Abstract
To screen isolates and to identify mecA alleles, published mecA sequences were analyzed, and a microarray for the rapid discrimination of mecA alleles was designed. A GenBank analysis yielded 135 full-length gene sequences annotated as mecA. These sequences clustered into 32 different alleles corresponding to 28 unique amino acid sequences and to 15 distinct hybridization patterns on this microarray. A collection of 78 clinical and veterinary isolates of Staphylococcus spp. was characterized using this assay. Nine of the 15 expected patterns, as well as one as-yet-unknown pattern, were identified. These patterns were detected in various epidemic methicillin-resistant Staphylococcus aureus strains, in S. pseudintermedius, and in coagulase-negative species such as S. epidermidis, S. fleurettii, or S. haemolyticus. There was no correlation between the different mecA hybridization patterns and the SCCmec type. Determination of MICs showed that mecA alleles corresponding to only four of these nine patterns were associated with β-lactam resistance. The mecA alleles that did not confer β-lactam resistance were largely restricted to coagulase-negative staphylococci of animal origin, such as S. sciuri and S. vitulinus. Because of the diversity of sequences and the different impact on β-lactam susceptibility, the existence of different mecA alleles needs to be taken into account when designing diagnostic assays for the detection of mecA.
INTRODUCTION
Methicillin resistance, accompanied by resistance to all β-lactam compounds in current clinical use, has become increasingly common in Staphylococcus aureus, as well as in other staphylococci. This phenomenon has been known for over 50 years. About 1 year after the introduction of penicillinase-resistant semisynthetic penicillin compounds, methicillin-resistant S. aureus (MRSA) was reported in the United Kingdom (7). Resistance is caused by an alternate penicillin-binding protein (PBP2′ or PBP2a) encoded by the gene mecA. Because of the high clinical relevance of methicillin/β-lactam resistance, phenotypic resistance in a clinical isolate of S. aureus can be confirmed either by a PCR for the detection of mecA or by an antibody-based test, i.e., a lateral flow or agglutination assay for the detection of PBP2a.
The classical mecA gene as known from MRSA is located on complex mobile genetic elements (6), known as SCCmec (i.e., a “staphylococcal cassette chromosome” or “staphylococcal chromosomal cassette” harboring mecA). SCCmec elements and mecA are not restricted to S. aureus but are commonly found in other staphylococci such as, for instance, S. epidermidis or S. haemolyticus (3) and, increasingly, S. pseudintermedius (11). SCC elements probably preceded MRSA and methicillin resistance as vectors for other genes in staphylococci. There are similar mobile genetic elements harboring capsule group 1 factors of S. aureus as well as the fusidic acid resistance gene fusB/Q6GD50, several heavy metal resistance operons, and the arginine catabolic mobile element, all of which can be found in different staphylococcal species. The provenance of mecA is not yet known. However, it appears to be likely that genes from animal commensals are ancestors to the methicillin resistance determinant, and S. sciuri (2), S. fleurettii (17), and Macrococcus caseolyticus (1, 16) might be sources of a mecA precursor.
Assuming a long existence of mecA in staphylococci other than S. aureus/MRSA, it can be expected that different alleles of this gene were detectable and that some of them might play a physiological role other than conferring antibiotic resistance. In addition, it is likely that more alleles actually exist than are currently known. Such a possible diversity of mecA alleles might be of significant practical relevance for the design of assays for the detection or confirmation of mecA/PBP2a as a marker for methicillin/β-lactam resistance in routine clinical diagnostics. For instance, deviant mecA alleles might not be detected by molecular methods with currently available assays, as recently observed for emerging CC130-MRSA-XI strains (4, 14) or, vice versa, allelic variants might result in false positives in antibody-based tests.
In order to screen isolates and to identify mecA alleles, mecA sequences deposited in the GenBank database were analyzed, and a microarray-based assay for the experimental discrimination of mecA alleles was designed. A collection of clinical and/or veterinary isolates of Staphylococcus spp. was characterized using this assay. In addition, MICs were determined to see how the different mecA alleles correlate to MICs of different β-lactam antibiotics.
MATERIALS AND METHODS
Isolates.
A total of 78 isolates were selected and genotyped for the present study. These included 34 S. aureus, 1 S. capitis, 5 S. epidermidis, 5 S. fleurettii, 3 S. haemolyticus, 3 S. pseudintermedius, 2 S. saprophyticus, 18 S. sciuri, 2 S. simulans, 1 S. succinus, and 4 S. vitulinus strains. All isolates were hybridized to previously described DNA arrays, the S. aureus genotyping kit (Alere Technologies, Jena, Germany) (8, 10), in order to detect and characterize SCCmec elements and, in the case of S. aureus, to determine their clonal complex and strain affiliations.
