Abstract
The MIC90 of RBx 14255, a novel ketolide, against Clostridium difficile was 4 μg/ml (MIC range, 0.125 to 8 μg/ml), and this drug was found to be more potent than comparator drugs. An in vitro time-kill kinetics study of RBx 14255 showed time-dependent bacterial killing for C. difficile. Furthermore, in the hamster model of C. difficile infection, RBx 14255 demonstrated greater efficacy than metronidazole and vancomycin, making it a promising candidate for C. difficile treatment.
TEXT
Clostridium difficile is a Gram-positive spore-forming anaerobic bacterium that causes a range of infections from mild and self-limiting diarrhea to life-threatening pseudomembranous colitis and death (6, 7). C. difficile spores excreted from infected patients are prevalent in hospitals and health care facilities and can persist in the lower intestinal tract of human beings (6, 16). The use of broad-spectrum antibiotics causes disruption of the normal gut flora and predisposes the gut to C. difficile intestinal colonization (11, 12). During the past decade, there has been a striking increase in the number of cases of C. difficile infection (CDI) due to the emergence of epidemic strains such as the C. difficile B1/NAP/027 strain (3, 5).
Metronidazole and vancomycin are the drugs of choice to treat CDI patients. Although both drugs are used for treating the infection, they have shortcomings, including unacceptably high rates of recurrence of infection (15 to 35%) (2, 18). The drawbacks of current CDI therapy have necessitated the search for new and improved therapies.
Ketolides belong to a novel class of macrolide antibiotics with improved antibacterial activity against macrolide-resistant organisms (17). In this study, we have investigated for the first time the activity of RBx 14255, a novel ketolide (Fig. 1), against C. difficile in vitro and in a hamster model of CDI and identified a prospective candidate for the treatment of C. difficile infection.
Fig 1.
Structure of RBx 14255.
The bacterial strains included in this study were from the Ranbaxy Research Laboratories (Gurgaon, India) culture collection, the American Type Culture Collection (ATCC; Manassas, VA), the National Collection of Type Cultures (London, United Kingdom), and a tertiary care medical center (PGIMER, Chandigarh, India). The BI/NAP1/027-type strain 2009155 (epidemic strain) was obtained from the Centers for Disease Control and Prevention (Atlanta, GA). Reference anaerobe strains Bacteroides fragilis ATCC 25285 and Bacteroides thetaiotaomicron ATCC 29741 were also included when performing antibiotic susceptibility assays.
Erythromycin, clarithromycin, vancomycin, and metronidazole were purchased from Sigma (St. Louis, MO). Telithromycin and clindamycin phosphate were obtained from a commercial source. RBx 14255 (Fig. 1) was synthesized at Ranbaxy Research Laboratories, Gurgaon, India.
MICs of RBx 14255, telithromycin, erythromycin, clarithromycin, vancomycin, metronidazole, and clindamycin were determined in accordance with Clinical and Laboratory Standards Institute (CLSI) guidelines by the agar dilution method (13). Further, the antimicrobial activities of RBx 14255 and comparators against the spores of C. difficile ATCC 43255 and C. difficile ATCC 9689 were determined by the broth microdilution method in 96-well plates. The spores of C. difficile ATCC 43255 and ATCC 9689 were prepared as described earlier (9).
Time-kill kinetics studies of RBx 14255, vancomycin, and telithromycin against C. difficile ATCC 43255 and C. difficile 6387 were performed at multiple concentrations ranging from 1× to 16× MIC as described earlier (4, 10).
The frequency of resistance development by C. difficile ATCC 43255 and C. difficile 6387 in the presence of 4× and 16× MICs of RBx 14255, vancomycin, metronidazole, and rifampin was determined by a previously described method (10). In order to determine the ability of C. difficile ATCC 43255 and C. difficile 6429 strains to develop resistance to RBx 14255, vancomycin, metronidazole, and telithromycin, a previously published serial passage method was used (10). All the above in vitro experiments were performed in an anaerobic chamber (Bactron IV; Sheldon Mfg., Cornelius, OR).
Golden Syrian hamsters weighing 80 to 100 g were used for the study. They were purchased from the National Institute of Nutrition (NIN, ICMR, Hyderabad, India). Hamsters were given a single subcutaneous dose of clindamycin phosphate (30 mg/kg of body weight), to render them susceptible to C. difficile infection (10, 14). On the following day, hamsters were infected by oral gavage with a 1-ml suspension (5 × 107 CFU/ml) of C. difficile ATCC 43596 prepared from a culture grown overnight. Treatment was started 24 h postinfection with vehicle, RBx 14255 (50 mg/kg), vancomycin (5 mg/kg), or metronidazole (150 mg/kg) and was given twice a day for 5 consecutive days. RBx 14255 and metronidazole were prepared in 0.25% methylcellulose, while vancomycin was prepared in sterile Milli-Q water. An infected and an uninfected control (administered clindamycin only) were also included in this study. Six hamsters per group were taken and monitored twice daily for morbidity and mortality. The statistical analysis was performed using GraphPad Prism-5.03 (GraphPad Software, San Diego, CA).
