 μg/ml (range, 0.002 to 0.015 μg/ml) for MSSA (Table 3).
Table 3.
Susceptibility of most common Gram-positive pathogens isolated from the infection site (ME population)
| Baseline pathogen | No. of isolates | MIC90 (rangea), in μg/ml |
|
|---|---|---|---|
| TD-1792 | Vancomycin | ||
| TD-1792 treatment group | |||
| Staphylococci | 47 | 0.015 (0.002–0.015) | 1 (≤0.25–1.0) |
| MRSA | 36 | 0.015 (0.008–0.015) | 1 (0.5–1.0) |
| MSSA | 11 | 0.008 (0.002–0.015) | 1 (≤0.25–1.0) |
| Staphylococcus epidermidis | 1 | 0.015 (—) | 2 (—) |
| Streptococcus agalactiae | 1 | 0.002 (—) | 0.5 (—) |
| Streptococcus anginosus | 1 | <0.001 (—) | 0.5 (—) |
| Vancomycin treatment group | |||
| Staphylococci | 48 | 0.015 (0.004–0.015) | 1 (0.5–1.0) |
| MRSA | 34 | 0.015 (0.008–0.015) | 1 (—) |
| MSSA | 14 | 0.008 (0.002–0.015) | 1 (0.5–1) |
| Streptococcus agalactiae | 3 | 0.001 (—) | 0.5 (—) |
| Streptococcus anginosus | 1 | 0.002 (—) | 0.5 (—) |
—, not available.
Pharmacokinetics.
The pharmacokinetic disposition of TD-1792 was evaluated in 64 patients. After the 1-h intravenous infusion in patients with cSSSI, TD-1792 concentrations declined in a bi-exponential manner. The mean maximum concentration in serum (Cmax) of TD-1792 (±standard deviation [SD]) was 4.17 (±1.96) μg/ml. The half-life determined for 52 patients was 7.5 (±1.5) h, and the area under the curve from 0 to 24 h (AUC0–24) was 16.4 ± 5.3 μg · h/ml. Mean trough levels assessed in 55 patients were 0.196 (±0.183) μg/ml.
Clinical response.
Clinical cure rates at the TOC visit were similar in all analysis populations (Table 4). In the AT population, cure rates were 80.6% and 82.8% in the TD-1792 and the vancomycin groups, respectively (95% CI for the difference, −13.0 and 8.6). In the CE population, cure rates were 91.7% in the TD-1792 group and 90.7% in the vancomycin group (95% CI for the difference, −7.9 and 9.7). In the ME patients, cure rates were 92.1% in both treatment groups. In patients infected with MRSA, cure was achieved in 94.7% in the TD-1792 group and 91.9% in the vancomycin group.
Table 4.