In the case of S. sciuri, S. vitulinus, and S. aureus CC130/SCCmec XI, all available isolates were included as sequence data indicated a possible presence of mecA alleles undetectable by the S. aureus genotyping kit. Isolates of the other species and of S aureus/MRSA (other than CC130) were selected in order to represent the different SCCmec types, as well as some major epidemic strains of MRSA.
Array procedures.
The DNA preparation was performed using reagents (lysostaphin, lysozyme, and RNase) and buffers from the S. aureus genotyping kit (Alere Technologies), as well as Qiagen spin columns (Qiagen, Hilden, Germany), according to a previously described protocol (8).
Labeling was performed by incorporation of biotin-dUTP in amplicons from a thermally synchronized multiplex primer elongation reaction (9). Reagents and buffers from the Alere Hybridization Plus kit (catalog no. 245400100; Alere Technologies) were used, adding 3.9 μl of B1 labeling buffer, 0.1 μl of B2 labeling enzyme, and 1 μl of a mix of all primers (each at 0.135 μM) to 5 μl (containing at least 0.5 μg) of target DNA. All primer sequences are listed in Table S1 in the supplemental material. Amplification was performed in a standard thermocycler (MasterCycler; Eppendorf, Hamburg, Germany) according to the following protocol: preheating of 5 min at 96°C, followed by 50 cycles consisting of 60 s at 96°C, 20 s at 50°C, and 40 s at 72°C.
The exact reproducibility of the test conditions, especially with regard to hybridization temperatures, is crucial, and several commercially available thermomixers yielded unsatisfying results due to inhomogeneous distributions of temperatures within heating blocks and/or due to differences between displayed and true temperatures in the cavities (data not shown). Finally, the hybridization of the single stranded biotin-labeled amplicons to the arrays was performed using a BioShake iQ Thermoshaker (QuantiFoil Instruments, Jena, Germany).
The hybridization probes are listed in Table S2 in the supplemental material. Prior to use, arrays were prewashed in 150 μl of distilled water and hybridization buffer C1 (the latter from the Alere Hybridization Plus kit; both steps for 5 min at 50°C and 550 rpm). For hybridization, 10 μl of biotin-labeled, single-stranded amplicon and 90 μl of hybridization buffer C1 were incubated with the array at 60 min, 50°C, and 550 rpm. This was followed by three washing steps (150 μl of washing buffer C2, pipetting up and down three times, and [twice] 150 μl of washing buffer C2 for 10 min at 45°C and 550 rpm). Conjugation was performed by adding 99 μl of C4 conjugation buffer and 1 μl of horseradish peroxidase-streptavidin conjugate (kit reagent C3) for 15 min at 30°C and 550 rpm. Again, this was followed by washing steps (150 μl of washing buffer C5 and pipetting up and down three times, followed by one incubation step with 150 μl of washing buffer C5 for 5 min at 30°C and 550 rpm). The washing buffer was discarded, and 100 μl of D1 substrate (a precipitating dye; prewarmed to 25°C) was added. After incubation (for 10 min at 25°C, without mixing or moving), the liquid was completely removed, and an image of the array was recorded using a designated reading device (ArrayMate; Alere Technologies), analyzed, and compared to reference experiments according to previously described algorithms (8, 10, 12). Most isolates were tested repeatedly using multiple batches of arrays, with an average of two to three experiments per isolate (see Table S5 in the supplemental material).
Susceptibility testing.
MICs were determined using Etest strips (bioMérieux S.A., Marcy l'Etoile, France), i.e., an agar dilution technique that uses plastic stripes impregnated with a gradient of an antibiotic compound in order to allow direct reading of the MIC. Etest strips were used according to manufacturer's instructions on Mueller-Hinton agar (Oxoid, Wesel, Germany) with or without 2% sodium chloride (Merck, Darmstadt, Germany). Incubation was performed overnight at 37°C.
Nucleotide sequence accession numbers.
A nucleotide sequence from a S. sciuri isolate from Propithecus verreauxi was submitted to the GenBank database under accession number JX094435.
RESULTS
Bioinformatic analysis of mecA gene polymorphisms.
