Abstract
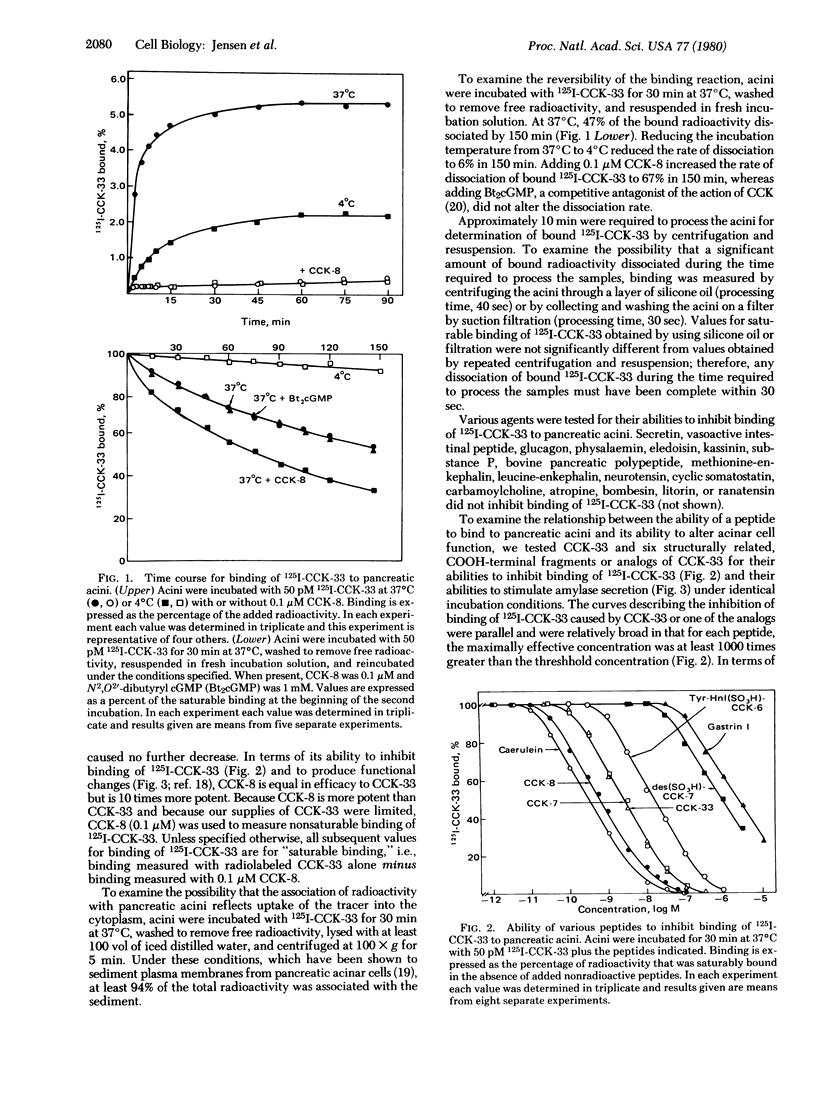
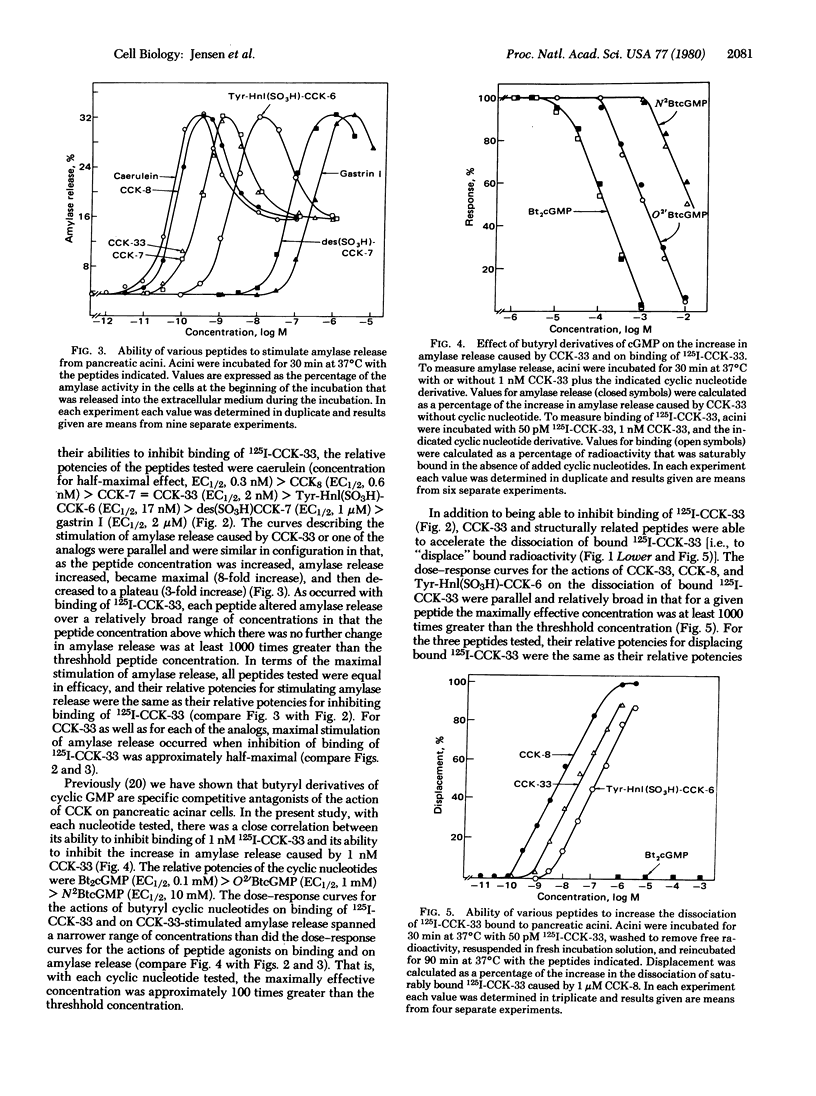
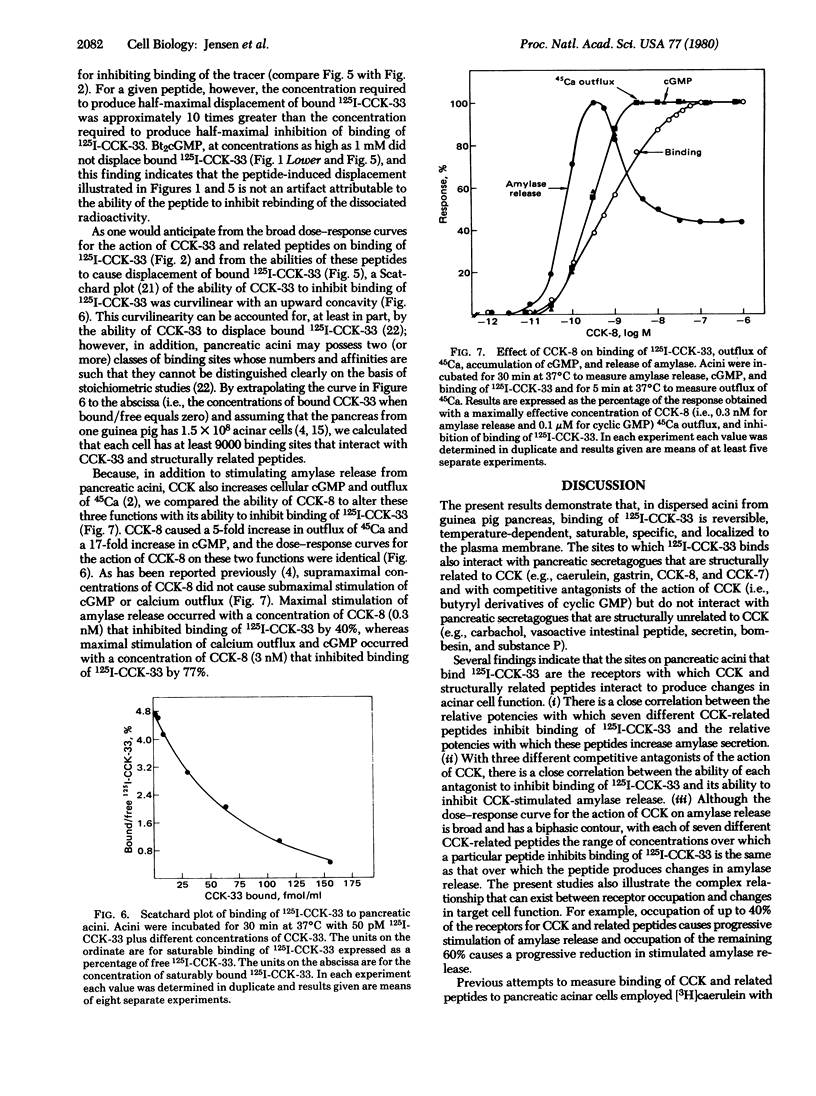
We have prepared 125I-labeled cholecystokinin and have examined the kinetics, stoichiometry, and chemical specificity with which the labeled peptide binds to dispersed acini from guinea pig pancreas. Binding of 125I-labeled cholecystokinin was reversible, temperature-dependent, saturable, specific, and localized to the plasma membrane. Each acinar cell possessed approximately 9000 binding sites, and binding of the labeled peptide to these sites could be inhibited by cholecystokinin and structurally related peptides (e.g., gastrin and caerulein) as well as by nonpeptide competitive antagonists of the action of cholecystokinin. Binding was not inhibited by other pancreatic secretagogues such as secretin, vasoactive intestinal peptide, glucagon, physalaemin, eledoisin, kassinin, substance P, carbamoylcholine, litorin, or ranatensin or by bovine pancreatic polypeptide, atropine, neurotensin, leucineenkephalin, methionine-enkephalin, or cyclic somatostatin. With agonists as well as antagonists there was a good correlation between occupation of cholecystokinin binding sites and changes in acinar cell function. With each of six different peptide agonists maximal stimulation of enzyme secretion occurred with 40% receptor occupation and occupation of the remaining 60% caused a progressive decrease in stimulated amylase release. Agonists, but not antagonists, accelerated the dissociation of bound 125I-labeled cholecystokinin, and these findings suggest that, in pancreatic acini, radiolabeled cholecystokinin binds to at least one class of interacting binding sites whose affinities are influenced by the extent to which these sites are occupied by agonists but not the extent to which they are occupied by antagonists.