The MIC range, MIC50s, and MIC90s for the tested organisms are presented in Table 1. RBx 14255 showed 2-fold-higher MICs against all the clinical and reference strains of C. difficile than did metronidazole, 2-fold-lower MICs than did vancomycin, and more-than-2-fold-lower MICs than did other comparators. RBx 14255 showed MICs of 0.25 μg/ml against C. difficile BI/NAP1/027, whereas MICs of clindamycin, erythromycin, and clarithromycin were 16, 8, and 4 μg/ml, respectively. Moreover, against other Clostridium spp., including C. perfringens, the activities of RBx 14255 were higher than those of reference antibiotics, with MIC90 being 0.125 μg/ml. RBx 14255 showed poor activity against Gram-negative anaerobes B. fragilis and Fusobacterium nucleatum (Table 1). RBx 14255 and comparator antibiotics showed comparable activities against spores of C. difficile ATCC 43255 and C. difficile ATCC 9689 (Table 2).
Table 1.
Antibacterial activity of RBx 14255 against Clostridium spp. and other anaerobic bacteria
| Organism (n) and antimicrobial agent | MIC (μg/ml) |
||
|---|---|---|---|
| MIC50 | MIC90 | Range | |
| C. difficile (28)a | |||
| RBx 14255 | 0.5 | 4 | 0.125–8 |
| Telithromycin | 0.5 | 8 | 0.5–16 |
| Erythromycin | 2 | 16 | 2–>16 |
| Clarithromycin | 1 | >16 | 1–>16 |
| Vancomycin | 1 | 4 | 0.5–8 |
| Metronidazole | 0.25 | 2 | 0.06–4 |
| Clindamycin | 8 | >16 | 2–>16 |
| Clostridium spp. (11)b | |||
| RBx 14255 | 0.06 | 0.125 | 0.03–0.25 |
| Telithromycin | 0.125 | 2 | 0.5–4 |
| Erythromycin | 2 | 8 | 2–16 |
| Clarithromycin | 0.5 | 16 | 0.06–16 |
| Vancomycin | 0.5 | 1 | 0.5–4 |
| Metronidazole | 0.25 | 1 | 0.03–1 |
| Clindamycin | 0.5 | 1 | 0.125–8 |
| Propionibacterium acnes (6)c | |||
| RBx 14255 | 0.03 | 1 | 0.015–1 |
| Telithromycin | 0.125 | 4 | 0.06–>4 |
| Erythromycin | 0.125 | 0.25 | 0.125–0.25 |
| Clarithromycin | 0.125 | 0.25 | 0.03–0.5 |
| Vancomycin | 1 | 1 | 0.125–1 |
| Metronidazole | 16 | 16 | 16 |
| Clindamycin | 0.25 | 1 | 0.25–1 |
| B. fragilis (17)d | |||
| RBx 14255 | 2 | 8 | 1–16 |
| Telithromycin | 2 | 16 | 2–16 |
| Erythromycin | 8 | >16 | 8–>16 |
| Clarithromycin | 8 | >16 | 8–>16 |
| Vancomycin | 16 | 16 | 16–>16 |
| Metronidazole | 0.5 | 2 | 0.25–2 |
| Clindamycin | 1 | 16 | 1–>16 |
| F. nucleatum (3)e | |||
| RBx 14255 | 8 | 16 | 8–>16 |
| Telithromycin | 2 | 4 | 2–16 |
| Erythromycin | >16 | >16 | >16 |
| Clarithromycin | >16 | >16 | >16 |
| Vancomycin | 16 | 16 | 8–>16 |
| Metronidazole | 0.25 | 0.5 | 0.06–0.5 |
| Clindamycin | 0.06 | 0.125 | 0.03–0.125 |
Clostridium difficile ATCC 43255, C. difficile ATCC 43596, C. difficile ATCC 9689, C. difficile NCTC 11223, C. difficile BI/NAP1/027-2001155, and C. difficile clinical isolates (23).
Clostridium perfringens ATCC 13124, C. perfringens clinical isolates (4), Clostridium cadaveris 6400, Clostridium bifermentans (3), and Clostridium subterminale (2).