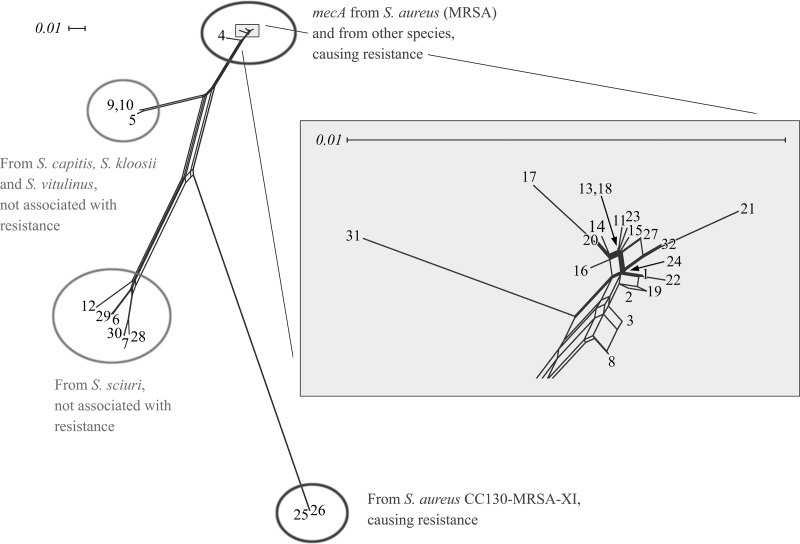
As of mid-2011, a GenBank database search yielded 135 full-length gene sequences (i.e., complete open reading frames) annotated as mecA. These sequences clustered into 32 unique alleles, which corresponded to 28 different amino acid sequences (Table 1; see also Tables S3 and S4 in the supplemental material). Visualization of the aligned DNA sequences by SplitsTree (version 4.11.3 on default settings; character transformation, uncorrected P/ignore ambiguous states; distance transformation, Neighbor-Net; and variance, ordinary least-squares [5]) showed them to cluster into four distinct branches (Fig. 1). One branch included sequences of well-known MRSA strains, such as N315 and COL, as well as methicillin-resistant coagulase-negative staphylococci. This also included some sequences (GU227428.1 [141:2147], X52593.1 [141:2147], and Y14051.1 [3472:5478]) harboring a frameshift mutation encompassing 17 amino acids (positions 198 to 214). These changes did not occur in the penicillin-binding domain of the PBP2a protein and did not affect resistance (13). Another branch comprised a recently described mecA allele from S. aureus element SCCmec IX, while the remaining two branches corresponded to different mecA alleles from coagulase-negative staphylococci such as S. vitulinus and S. sciuri.
Table 1.
For universal sequence allocators, see the supplemental material.
Fig 1.
SplitsTree analysis of the different alleles of mecA from staphylococci. Allele numbers are the same as in Table 1.
Primers and hybridization probes (see Tables S1 and S2 in the supplemental material) were designed to generate type-specific hybridization patterns under uniform experimental conditions at the stringency optimum for primer and probe binding. It was possible to predict actual hybridization patterns (Fig. 2) in theoretical experiments based on the following assumptions. A perfect match of a probe to a target sequence should result in 100% signal intensity, while three or more mismatches would prevent detection of any signal (0% signal intensity). One or two mismatches would result in weaker, intermediate, signal intensities, e.g., 60% for one mismatch and 30% for two mismatches, although actual signal intensities would also be influenced by the localization of the mismatch within the binding site. With 29 hybridization probes and 17 primers, as listed in the supplemental material, 16 different hybridization patterns can theoretically be generated. However, two of the predicted patterns, [AB546266] and [CP000046], were too similar as to allow a robust practical separation (see the supplemental material). Thus, assignment of experimental data sets to 15 different mecA types was possible (Fig. 1 and Table 1; see Table S4 in the supplemental material).
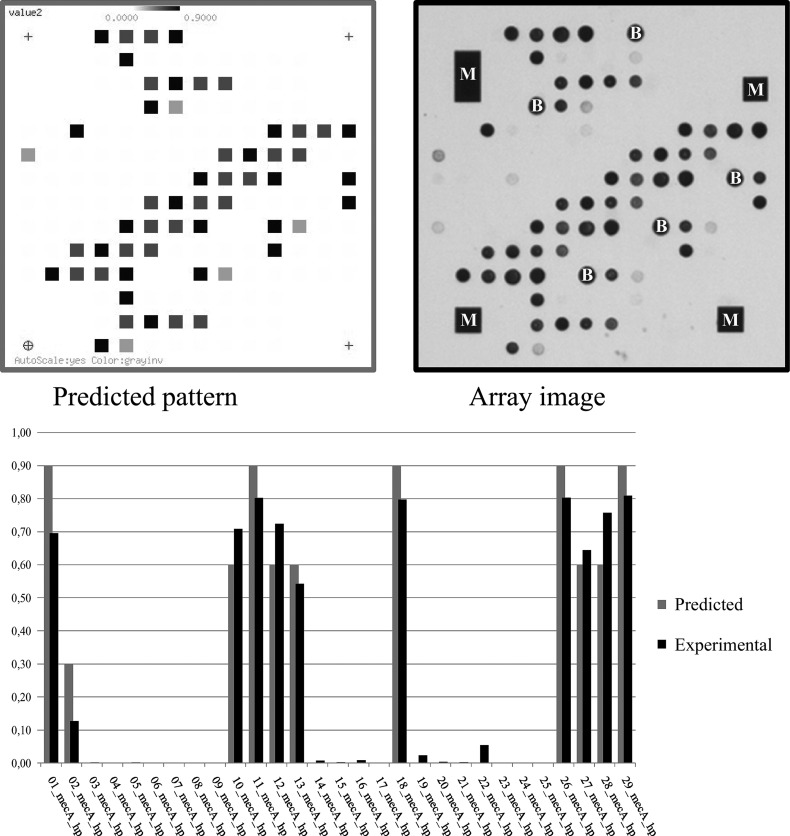
Fig 2.