Keywords: pancreatic secretagogues, amylase secretion, calcium transport, cyclic GMP
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J., Bhoola K. D., Harvey R. F. Intracellular messenger role of cyclic GMP in exocrine pancreas. Nature. 1976 Jul 29;262(5567):404–406. doi: 10.1038/262404a0. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophe J. P., Conlon T. P., Gardner J. D. Interaction of porcine vasoactive intestinal peptide with dispersed pancreatic acinar cells from the guinea pig. Binding of radioiodinated peptide. J Biol Chem. 1976 Aug 10;251(15):4629–4634. [PubMed] [Google Scholar]
- Christophe J. P., Frandsen E. K., Conlon T. P., Krishna G., Gardner J. D. Action of cholecystokinin, cholinergic agents, and A-23187 on accumulation of guanosine 3':5'-monophosphate in dispersed guinea pig pancreatic acinar cells. J Biol Chem. 1976 Aug 10;251(15):4640–4645. [PubMed] [Google Scholar]
- Christophe J., De Neef P., Deschodt-Lanckman M., Robberecht P. The interaction of caerulein with the rat pancreas. 2. Specific binding of [3H]caerulein on dispersed acinar cells. Eur J Biochem. 1978 Nov 2;91(1):31–38. doi: 10.1111/j.1432-1033.1978.tb20933.x. [DOI] [PubMed] [Google Scholar]