Clinical cure rates at test-of-cure visit
| Study population | No. cured with indicated drug/no. tested (%) |
Difference (95% CI) | |
|---|---|---|---|
| TD-1792 | Vancomycin | ||
| All-treated | 79/98 (80.6) | 82/99 (82.8) | −2.2 (−13.0, 8.6) |
| Modified all-treated | 62/76 (81.6) | 63/76 (82.9) | −1.3 (−13.5, 10.8) |
| Clinically evaluable | 77/84 (91.7) | 78/86 (90.7) | 1.0 (−7.9, 9.7) |
| Microbiologically evaluable | 58/63 (92.1) | 58/63 (92.1) | 0.0 (−10.0, 10.0) |
| Microbiologically evaluable patients infected with MRSA | 36/38 (94.7) | 34/37 (91.9) | 2.8 (−9.8, 15.3) |
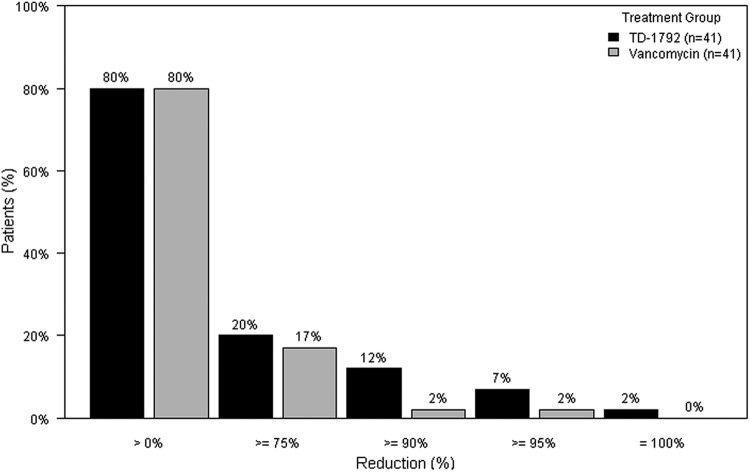
The proposed primary endpoint from draft FDA guidance issued August 2010 (early clinical endpoint) was evaluated post hoc in 82 patients with Gram-positive infection and a lesion size of ≥75 cm2 (41 in the TD-1792 group and 41 in the vancomycin group). The success rate using the new endpoint was 80% in both treatment groups (Fig. 1).
Fig 1.
Percentage of patients achieving cessation of spread or reduction of lesion size and temperature of <37.7°C at 72 h after initiation of treatment (early clinical endpoint). Only patients with baseline lesions ≥75 cm2 and Gram-positive infection were included. Patients with missing percentage of change and/or missing temperature were excluded.
Microbiological response.
A total of 126 patients were microbiologically evaluable. Among these patients, pathogens were eradicated (or presumed eradicated) at TOC in 93.7% in the TD-1792 group and in 92.1% in the vancomycin group (95% CI for the difference, −8.0 and 11.1) (Table 5). In patients infected with MRSA, eradication rates were 94.7% in the TD-1792 group and 91.9% in the vancomycin group (95% CI for the difference, −9.8% and 15.3%).
Table 5.
Microbiological response of eradication at test-of-cure visit (ME population)
| Baseline pathogen | No. with eradication with drug/no. tested (%) |
% Difference (95% CI) | |
|---|---|---|---|
| TD-1792 | Vancomycin | ||
| Any pathogen | 59/63 (93.7) | 58/63 (92.1) | 1.6 (−8.0, 11.1) |
| S. aureus | 49/52 (94.2) | 51/55 (92.7) | 1.5 (−8.8, 11.5) |
| MRSA | 36/38 (94.7) | 34/37 (91.9) | 2.8 (−9.8, 15.3) |
| MSSA | 11/12 (91.7) | 17/18 (94.4) | −2.8 (−26.8, 18.3) |
| Streptococcus agalactiae | 3/3 (100) | 3/3 (100) | |
Safety and tolerability.
The types and frequency of adverse events (AEs) were similar between the two treatment groups (Table 6). AEs were documented for 68% of patients receiving TD-1792 and 75% of patients receiving vancomycin. No serious AEs (SAEs) were reported for patients receiving TD-1792 but occurred in 2 patients receiving vancomycin. One patient had an increase in serum creatinine reaching a maximum of 2.0 mg/dl, with partial recovery after stopping therapy; the other patient had deep venous thrombosis. Seven patients were discontinued from study medication due to AEs. Two patients in the TD-1792 group discontinued because of an AE, including one with rash and one with palpitations. Both cases were considered mild in nature and resolved or were resolving at the follow-up visit. Five vancomycin-treated patients discontinued study participation secondary to red man syndrome (n = 2), pyrexia (n = 1), elevated liver enzymes (n = 1), and increased serum creatinine (n = 1). There were no deaths in either treatment group.
Table 6.