Predicted and actual array image for S. aureus strain COL (GenBank accession no. CP000046.1, pattern [AB546266]/[CP000046]) and bar graph diagram of predicted and experimental spot intensities for the different probes. M, asymmetric metallic markers that provide orientation to the image analysis software; B, biotin spots serving as controls for the staining procedure.
Screening of clinical isolates.
Experimental conditions were optimized using known, fully sequenced, reference strains (such as COL and N315) so that theoretical expectations and experimental results matched optimal for the given set of primers and probes (Fig. 2). Subsequently, a collection of clinical and veterinary isolates was screened using the microarray-based approach as described. Nine of the fifteen predicted patterns were experimentally identified (Table 2 and see Table S5 in the supplemental material). The most common and widespread alleles were those that corresponded to hybridization patterns representing sequences known from MRSA strains. The pattern [AB546266]/[CP000046] was identified in isolates of S. aureus, S. epidermidis, S. fleurettii, S. pseudintermedius, S. saprophyticus, and S. simulans. Pattern [BA000018] was found in S. aureus, S. capitis, S. fleurettii, S. epidermidis, S. pseudintermedius, and S. saprophyticus isolates, while [GQ902038] was observed in isolates of S. aureus, S. haemolyticus, S. succinus, and S. pseudintermedius.
Table 2.
Detected hybridization patterns and strains in which these patterns were observed
| Hybridization pattern | Strains in which this pattern was observeda |
|---|---|
| [AB546266]/[CP000046] | S. aureus ST250-MRSA-I, Early/Ancestral MRSA, COL (CP000046.1) |
| S. aureus ST239-MRSA-III (two clinical isolates; one of them from Germany but related to medical care in Greece) | |
| S. aureus CC22-MRSA-IV, UK-EMRSA-15/Barnim EMRSA (2 medical isolates, from Germany and from Ireland) | |
| S. aureus CC398-MRSA-V (two veterinary isolates from Germany) | |
| S. aureus CC5-MRSA-VI, New Pediatric Clone (1 isolate) | |
| S. aureus ST5-MRSA-VII/SCC-JCSC6082 (AB373032) | |
| S. epidermidis (one veterinary isolate from Germany) | |
| S. fleurettii, ATCC_BAA-274/CCUG43834 (AB546266) | |
| S. fleurettii (one veterinary isolate from Germany and one screening isolate from a human with animal contact) | |
| S. pseudintermedius (one veterinary isolate from Germany) | |
| S. saprophyticus (one veterinary isolate from Germany) | |
| S. simulans (one medical isolate from Sweden) | |
| [AB546780] | S. vitulinus (four veterinary isolates) |
| [AB547235] | S. sciuri, DSM16827/ATCC700061 (AB547235) |
| [Y13094] | S. sciuri (DSM15613 and four veterinary isolates from Germany) |
| [BA000018] | S. aureus ST247-MRSA-I, North German/Iberian EMRSA (two isolates, one from Germany and one from Ireland) |
| S. aureus South German EMRSA/Italian Clone (three medical isolates from Germany) | |
| S. aureus ST5-MRSA-II, UK-EMRSA-3/New York-Japan Clone, N315 (BA000018) | |
| S. aureus ST5-MRSA-II, UK-EMRSA-3/New York-Japan, Mu50 (BA000017) | |
| S. aureus ST8-MRSA-IIB&SCC-M1, AR05_0.1345 (AJ810123) | |
| S. aureus ST239-MRSA-III (one medical isolate from Australia) | |
| S. aureus ST8-MRSA-IV [PVL+], USA300-TCH1516 (CP000730) | |
| S. aureus ST8-MRSA-IV [PVL+], USA300 (one clinical isolate from Germany and three from the USA) | |
| S. aureus CC5-MRSA-IV, Pediatric clone (one isolate from the USA) | |
| S. aureus CC22-MRSA-IV [PVL+] (one medical isolate from Sweden) | |
| S. aureus ST45-MRSA-IV, Berlin EMRSA (one medical isolate from Germany) | |
| S. aureus CC8-MRSA-VIII (one medical isolate from Ireland) | |
| S. capitis (one veterinary isolate from Germany) | |
| S. epidermidis (one veterinary and three medical isolates from Germany) | |
| S. fleurettii (two veterinary isolates from Germany) | |
| S. pseudintermedius (one veterinary isolate from Germany) | |
| S. saprophyticus (one medical isolate from Germany) | |
| [AY820253] | S. sciuri (DSM6671, one veterinary isolate from Germany, two isolates from sifakas from Madagascar) |
| [GQ902038] | S. aureus CC8-MRSA-V (one medical isolate from Germany) |
| S. aureus CC45-MRSA-V (two medical isolates, from Germany and Ireland) | |
| S. aureus CC398-MRSA-V (two veterinary isolates from Germany) | |
| S. haemolyticus (one medical and two veterinary isolates from Germany) | |
| S. pseudintermedius (one veterinary isolate from Germany) | |
| S. succinus (one isolate from the USA) | |
| [FR823292] | S. aureus CC130-MRSA-XI, M10/0061 (FR823292) |
| S. aureus CC130-MRSA-XI (one isolate from a hedgehog from Sweden) | |
| [Y09223] | S. sciuri, DSM20345/ATCC 29062 (Y09223) |
| S. sciuri (four veterinary isolates from Germany, including one from a mink, Mustela sp., and one isolate from a sifaka from Madagascar) | |
| Unknown pattern | S. sciuri (two isolates from sifakas from Madagascar) |
Strains for which mecA sequences are published are indicated in boldface.