Propionibacterium acnes ATCC 6919, P. acnes clinical isolates (5).
Bacteroides fragilis ATCC 25285, B. fragilis (16).
Fusobacterium nucleatum 5356, F. nucleatum (2).
Table 2.
Antibacterial activity of RBx 14255 against C. difficile vegetative cells and spores
| Antimicrobial agent | MIC against C. difficile (μg/ml) |
|||
|---|---|---|---|---|
| ATCC 43255 |
ATCC 9689 |
|||
| Vegetative cells | Spores | Vegetative cells | Spores | |
| RBx 14255 | 0.5 | 2 | 0.25 | 2 |
| Telithromycin | 0.25 | 2 | 0.25 | 2 |
| Erythromycin | 1 | 2 | 1 | 2 |
| Clarithromycin | 0.5 | 2 | 32 | >32 |
| Vancomycin | 1 | 4 | 2 | 4 |
| Metronidazole | 0.125 | 0.25 | 0.25 | 2 |
| Clindamycin | 8 | >16 | 8 | >32 |
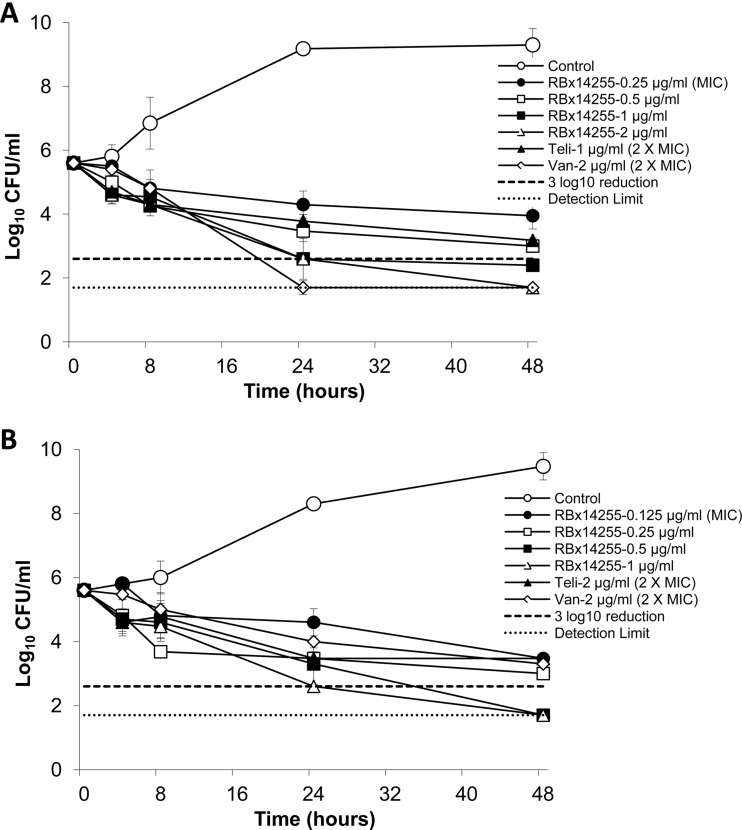
The time-kill studies demonstrated that RBx 14255 at 4× MIC and above showed bactericidal (99.9%) activity against both strains with no regrowth even at MIC level (Fig. 2). Vancomycin showed bactericidal activity against C. difficile ATCC 43255 and bacteriostatic activity against C. difficile 6387 at 2× MIC, whereas telithromycin at 2× MIC produced a bacteriostatic effect.
Fig 2.
In vitro time-kill kinetics of RBx 14255 against C. difficile ATCC 43255 (A) and C. difficile 6387 (B). Viability counts are presented at various time periods between 0 and 48 h.
The frequency of resistance to RBx 14255 observed for C. difficile ATCC 43255 was 2 × 10−8 and <7 × 10−9 whereas that for C. difficile 6387 was 1.5 × 10−9 and 4.0 × 10−9 at 4× and 16× MIC, respectively. The frequency of resistance to vancomycin and metronidazole was also comparable to that to RBx 14255 for both the C. difficile strains (Table 3). Twenty-five serial passages of C. difficile ATCC 43255 and C. difficile 6429 resulted in no significant increase in RBx 14255 MICs. Similarly, C. difficile strains did not develop any kind of shift for vancomycin and metronidazole MICs (data not shown).
Table 3.
