Abstract
The role played by alpha-fetoprotein (AFP) during the perinatal period in rats has not yet been ascertained despite earlier suggestions that this plasma protein affected the multiplication, in an "in animal-in culture" system of tumor cells that are stimulated by estrogen (E) for growth. To test this inference developed from our previous work, AFP was purified by reverse affinity chromatography to homogeneity by electrophoretic and immunochemical criteria. Purified AFP was added at different concentrations to horse serum-supplemented medium which by itself is able to allow maximal exponential growth of a rat pituitary tumor cloned cell line C29RAP. These cells carry estrophilins and their growth is stimulated by E in animals but not in culture. At 3 mg/ml in culture media, AFP prevented growth of C29RAP cells; the effect was dose dependent. F4C1, a rat pituitary cloned cell line that carries estrophilins but shows autonomous behavior when injected into male and female Fisher rats, was not affected in cultured by comparable concentrations of AFP in the culture media. The effect of AFP on the growth of E-sensitive cells in culture was not reversed by E administration. We conclude from these experiments that (i) AFP is a specific inhibitor of the cell multiplication of cells that are E-sensitive for growth (as defined in this presentation), (ii) estrophilins seem not to play a significant role in this inhibition, and (iii) E appears not to be a growth-promoting hormone per se.
Full text
PDF
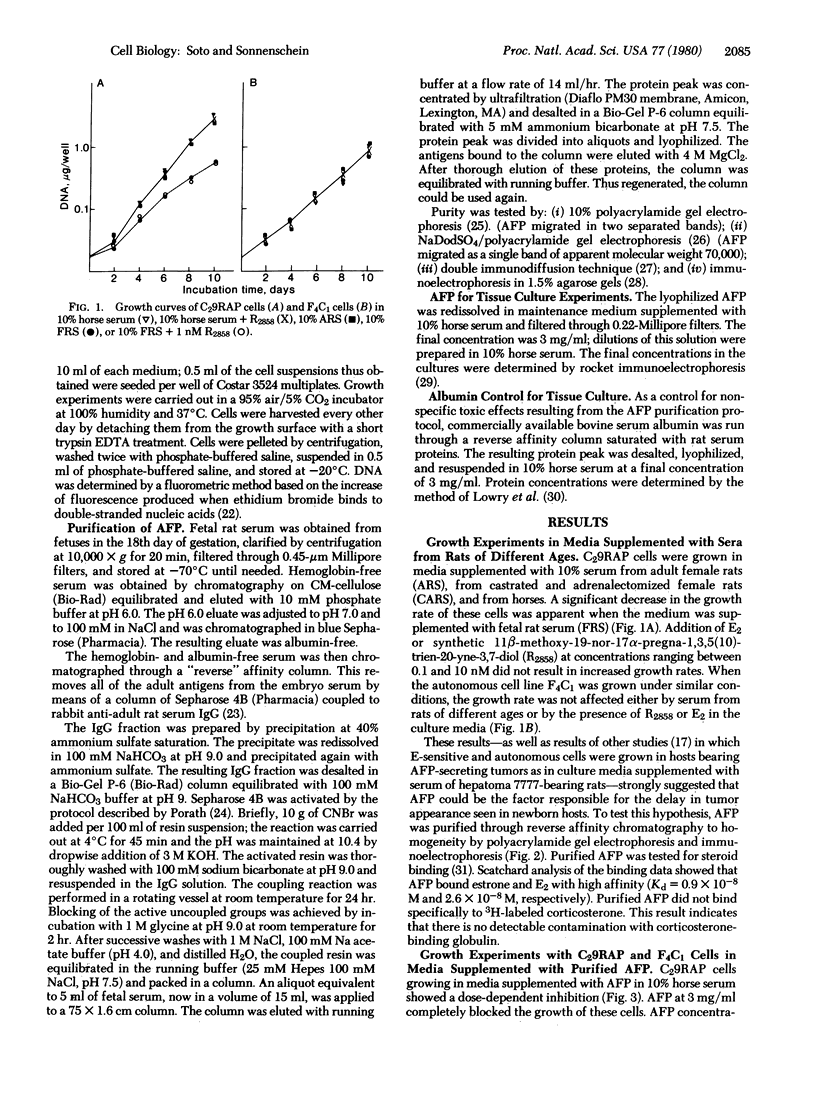
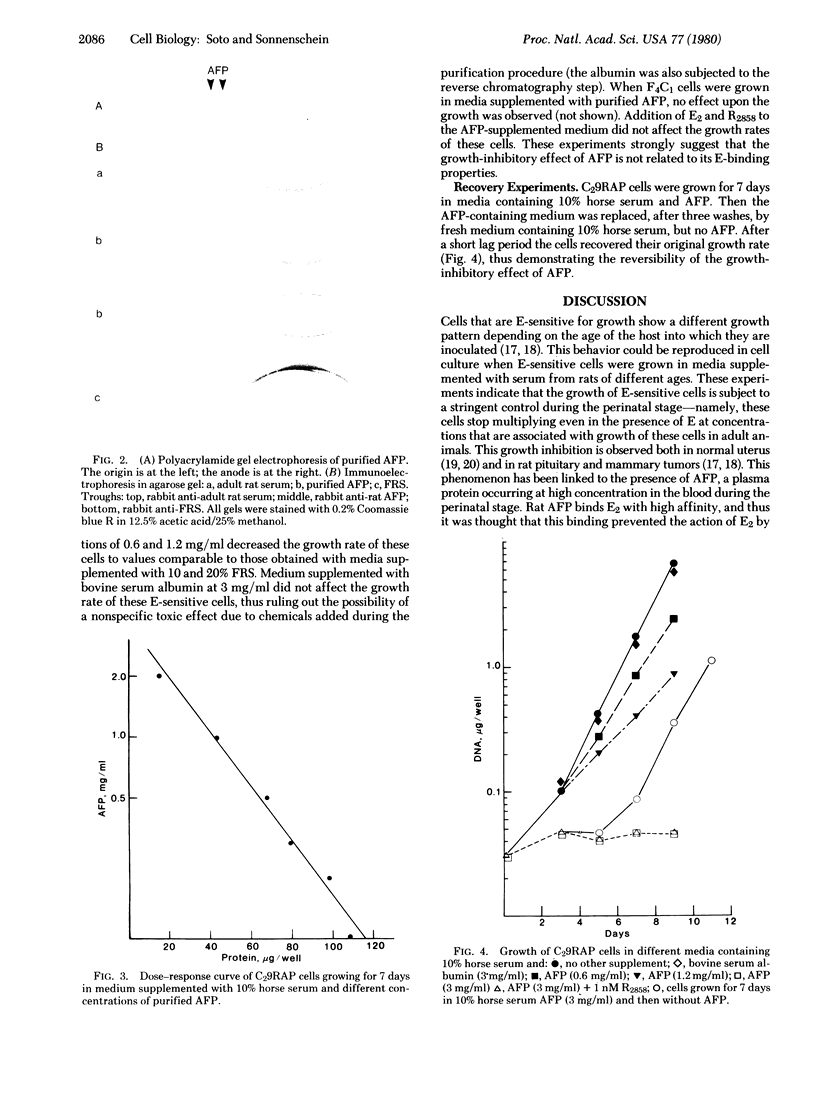

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. A., Kang Y. H., DeSombre E. R. Endogenous peroxidase: specific marker enzyme for tissues displaying growth dependency on estrogen. J Cell Biol. 1975 Mar;64(3):668–681. doi: 10.1083/jcb.64.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews W. W., Ojeda S. R. On the feedback actions of estrogen on gonadotropin and prolactin release in infantile female rats. Endocrinology. 1977 Nov;101(5):1517–1523. doi: 10.1210/endo-101-5-1517. [DOI] [PubMed] [Google Scholar]
- Cittanova N., Grigorova A. M., Benassayag C., Nunez E., Jayle M. F. Affinity chromatography purification of rat alpha 1-foetoprotein. FEBS Lett. 1974 Apr 15;41(1):21–24. doi: 10.1016/0014-5793(74)80944-0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GRABAR P., WILLIAMS C. A. Méthode permettant l'étude conjuguée des proprietés électrophorétiques et immunochimiques d'un mélange de protéines; application au sérum sanguin. Biochim Biophys Acta. 1953 Jan;10(1):193–194. doi: 10.1016/0006-3002(53)90233-9. [DOI] [PubMed] [Google Scholar]