Pattern [FR823292] was exclusively found in S. aureus CC130-MRSA-XI.
The other four hybridization patterns were detected in coagulase-negative staphylococci. Pattern [AB546780] was found in S. vitulinus. Sixteen of eighteen S. sciuri isolates yielded patterns [AY820253], [Y13094], or [Y09223].
In two S. sciuri isolates from Madagascan Verreaux's sifaka (Propithecus verreauxi), a distinct, unexpected hybridization pattern was noted. Sequencing of the region of its mecA gene that included the probe binding sites was in accordance with the observed hybridization pattern (GenBank accession no. JX094435).
SCCmec elements and mecA alleles.
The carriage of SCCmec elements did not necessarily coincide with the carriage of mecA alleles. Strains harboring SCCmec I elements yielded patterns [AB546266]/[CP000046] in ST250-MRSA-I “Early/Ancestral MRSA” or [BA000018] in ST247-MRSA-I “North German/Iberian EMRSA” and ST228-MRSA-I “South German EMRSA/Italian Clone.”
For SCCmec III, sequence analysis showed the presence of two different mecA alleles in two different sets of sequence data of ST239-MRSA-III (see Table S5 in the supplemental material and GenBank entries ABSA01000066 in JKD6008-ST239 and FN433596 in TW20-ST239). Accordingly, both patterns, [AB546266]/[CP000046] and [BA000018], were observed when testing different isolates of that strain.
SCCmec IV elements yielded mecA patterns [AB546266]/[CP000046] in CC22-MRSA-IV “UK-EMRSA-15/Barnim EMRSA” or [BA000018] in ST8-MRSA-IV “USA300,” CC5-MRSA-IV “Pediatric Clone,” and ST45-MRSA-IV “Berlin EMRSA” and in PVL-positive CC22-MRSA-IV.
Hybridization patterns [GQ902038] and [AB546266]/[CP000046] were observed in strains with SCCmec V/VT elements. In the epidemic livestock-associated CC398-MRSA-V, either of both alleles was observed.
Other SCCmec elements appeared to be more uniform with regard to mecA alleles, with [BA000018] being detected in SCCmec II and VIII and [AB546266]/[CP000046] being detected in SCCmec VI and VII, as well as [FR823292] in SCCmec XI. However, a variability of mecA within these elements cannot yet be completely ruled out due to the limited number of isolates tested.
Resistance tests for isolates with different mecA alleles.
The MICs of oxacillin, cefoxitin, cefepime, and imipenem were determined using Etest on Mueller-Hinton agar and on Mueller-Hinton agar supplemented with sodium chloride. These MIC values are summarized in Table 3.
Table 3.