In vitro frequency of resistance by C. difficile against RBx 14255 and other standard drugs
| C. difficile strain/drug | Frequency of spontaneous resistance |
|
|---|---|---|
| 4× MIC (mg/liter) | 16× MIC (mg/liter) | |
| ATCC 43255 | ||
| RBx 14255 | 2 × 10−8 | <7.0 × 10−9 |
| Vancomycin | 3.5 × 10−9 | <7.0 × 10−9 |
| Metronidazole | 2.0 × 10−9 | 6.0 × 10−9 |
| Rifampin | 1.0 × 10−7 | 3.5 × 10−7 |
| 6387 | ||
| RBx 14255 | 1.5 × 10−9 | 4.0 × 10−9 |
| Vancomycin | 4.5 × 10−9 | <6.5 × 10−9 |
| Metronidazole | 1.0 × 10−8 | <6.5 × 10−9 |
| Rifampin | 1.5 × 10−7 | 2.0 × 10−7 |
As expected from a ketolide, the sub-MIC of RBx 14255 in a mechanism of action (MOA) study resulted in 70% inhibition of protein synthesis at 6 h of incubation (data not shown). Moreover, RBx 14255 inhibited the [14C]erythromycin binding to 70S ribosome with a 50% inhibitory concentration (IC50) of 0.23 μM; in comparison, telithromycin showed the same inhibition at 0.65 μM (8). RBx 14255 displayed greater potency for the 70S ribosome than did telithromycin. This finding supports the excellent in vitro activity and time-kill kinetics profile of RBx 14255.
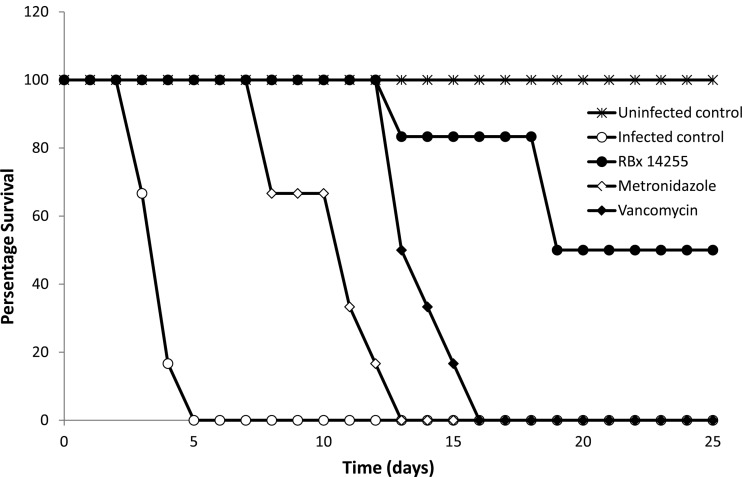
The in vivo efficacy of RBx 14255 in the hamster model of CDI is presented in Fig. 3. All infected hamsters treated with placebo developed clinical symptoms of infection, including a wet tail, within 4 days of infection and died by day 5. The feces and cecal contents collected from dead animals were found to be positive for C. difficile vegetative cells and C. difficile toxins (A/B). The first mortality in the RBx 14255-treated group occurred at day 13 with no sign of diarrhea; however, the cecal content of the dead animal was found to be positive for C. difficile and its toxin. The remaining hamsters in the RBx 14255-treated group showed no mortality until day 18. In the metronidazole-treated group, no mortality occurred during treatment; however, within 4 days of stoppage of treatment, hamsters started showing signs of diarrhea, followed by 100% mortality by day 13. While no mortality occurred in the vancomycin-treated group until 7 days after treatment, all animals subsequently succumbed to diarrhea within the next 3 days. The dose of vancomycin in our study was low (5 mg/kg); however, another study reported that a higher dose of vancomycin (50 mg/kg), which is equal to the dose of RBx 14255 administered in this study, resulted in comparable efficacy (1).
Fig 3.
In vivo efficacy of RBx 14255 in a hamster model of C. difficile infection. Hamsters were pretreated with 30 mg/kg of clindamycin subcutaneously on day −1 and infected orally with C. difficile on day 0. Hamsters were treated with RBx 14255 (50 mg/kg), metronidazole (150 mg/kg), and vancomycin (5 mg/kg) orally twice a day on day 1 until day 5 and observed for clinical signs of disease and mortality. All RBx 14255-treated animals showed extended survival compared to metronidazole-treated animals (P < 0.0001) and vancomycin-treated animals (P < 0.002).