Adverse events reported in ≥3% of any treatment group (safety population)
| Adverse eventsa | No. (% of total treated with drug) |
|
|---|---|---|
| TD-1792 (n = 98) | Vancomycin (n = 99) | |
| At least one AE | 67 (68) | 74 (75) |
| At least one SAE | 0 | 2 (2) |
| Discontinuation due to an AE | 2 (2) | 5 (5) |
| Frequency of AEs | ||
| Headache | 18 (18) | 14 (14) |
| Nausea | 14 (14) | 12 (12) |
| Pruritus | 13 (7) | 27 (19) |
| Increased AST | 12 (12) | 9 (9) |
| Increased ALT | 9 (9) | 11 (11) |
| Diarrhea | 8 (8) | 9 (9) |
| Infusion site pain | 5 (5) | 7 (7) |
| Infusion site pruritus | 4 (4) | 7 (7) |
| Dysgeusia | 7 (7) | 4 (4) |
| Constipation | 2 (2) | 7 (7) |
| Insomnia | 5 (5) | 4 (4) |
| Vomiting | 4 (4) | 4 (4) |
| Infusion site erythema | 5 (5) | 3 (3) |
| Rash | 2 (2) | 4 (4) |
| Dizziness | 2 (2) | 3 (3) |
| Cough | 2 (2) | 3 (3) |
| Increased blood pressure | 3 (3) | 1 (1) |
| Red man syndrome | 1 (1) | 3 (3) |
| Pain in extremity | 1 (1) | 3 (3) |
| Allergic dermatitis | 0 | 4 (4) |
| Fatigue | 0 | 3 (3) |
| Abdominal pain, upper | 0 | 3 (3) |
| Infusion site reaction | 3 (3) | 0 |
| Abscess, limb | 0 | 3 (3) |
| Hypoglycemia | 0 (0) | 3 (3) |
Sorted by frequency from the greatest to the least.
The most common AEs in both treatment groups were headache, pruritus, nausea, increased liver enzymes, and diarrhea (Table 6), and AEs were generally mild in both treatment groups. Pruritus was more common in the vancomycin group (13 patients in the TD-1792 group versus 27 patients in the vancomycin group). AEs considered moderate in severity occurred in 18 patients receiving TD-1792 and 48 patients receiving vancomycin, and severe AEs were reported for 6 and 11 patients, respectively. These events were largely gastrointestinal disorders (primarily constipation, diarrhea, nausea, and vomiting), administrative-site conditions (primarily infusion site reactions), investigations (primarily elevated aspartate transaminase [AST] and alanine aminotransferase [ALT]), nervous system disorders (primarily headaches), and skin and soft tissue disorders (primarily red man syndrome). Clinically significant laboratory abnormalities were infrequent and are shown in Table 7.
Table 7.
Incidence of potentially clinically significant laboratory abnormalities
| Laboratory abnormality | No. of occurrences in treatment group |
|
|---|---|---|
| TD-1792 (n = 98) | Vancomycin (n = 99) | |
| Hematology | ||
| Hemoglobin (male, <10 g/liter) | 1 | 0 |
| WBC (<2.8 × 109/liter) | 0 | 1 |
| Eosinophils (≥10%) | 2 | 1 |
| Platelet count (<75 × 109/liter) | 1 | 0 |
| Chemistry | ||
| Potassium (<3.0 mmol/liter) | 1 | 1 |
| Potassium (>5.5 mmol/liter) | 3 | 1 |
| Calcium (<1.5 mmol/liter) | 2 | 0 |
| Chloride (>115.0 mmol/liter) | 2 | 1 |
| Serum creatinine (>177 mmol/liter) | 0 | 1 |
| BUN (>11mmol/liter) | 2 | 1 |
| Serum creatinine (≥133 mmol/liter and 50% greater than baseline) | 0 | 1 |
| Liver function tests | ||
| SGOT/AST (≥3× ULN) | 3 | 3 |
| SGPT/ALT (≥3× ULN) | 2 | 3 |
| Total bilirubin (≥1.5× ULN) | 0 | 0 |
WBC, white blood count; BUN, blood urea nitrogen; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; ULN, upper limit norm.