MICs for strains with different hybridization patterns/mecA alleles
| Hybridization pattern/mecA allele | Isolate | Species, strain, and/or origin | MIC (μg/ml)a |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OXA | FOX | FEP | IPM | OXA-NaCl | FOX-NaCl | FEP-NaCl | IPM-NaCl | |||
| [B546266]/[CP000046] | COL | S. aureus ST250-MRSA-I | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| FLI-Neustadt-Ans46 | S. aureus ST239-MRSA-III | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | |
| SG-01 | S. fleurettii, veterinary isolate from Germany | 2 | 1 | 2 | 0.064 | 6 | 3 | 12 | 0.25 | |
| ATCC BAA-274 | S. fleurettii | 0.75 | 0.75 | 1 | 0.032 | >256 | 16 | 1 | 0.5 | |
| FLI-Neustadt-07 | S. saprophyticus, veterinary isolate from Germany | 8 | 8 | 32 | 0.125 | >256 | 16 | >256 | 0.25 | |
| [AB546780] | 88_C173_R34 | S. vitulinus, veterinary isolate from Germany | 0.38 | 0.75 | 0.38 | 0.023 | 0.75 | 1.5 | 1 | 0.032 |
| FLI-Neustadt-09-385 | S. vitulinus, veterinary isolate from Germany | 0.25/0* | NA | 0.38 | 0.016/0.03* | 0.25/0.38 | 0.75 | 0.75 | 0.032 | |
| SG-05_24635_FE63-1 | S. vitulinus, veterinary isolate from Germany | 0.38 | 1 | 0.5 | 0.023 | 0.38 | 1.5 | 1.5 | 0.047 | |
| [Y13094] | FLI-Neustadt-73_A215 | S. sciuri, veterinary isolate from Germany | 0.5/0.75 | 1 | 1/1.5 | 0.032/0.04* | 0.75 | 1 | 0.75 | 0.064 |
| FLI-Neustadt-10_24T | S. sciuri, veterinary isolate from Germany | 0.75 | 1.5 | 2 | 0.047 | 0.75 | 1.5 | 1.5 | 0.094 | |
| FLI-Neustadt-06_Rd.120 | S. sciuri, veterinary isolate from Germany | 0.5 | 1 | 1 | 0.032 | 0.5 | 1 | 1.5 | 0.047 | |
| [BA000018] | N315 | S. aureus ST5-MRSA-II | 16 | 32 | 32 | 4 | >256 | >256 | >256 | >256 |
| FLI-Neustadt-09_Rd.294 | S. aureus ST45-MRSA-IV, Berlin EMRSA, veterinary isolate from Germany | >256 | 128 | >256 | 3 | >256 | 64 | >256 | >256 | |
| FLI-Neustadt-24_P-LM-2 | S. aureus CC398-MRSA-V, isolate from poultry meat from Germany | 6 | 8 | 12 | 0.064 | 6 | 6 | 8 | 0.032 | |
| AT-Screen3_9-I | S. epidermidis, nasal swab from a healthy human from Germany | 0.75 | 6 | 1.5 | 0.125 | 0.5 | 4 | 2 | 0.094 | |
| FLI-Neustadt-05_Rd.255 | S. haemolyticus, veterinary isolate from Germany | >256 | 12 | 24 | 0.25 | >256 | 16 | >256 | 2 | |
| FLI-Neustadt-49_coa114 | S. epidermidis | 1 | 6 | 2 | 0.125 | 3 | 6 | 3 | 0.5 | |
| SG-02_24545 | S. fleurettii, veterinary isolate from Germany | 4 | 1.5 | 3 | 0.125 | 24 | 3 | 8 | 0.25 | |
| SG-03_24654 | S. fleurettii, veterinary isolate from Germany | 2 | 1.5 | 2 | 0.094 | 8 | 2 | 8 | 0.19 | |
| FLI-Neustadt-58M06-12_ | S. sciuri, veterinary isolate from Germany | 0.75 | 1 | 6 | 0.064 | 1.5 | 1 | 6 | 0.094 | |
| [AY820253] | CoNS-Isolate-20 | S. sciuri, isolate from Sweden | 1 | 1.5 | 1.5 | 0.032/0.06* | 1/1.5 | 1.5 | 2 | 0.064 |
| [GQ902038] | UKD-10C5347 | S. haemolyticus, clinical isolate from Germany | 128 | 16 | 96 | 0.25 | >256 | >256 | >256 | >256 |
| [FR823292] | Dublin 10/0061 | S. aureus CC130-MRSA-XI, clinical isolate from Ireland (14) | 1.5 | 8 | 6 | 0.064 | 1 | 4 | 12 | 0.125 |
| Hedgehog | S. aureus CC130-MRSA-XI, wildlife isolate from Sweden | 6 | 16 | 16 | 0.38 | 4 | 12 | 24 | 0.75 | |
| [Y09223] | FLI-Neustadt-48_coa111_151210 | S. sciuri, veterinary isolate from Germany | 0.75/1* | 1.5 | 1/1.5 | 0.047/0.06* | 0.5/0.75 | 1 | 1.5 | 0.064 |
| FLI-Neustadt-03_coa252 | S. sciuri, veterinary isolate from Germany | 1 | 2 | 1.5 | 0.047 | 0.5 | 1.5 | 1.5 | 0.064 | |
| Unknown pattern | 09-LEM-1/3 | S. sciuri from a sifaka from Madagascar | 2 | NA | NA | 0.047 | 1,5 | NA | NA | 0.094 |
OXA, oxacillin; FOX, cefoxitin; FEP, cefepime; IPM, imipenem. Asterisks indicate that multiple measurements were performed. NA, not applicable.