- Deschodt-Lanckman M., Robberecht P., Camus J., Christophe J. The interaction of caerulein with the rat pancreas. 1. Specific binding of [3H]caerulein on plasma membranes and evidence for negative cooperativity. Eur J Biochem. 1978 Nov 2;91(1):21–29. doi: 10.1111/j.1432-1033.1978.tb20932.x. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Hahne W. F. Calcium transport in dispersed acinar cells from rat pancreas. Biochim Biophys Acta. 1977 Dec 15;471(3):466–476. doi: 10.1016/0005-2736(77)90050-5. [DOI] [PubMed] [Google Scholar]
- Gardner J. D. Regulation of pancreatic exocrine function in vitro: initial steps in the actions of secretagogues. Annu Rev Physiol. 1979;41:55–66. doi: 10.1146/annurev.ph.41.030179.000415. [DOI] [PubMed] [Google Scholar]
- Haymovits A., Scheele G. A. Cellular cyclic nucleotides and enzyme secretion in the pancreatic acinar cell. Proc Natl Acad Sci U S A. 1976 Jan;73(1):156–160. doi: 10.1073/pnas.73.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. T., Moody T., Pert C., Rivier J. E., Gardner J. D. Interaction of bombesin and litorin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6139–6143. doi: 10.1073/pnas.75.12.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin R. N., Gardner J. D. Effects of calcium and chelating agents on the ability of various agonists to increase cyclic GMP in pancreatic acinar cells. Biochim Biophys Acta. 1978 Nov 1;543(4):465–475. doi: 10.1016/0304-4165(78)90301-x. [DOI] [PubMed] [Google Scholar]
- May R. J., Conlon T. P., Erspamer V., Gardner J. D. Actions of peptides isolated from amphibian skin on pancreatic acinar cells. Am J Physiol. 1978 Aug;235(2):E112–E118. doi: 10.1152/ajpendo.1978.235.2.E112. [DOI] [PubMed] [Google Scholar]