No patient had a Fridericia-corrected QT interval (QTcF) greater than 480 ms, and none had a QTcF value that increased by more than 60 ms from baseline. Fourteen patients in the TD-1792 group (14%) and 12 patients in the vancomycin group (12%) experienced heart rate increases of >20 beats per minute (bpm) any time after dosing. Three patients in each group experienced a heart rate increase of >30 bpm. The mean changes in heart rate relative to predose baseline at day 4 were 3 ± 8.1 bpm for TD-1792 and 0 ± 8.0 bpm for vancomycin and at day 7 were 3 ± 7.0 bpm and 2 ± 6.7 bpm, respectively.
DISCUSSION
This is the first study exploring safety and efficacy of TD-1792 at 2 mg/kg in patients with cSSSI. MRSA was the most common pathogen, reflecting the contemporary epidemiology of Gram-positive bacteria and complicated skin and soft tissue infections (12, 17), and accounted for approximately 60% of all bacterial isolates among microbiologically evaluable patients.
Analysis of the data from this study provides several important findings. TD-1792 and vancomycin had similar cure rates for the treatment of patients with cSSSI due to suspected or proven Gram-positive pathogens. This observation was consistent in all study populations for both primary and secondary endpoints. The highest cure rates achieved by TD-1792 were observed in those patients infected with MRSA, the targeted pathogen. In testing isolates from the present study, TD-1792 was found to be >60-fold more potent in vitro than vancomycin against clinical strains of MRSA isolated at baseline (MIC90 0.015 μg/ml for TD-1792 versus 1.0 μg/ml for vancomycin). These findings are in agreement with neutropenic murine thigh and subcutaneous thigh infection models with S. aureus in whichTD-1792 was found to be severalfold more potent than vancomycin or linezolid (10). In the clinical study, eradication rates for TD-1792-treated patients for MRSA and MSSA were also at least comparable to those for vancomycin-treated patients.
Recent FDA draft guidance has recommended a primary efficacy endpoint of resolution or absence of fever and no increase in lesion size at 72 h after starting therapy in the target population (26). Applying this newly recommended endpoint retrospectively, we demonstrated similar efficacies for TD-1792 and vancomycin.
With the exception of pruritus, which was more common in the vancomycin group, the incidences of the most frequent adverse events were comparable between the two treatment groups. The most common AEs in both treatment groups, such as headache, nausea, and diarrhea, were generally mild and transient. Importantly, no SAEs were reported in the TD-1792 group. Laboratory abnormalities were also comparable between the two groups, although increases in liver function tests were more common in the vancomycin group. There was no biochemical or clinical sign of any kidney injury in patients who received TD-1792 at 2 mg/kg/day.
Vital signs were unremarkable with the exception of heart rate, for which there was a small mean increase postdose on day 4 versus predose baseline in the TD-1792 group (3 bpm) versus the vancomycin group (0 bpm); however, the proportion of patients with heart rate increases of >20 bpm and >30 bpm were similar between the two treatment groups. Pharmacological studies have demonstrated that TD-1792 is a moderate-affinity (KB [affinity exhibited by the antagonist for the receptor] = 105 nM) antagonist of human M2 muscarinic receptors and produces dose-dependent increases in heart rate in nonclinical species (Theravance, Inc., data on file). Antagonism of M2 muscarinic receptors in the sino-atrial node causes suppression of vagal parasympathetic drive to the heart, leading to elevation in heart rate, as observed with muscarinic antagonists, such as tolterodine, that are used for the treatment of overactive bladder (18).
Limitations of this study include the high proportion of patients with abscesses. Small abscesses can be successfully treated with drainage alone; however, in this study the median size of abscesses (120 cm2) was substantially larger than is typically managed without antibiotics (14, 19). In addition, patients enrolled in this study were those that the treating physician considered to require at least 7 days of intravenous antibiotics. The high proportion of abscesses observed in this study likely reflects the high prevalence of CA-MRSA infection (25).