In general, elevated MIC values were noted only for isolates that yielded hybridization patterns [B546266]/[CP000046], [BA000018], [GQ902038], or [FR823292], the latter one corresponding to the novel mecA from strains M10 and LGA251. Induction by sodium chloride was observed only for isolates with mecA alleles corresponding to hybridization patterns [B546266]/[CP000046], [BA000018], and [GQ902038]. For isolates carrying other mecA alleles, the MIC values were low, and no inducibility was observed.
DISCUSSION
This study identified a considerable diversity of mecA genes among the published sequences. This led to the design of a DNA microarray that can be used to rapidly and reliably differentiate between these alleles based on a pattern recognition algorithm rather than sequencing. This allows high-throughput screening of clinical isolates in order to identify their mecA alleles and identification of novel alleles based on the observation of unpredicted hybridization patterns followed by sequencing.
The observations described here are also of interest for the understanding of the evolution of methicillin resistance in S. aureus and staphylococci in general. The species with the highest degree of diversity of mecA alleles was S. sciuri. Moreover, there are hardly any S. sciuri isolates without a mecA gene (we did not find a single one), although the mecA alleles in S. sciuri apparently do not confer a high level of methicillin resistance. This indicates that S. sciuri might have been the staphylococcal species in which mecA first appeared and evolved, although it fulfilled probably another physiological function. It would also be interesting to generate mecA knockout mutants of wild-type S. sciuri to study the function of this gene in the absence of methicillin resistance. In S. fleurettii, S. sciuri, and S. vitulinus, mecA does not reside in a SCC element but rather within the xyl gene cluster (17, 18). Resistance-conferring, SCCmec-associated mecA is, indeed, still linked to a xylR gene in SCCmec elements of types II, III, and VIII. Another step toward resistance-conferring, SCCmec-associated mecA was probably a recombination event linking mecA to regulatory genes derived from a β-lactamase operon (15). Indeed, a β-lactamase gene is still present in the SCCmec XI element. However, because of the great divergence of the mecA alleles from SCCmec XI (FR823292, FR821779) compared to other mecA alleles from methicillin-resistant staphylococci (4, 14) (see Fig. 1), it can be assumed that the evolution of a mecA precursor to a gene that confers resistance toward β-lactam compounds occurred at least twice.
Several SCCmec types (I, III, IV, and V) might harbor diverse alleles of resistance-conferring mecA. In CC22-MRSA-IV, two different mecA alleles were observed ([B546266]/[CP000046] and [BA000018]) with UK-EMRSA-15 carrying the former and two PVL-positive isolates carrying the latter. For two epidemic strains of MRSA (ST239-MRSA-III and CC398-MRSA-V), it was observed that isolates differed in the carriage of mecA alleles. There are two possible explanations for the observations.
First, each of these SCCmec types and strains could be polyphyletic. Multiple and independent recombination events might have resulted in very similar SCCmec elements that are merged together as one single SCCmec type. S. aureus from the same clonal background might have acquired such elements on different and independent occasions, thus giving the impression of belonging to the same “strain.” This hypothesis might be confirmed if genome sequencing of multiple isolates of one “strain” identified additional polymorphic markers that correlate to the mecA alleles substantiating the concept of a phylogenetic origin.
Second, the differences between the mecA alleles are caused by a number of secondary mutations that at random might occur independently of SCCmec type and strain affiliation. Their sites may represent mutational “hot spots” where mutations do not affect the functionality of the gene product. This possibility could experimentally be confirmed by continuous culture or serial transfer experiments.
Interestingly, several mecA alleles were found that are not known from MRSA strains and that apparently did not cause significant β-lactam resistance. For S. sciuri, this has previously been observed (18). It is not yet clear which physiological role these mecA alleles actually play and whether they also might occasionally be encountered in staphylococci of medical relevance, including S. aureus. Regarding nomenclature of these genes, we adhered to GenBank accession numbers for the sake of unambiguousness, but it should be considered to introduce another designation than mecA for alleles that do not confer phenotypic resistance to β-lactams.
The observation of sequence diversity within mecA could be of high relevance for the design of assays for the detection of mecA or of PBP2a. Alleles that cause resistance might remain undetected due to polymorphisms affecting primer binding sites or epitopes of antibodies. This has already been observed recently when a number of commercial tests failed to identify the mecA allele in emerging SCCmec XI strains (4, 14). On the other hand, a possible presence of mecA alleles and corresponding PBP2a variants that are not associated with resistance might render diagnostic tests essentially false positive. Thus, the presence of diverse mecA alleles, especially in coagulase-negative staphylococci, needs to be considered when designing diagnostic assays for the detection of mecA or PBP2a.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge A. Ruppelt, S. Schubert (Technical University of Dresden), as well as I. Engelmann, G. Roessler, and J. Sachtschal (Alere Technologies, Jena, Germany) for excellent technical assistance. We are indebted to T. Ellinger (Alere Technologies), B. Walther and S. Günther (Institute of Microbiology and Epizootics, Department of Veterinary Medicine, Freie Universität Berlin, Berlin, Germany), C. Berglund (Aleris Medilab, Stockholm, Sweden), A. Moodley (Department of Veterinary Disease Biology, Faculty of Life Sciences, University of Copenhagen, Copenhagen, Denmark), A. Shore (Dublin Dental University Hospital, Trinity College Dublin, Dublin, Ireland), L. Skakni (King Fahad Medical City, Riyadh, Kingdom of Saudi Arabia), B. Söderquist (Department of Clinical Microbiology, Orebro University Hospital, Orebro, Sweden), and D. Gavier-Widén (for the Wildtech project, EU 7th Framework Program for Research and Technological Development, grant agreement 222633) for providing clinical isolates. We also thank P. Slickers and V. Baier (Alere Technologies) for creating software tools used for the prediction of hybridization patterns. We thank E. Jacobs (Technical University of Dresden) and E. Ermantraut (Alere Technologies) for supporting this work.