In summary, we have investigated and reported the activity of RBx 14255, a novel ketolide, against anaerobic bacteria for the first time. It was found that RBx 14255 is active against erythromycin- and clarithromycin-resistant C. difficile strains, including the epidemic BI/NAP1/027 strain, and several bacterial species with different resistance patterns; these findings could indicate a special advantage of RBx 14255 over existing macrolides and ketolides (15, 17). RBx 14255 is a novel antibacterial agent that prevents the growth of C. difficile by inhibiting protein synthesis and could be investigated for C. difficile treatment. Based on the above promising data, RBx 14255, a novel investigational antibacterial agent, may be explored further for treatment of CDI in humans.
ACKNOWLEDGMENTS
We thank Pankaj Mathur (Medical Superintendent, ONGC Ltd. Hospital, Dehradun, India) for providing clinical C. difficile strains for our study.
This research was supported by Ranbaxy Research Laboratories, Gurgaon, India.
There are no competing interests to declare.
The institutional animal ethics committee (IAEC) of Ranbaxy Research Laboratories, Gurgaon, India, approved all experimental protocols (no. 159/07 E2/10) for the use of animals.
Footnotes
Published ahead of print 6 August 2012
REFERENCES
- 1. Anton PM, et al. 2004. Rifalazil treats and prevents relapse of Clostridium difficile-associated diarrhea in hamsters. Antimicrob. Agents Chemother. 48:3975–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barbut F, et al. 2000. Epidemiology of recurrences or reinfection of Clostridium difficile-associated diarrhea. J. Clin. Microbiol. 38:2386–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention 2005. Clostridium difficile information for healthcare providers. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/ncidod/dhqp/id_CdiffFAQ_HCP.html Accessed 26 November 2007 [Google Scholar]
- 4. Credito KL, Ednie LM, Appelbaum PC. 2008. Comparative antianaerobic activities of doripenem determined by MIC and time-kill analysis. Antimicrob. Agents Chemother. 52:365–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deneve C, Janoira C, Poilaneb I, Fantinatob C, Collignona A. 2009. New trends in Clostridium difficile virulence and pathogenesis. Int. J. Antimicrob. Agents 33:S24–S28 [DOI] [PubMed] [Google Scholar]
- 6. Gerding DN, Jr, et al. 1986. Clostridium difficile-associated diarrhea and colitis in adults: a prospective case-controlled epidemiologic study. Arch. Intern. Med. 146:95–100 [PubMed] [Google Scholar]
- 7. Hookman P, Barkin JS. 2009. Clostridium difficile associated infection, diarrhea and colitis. World J. Gastroenterol. 15:1554–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalia V, et al. 2009. Mode of action of ranbezolid against staphylococci and structural modeling studies of its interaction with ribosomes. Antimicrob. Agents Chemother. 53:1427–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lyras D, et al. 2009. Toxin B is essential for virulence of Clostridium difficile. Nature 458:1176–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mathur T, et al. 2011. Activity of RBx 11760, a novel biaryl oxazolidinone, against Clostridium difficile. J. Antimicrob. Chemother. 66:1087–1095 [DOI] [PubMed] [Google Scholar]
- 11. McCusker ME, Harris AD, Perencevich EE, Roghmann MC. 2003. Fluoroquinolone use and Clostridium difficile-associated diarrhea. Emerg. Infect. Dis. 9:730–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Modena S, Bearelly D, Swartz K, Friedenberg FK. 2005. Clostridium difficile among hospitalized patients receiving antibiotics: a case-control study. Infect. Control Hosp. Epidemiol. 26:685–690 [DOI] [PubMed] [Google Scholar]
- 13. National Committee for Clinical Laboratory Standards 2004. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 6th ed: approved standard M11-A6. National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 14. Ochsner UA, et al. 2009. Inhibitory effect of REP3123 on toxin and spore formation in Clostridium difficile, and in vivo efficacy in a hamster gastrointestinal infection model. J. Antimicrob. Chemother. 63:964–971 [DOI] [PubMed] [Google Scholar]
- 15. Pandya M, et al. 2010. Activity of a novel series of acylides active against community acquired respiratory pathogens. Int. J. Antimicrob. Agents. 36:169–174 [DOI] [PubMed] [Google Scholar]
- 16. Siegel DL, Edelstein PH, Nachamkin I. 1990. Inappropriate testing for diarrheal diseases in the hospital. JAMA 263:979–982 [PubMed] [Google Scholar]
- 17. Tanikawa T, et al. 2003. Synthesis and antibacterial activity of a novel series of acylides: 3-O-(3-pyridyl) acetylerythromycin A derivatives. J. Med. Chem. 46:2706–2715 [DOI] [PubMed] [Google Scholar]
- 18. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. 2007. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin. Infect. Dis. 45:302–307 [DOI] [PubMed] [Google Scholar]