Given the high and increasing incidence of MRSA infections in the United States as well as other countries around the world, new drugs are needed to assist clinicians in treating this challenging pathogen. Results from this study support advancing the clinical development of TD-1792.
ACKNOWLEDGMENTS
This study was conducted under the auspices of Theravance, Inc., and coordinated by the Duke Clinical Research Institute, Durham, NC.
The research and publication process was supported by Theravance, Inc.
G.R.C. was principal investigator for the study. M.E.S. advised on the protocol and wrote the paper with P.D.P, with review and comment from all other authors. All authors saw and agreed on the final manuscript prior to submission. A.C. and J.K. were study investigators and represent the TD-1792 study group. M.E.S. is currently a consultant to Theravance, Inc., The Medicines Company, PRA International, Cerexa, and Furiex; he has provided consulting services to Astellas, Nabriva, and Trius Therapeutics in the past; and he has received an honorarium from Cempra and an NIH grant through Duke University.
J.K. has served as a consultant to Trius Therapeutics, Rib-X, and Furiex and has received or has grants pending with the following companies: Trius Therapeutics, Durata, Rib-X, Furiex, Achaogen, Replidyne, Cempra, Nabriva Therapeutics, Novexel, GlaxoSmithKline, Paratek, Cerexa, Cubist, and Theravance and has purchased stock from Trius Therapeutics.
G.R.C. has received research grants from Theravance, The Medicine Company, Cubist Pharmaceuticals, Cerexa, Innocol, and has served as a consultant to the following companies: Theravance, Astellas, The Medicine Company, Cubist Pharmaceuticals, Cerexa/Forest Pharmaceuticals, Cempra, Innocol, Iminex, AstraZeneca, Furiex, and Dr. Reddy's Labotories. Additionally, he has participated in advisory boards for the following companies: Pfizer, GlaxoSmithKline, Merck, Polymedix, Achoagen, Trius, Rib-X, Nabriva, Seachaid, BioCryst, Durata, and Gilead.
The TD-1792 Study Group also includes Christopher Bunce, Indianapolis, IN; Curtis Clark, Columbus, GA; Allan Churukian, San Diego, CA; John Gezon, Salt Lake City, UT; Roger Harvey, Des Moines, IA; Stanley Klein, Torrance, CA; Hector Labrada, Miami, FL; Patrick C. Lee, Springfield, MA; Purvi Mehra, San Diego, CA; Richard Pollak, San Antonio, TX; John Pullman, Butte, MT; Christian Schrock, Minneapolis, MN; and Shirin Towfigh, Los Angeles, CA.