S.S. was supported by the German Federal Ministry of Education and Research through the German Aerospace Center (grant 01KI1014D [MedVet-Staph]).
S.M., E.M., and R.E. are employees of Alere Technologies. This had no influence on the study design, the analysis, and the interpretation of the data.
Footnotes
Published ahead of print 13 August 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Baba T, et al. 2009. Complete genome sequence of Macrococcus caseolyticus strain JSCS5402, reflecting the ancestral genome of the human-pathogenic staphylococci. J. Bacteriol. 191:1180–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Couto I, et al. 1996. Ubiquitous presence of a mecA homologue in natural isolates of Staphylococcus sciuri. Microb. Drug Resist. 2:377–391 [DOI] [PubMed] [Google Scholar]
- 3. Feßler AT, Billerbeck C, Kadlec K, Schwarz S. 2010. Identification and characterization of methicillin-resistant coagulase-negative staphylococci from bovine mastitis. J. Antimicrob. Chemother. 65:1576–1582 [DOI] [PubMed] [Google Scholar]
- 4. Garcia-Alvarez L, et al. 2011. Methicillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infect. Dis. 11:595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23:254–267 [DOI] [PubMed] [Google Scholar]
- 6. IWG-SCC 2009. Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob. Agents Chemother. 53:4961–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jevons MP. 1961. “Celbenin”-resistant staphylococci. BMJ 1:1924–1925 [Google Scholar]
- 8. Monecke S, et al. 2011. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS One 6:e17936 doi:10.1371/journal.pone.0017936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monecke S, Ehricht R. 2005. Rapid genotyping of methicillin-resistant Staphylococcus aureus (MRSA) isolates using miniaturised oligonucleotide arrays. Clin. Microbiol. Infect. 11:825–833 [DOI] [PubMed] [Google Scholar]
- 10. Monecke S, Slickers P, Ehricht R. 2008. Assignment of Staphylococcus aureus isolates to clonal complexes based on microarray analysis and pattern recognition. FEMS Immunol. Med. Microbiol. 53:237–251 [DOI] [PubMed] [Google Scholar]
- 11. Perreten V, et al. 2010. Clonal spread of methicillin-resistant Staphylococcus pseudintermedius in Europe and North America: an international multicentre study. J. Antimicrob. Chemother. 65:1145–1154 [DOI] [PubMed] [Google Scholar]
- 12. Ruettger A, et al. 2011. Genotyping of Chlamydia trachomatis strains from culture and clinical samples using an ompA-based DNA microarray assay. Mol. Cell. Probes 25:19–27 [DOI] [PubMed] [Google Scholar]
- 13. Ryffel C, et al. 1990. Sequence comparison of mecA genes isolated from methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. Gene 94:137–138 [DOI] [PubMed] [Google Scholar]
- 14. Shore AC, et al. 2011. Detection of staphylococcal cassette chromosome mec type XI encoding highly divergent mecA, mecI, mecR1, blaZ, and ccr genes in human clinical clonal complex 130 methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 55:3765–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song MD, Wachi M, Doi M, Ishino F, Matsuhashi M. 1987. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 221:167–171 [DOI] [PubMed] [Google Scholar]
- 16. Tsubakishita S, Kuwahara-Arai K, Baba T, Hiramatsu K. 2010. Staphylococcal cassette chromosome mec-like element in Macrococcus caseolyticus. Antimicrob. Agents Chemother. 54:1469–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsubakishita S, Kuwahara-Arai K, Sasaki T, Hiramatsu K. 2010. Origin and molecular evolution of the determinant of methicillin resistance in staphylococci. Antimicrob. Agents Chemother. 54:4352–4359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu S, Piscitelli C, de Lencastre H, Tomasz A. 1996. Tracking the evolutionary origin of the methicillin resistance gene: cloning and sequencing of a homologue of mecA from a methicillin-susceptible strain of Staphylococcus sciuri. Microb. Drug Resist. 2:435–441 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.