- GREENGARD O., GORDON M., SMITH M. A., ACS G. STUDIES ON THE MECHANISM OF DIETHYLSTILBESTROL-INDUCED FORMATION OF PHOSPHOPROTEIN IN MALE CHICKENS. J Biol Chem. 1964 Jun;239:2079–2082. [PubMed] [Google Scholar]
- Karsten U., Wollenberger A. Determination of DNA and RNA in homogenized cells and tissues by surface fluorometry. Anal Biochem. 1972 Mar;46(1):135–148. doi: 10.1016/0003-2697(72)90405-8. [DOI] [PubMed] [Google Scholar]
- Kaye A. M., Sömjen D., King R. J., Sömjen G., Icekson I., Lindner H. R. Sequential gene expression in response to estradiol-17 beta during post-natal development of rat uterus. Adv Exp Med Biol. 1974 Jun;44(1):383–402. doi: 10.1007/978-1-4684-3246-6_24. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lu K. H., Koch Y., Meites J. Direct inhibition by ergocornine of pituitary prolactin release. Endocrinology. 1971 Jul;89(1):229–233. doi: 10.1210/endo-89-1-229. [DOI] [PubMed] [Google Scholar]
- Lyttle C. R., Garay R. V., DeSombre E. R. Ontogeny of the estrogen inducibility of uterine peroxidase. J Steroid Biochem. 1979 Apr;10(4):359–363. doi: 10.1016/0022-4731(79)90320-0. [DOI] [PubMed] [Google Scholar]
- MUELLER G. C., HERRANEN A. M., JERVELL K. F. Studies on the mechanism of action of estrogens. Recent Prog Horm Res. 1958;14:95–139. [PubMed] [Google Scholar]
- Meijs-Roelofs H. M., Uilenbroek J. T., de Jong F. H., Welschen R. Plasma oestradiol-17beta and its relationship to serum follicle-stimulating hormone in immature female rats. J Endocrinol. 1973 Nov;59(2):295–304. doi: 10.1677/joe.0.0590295. [DOI] [PubMed] [Google Scholar]
- Milgrom E., Atger M., Perrot M., Baulieu E. E. Progesterone in uterus and plasma. VI. Uterine progesterone receptors during the estrus cycle and implantation in the guinea pig. Endocrinology. 1972 Apr;90(4):1071–1078. doi: 10.1210/endo-90-4-1071. [DOI] [PubMed] [Google Scholar]
- Notides A., Gorski J. Estrogen-induced synthesis of a specific uterine protein. Proc Natl Acad Sci U S A. 1966 Jul;56(1):230–235. doi: 10.1073/pnas.56.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L., Kohler P. O., Korenman S. G. Studies on the mechanism of steroid hormone regulation of synthesis of specific proteins. Recent Prog Horm Res. 1969;25:105–160. doi: 10.1016/b978-0-12-571125-8.50006-5. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L. Studies on the mechanism of estrogen-mediated tissue differentiation: regulation of nuclear transcription and induction of new RNA species. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1527–1534. doi: 10.1073/pnas.60.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Antigen-antibody reactions in gels. IV. Types of reactions in coordinated systems of diffusion. Acta Pathol Microbiol Scand. 1953;32(2):230–240. [PubMed] [Google Scholar]
- Oka T., Schimke R. T. Interaction of estrogen and progesterone in chick oviduct development. II. Effects of estrogen and progesterone on tubular gland cell function. J Cell Biol. 1969 Oct;43(1):123–137. doi: 10.1083/jcb.43.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson U. I. Establishment in culture of a multihormone-secreting cell strain derived from the MtT/F4 rat pituitary tumor. J Cell Physiol. 1976 Jul;88(3):287–296. doi: 10.1002/jcp.1040880304. [DOI] [PubMed] [Google Scholar]
- Savu L., Crepy O., Guerin M. A., Nunez E., Engelmann F., Benassayag C., Jayle M. F. Etude des constantes de liaison entre les oestrogenes et 1'alpha(1)-foetoproteine de rat. FEBS Lett. 1972 Apr 15;22(1):113–116. doi: 10.1016/0014-5793(72)80233-3. [DOI] [PubMed] [Google Scholar]
- Sonnenschein C., Posner M., Sahr K., Farookhi R., Brunelle R. Estrogen sensitive cell lines: establishment and characterization of new cell lines from estrogen-induced rat pituitary tumors. Exp Cell Res. 1974 Mar 15;84(1):399–411. doi: 10.1016/0014-4827(74)90422-4. [DOI] [PubMed] [Google Scholar]
- Sonnenschein C., Soto A. M. But ... are estrogens per se growth-promoting hormones? J Natl Cancer Inst. 1980 Feb;64(2):211–215. doi: 10.1093/jnci/64.2.211. [DOI] [PubMed] [Google Scholar]
- Sonnenschein C., Weiller S., Farookhi R., Soto A. M. Characterization of an estrogen-sensitive cell line established from normal rat endometrium. Cancer Res. 1974 Nov;34(11):3147–3154. [PubMed] [Google Scholar]
- Soto A. M., Rosner A. L., Farookhi R., Sonnenschein C. Characterization of estrogen-binding proteins in sex steroid target cells growing in long-term culture. Methods Cell Biol. 1976;13:195–211. doi: 10.1016/s0091-679x(08)61803-8. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W. Studies on amphibian yolk. VII. Serum phosphoprotein synthesis by vitellogenic females and estrogen-treated males of Xenopus laevis. Can J Biochem. 1968 Aug;46(8):953–959. doi: 10.1139/o68-142. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]