Footnotes
Published ahead of print 6 August 2012
REFERENCES
- 1. Agresti A, Caffo B. 2007. Simple and effective confidence intervals for proportions and difference of proportions result from adding two successes and two failures. Am. Statistician 54:280–288 [Google Scholar]
- 2. Bae IG, et al. 2009. Heterogeneous vancomycin-intermediate susceptibility phenotype in bloodstream methicillin-resistant Staphylococcus aureus isolates from an international cohort of patients with infective endocarditis: prevalence, genotype, and clinical significance. J. Infect. Dis. 200:1355–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blais J, Li YP, Barriere S. 2009. Activity of Td-1792, a multivalent glycopeptide-cephalosporin antibiotic, against Gram-positive isolates from a recently completed phase 2 study in complicated skin and skin structure infection, abstr. E-209. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA [Google Scholar]
- 4. Boucher HW, Corey GR. 2008. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 46(Suppl 5):S344–S349 [DOI] [PubMed] [Google Scholar]
- 5. Burton DC, Edwards JR, Horan TC, Jernigan JA, Fridkin SK. 2009. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA 301:727–736 [DOI] [PubMed] [Google Scholar]
- 6. Difuntorum S, et al. 2007. In vitro and in vivo evidence for a multivalent mode of action of Td-1792 against Staphylococcus aureus, abstr. F1-2110. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL [Google Scholar]
- 7. Fritsche TR, Blais J, Jones RN. 2007. In vitro evaluation of Td-1792, a novel antimicrobic with potent Gram-positive activity, abstr. E-1627. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL [Google Scholar]
- 8. Gonzalez BE, et al. 2006. Community-associated strains of methicillin-resistant Staphylococccus aureus as the cause of healthcare-associated infection. Infect. Control Hosp. Epidemiol. 27:1051–1056 [DOI] [PubMed] [Google Scholar]
- 9. Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. 2006. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368:874–885 [DOI] [PubMed] [Google Scholar]
- 10. Hedge SS, et al. 2007. Efficacy of Td-1792, a novel multivalent glycopeptide, against Gram-positive pathogens in the neutropenic thigh model, abstr. A-42. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL [Google Scholar]
- 11. Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. 2006. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch. Intern. Med. 166:2138–2144 [DOI] [PubMed] [Google Scholar]
- 12. King MD, et al. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus U. S. A. 300 clone as the predominant cause of skin and soft-tissue infections. Ann. Intern. Med. 144:309–317 [DOI] [PubMed] [Google Scholar]
- 13. Klevens RM, et al. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 14. Lee MC, et al. 2004. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr. Infect. Dis. J. 23:123–127 [DOI] [PubMed] [Google Scholar]
- 15. Long DD, et al. 2008. Exploring the positional attachment of glycopeptide/beta-lactam heterodimers. J. Antibiot. (Tokyo) 61:603–614 [DOI] [PubMed] [Google Scholar]
- 16. Lunde CS, Lewis SR, Benton BM, Mammen M, Blais J. 2009. Td-1792, mode of action of a multivalent glycopeptide-cephalosporin antibiotic, abstr. C1-1344. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA [Google Scholar]
- 17. Moran GJ, et al. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666–674 [DOI] [PubMed] [Google Scholar]
- 18. Olshansky B, et al. 2008. Differential pharmacological effects of antimuscarinic drugs on heart rate: a randomized, placebo-controlled, double-blind, crossover study with tolterodine and darifenacin in healthy participants > or = 50 years. J. Cardiovasc. Pharmacol. Ther. 13:241–251 [DOI] [PubMed] [Google Scholar]
- 19. Rajendran PM, et al. 2007. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob. Agents Chemother. 51:4044–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenthal VD, et al. 2008. International nosocomial infection control consortium report, data summary for 2002–2007, issued January 2008. Am. J. Infect. Control 36:627–637 [DOI] [PubMed] [Google Scholar]
- 21. Sakoulas G, et al. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42:2398–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seybold U, et al. 2006. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin. Infect. Dis. 42:647–656 [DOI] [PubMed] [Google Scholar]
- 23. Shaw JP, et al. 2007. Pharmacokinetics of Td-1792, a novel multivalent glycopeptide, in preclinical animal species, abstr. A-43. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL [Google Scholar]
- 24. Soriano A, et al. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46:193–200 [DOI] [PubMed] [Google Scholar]
- 25. Stryjewski ME, Chambers HF. 2008. Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 46(Suppl 5):S368–S377 [DOI] [PubMed] [Google Scholar]
- 26. US Food and Drug Administration 2010. Guidance for industry. Acute bacterial skin and skin structure infections: developing drugs for treatment. http://www.fda.gov/Downloads/Drugs/Guidancecomplianceregulatoryinformation/Guidances/Ucm071185.Pdf Accessed 30 March 2012
- 27. Wong SL, et al. 2007. Pharmacokinetic (Pk) disposition of a novel multivalent glycopeptide, Td-1792, abstr. A-44. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL [Google Scholar]