- Peikin S. R., Costenbader C. L., Gardner J. D. Actions of derivatives of cyclic nucleotides on dispersed acini from guinea pig pancreas. Discovery of a competitive antagonist of the action of cholecystokinin. J Biol Chem. 1979 Jun 25;254(12):5321–5327. [PubMed] [Google Scholar]
- Peikin S. R., Rottman A. J., Batzri S., Gardner J. D. Kinetics of amylase release by dispersed acini prepared from guinea pig pancreas. Am J Physiol. 1978 Dec;235(6):E743–E749. doi: 10.1152/ajpendo.1978.235.6.E743. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Electrophysiology of mammalian gland cells. Physiol Rev. 1976 Jul;56(3):535–577. doi: 10.1152/physrev.1976.56.3.535. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Ueda N. Pancreatic acinar cells: effect of acetylcholine, pancreozymin, gastrin and secretin on membrane potential and resistance in vivo and in vitro. J Physiol. 1975 May;247(2):461–471. doi: 10.1113/jphysiol.1975.sp010941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. I. Development of sequence-specific radioimmunoassays for porcine triacontatriapeptide cholecystokinin. J Biol Chem. 1978 Jun 10;253(11):4016–4021. [PubMed] [Google Scholar]
- Robberecht P., Deschodt-Lackman M., Morgat J. L., Christophe J. The interaction of caerulein with the rat pancreas. 3. Structural requirements for in vitro binding of caerulein-like peptides and its relationship to increased calcium outflux, adenylate cyclase activation, and secretion. Eur J Biochem. 1978 Nov 2;91(1):39–48. doi: 10.1111/j.1432-1033.1978.tb20934.x. [DOI] [PubMed] [Google Scholar]
- Sjodin L., Gardner J. D. Effect of cholecystokinin variant (CCK39) on dispersed acinar cells from guinea pig pancreas. Gastroenterology. 1977 Nov;73(5):1015–1018. [PubMed] [Google Scholar]



