Abstract
T-2307, an arylamidine compound, has been previously reported to have broad-spectrum in vitro and in vivo antifungal activities against clinically significant pathogens, including Candida species, Cryptococcus neoformans, and Aspergillus species, and is now undergoing clinical trials. Here we investigated the mechanism of action of T-2307 using yeast cells and mitochondria isolated from yeast and rat liver. Nonfermentative growth of Candida albicans and Saccharomyces cerevisiae in glycerol medium, in which yeasts relied on mitochondrial respiratory function, was inhibited at 0.001 to 0.002 μg/ml (0.002 to 0.004 μM) of T-2307. However, fermentative growth in dextrose medium was not inhibited by T-2307. Microscopic examination using Mitotracker fluorescent dye, a cell-permeant mitochondrion-specific probe, demonstrated that T-2307 impaired the mitochondrial function of C. albicans and S. cerevisiae at concentrations near the MIC in glycerol medium. T-2307 collapsed the mitochondrial membrane potential in mitochondria isolated from S. cerevisiae at 20 μM. On the other hand, in isolated rat liver mitochondria, T-2307 did not have any effect on the mitochondrial membrane potential at 10 mM. Moreover, T-2307 had little inhibitory and stimulatory effect on mitochondrial respiration in rat liver mitochondria. In conclusion, T-2307 selectively disrupted yeast mitochondrial function, and it was also demonstrated that the fungal mitochondrion is an attractive antifungal target.
INTRODUCTION
Invasive mycoses are major and serious life-threatening infections in immunocompromised patients and have become more prevalent as the population of immunocompromised patients has increased. Fungal infections are treated primarily with azoles (e.g., fluconazole), candins (e.g., micafungin), or polyenes (e.g., amphotericin B). There are still many mycoses which are hard to treat even with the application of these antifungal agents (1, 5, 21, 24, 25); accordingly, it is imperative that new antifungal agents be developed with broad-spectrum activities, novel mechanisms of action, and fewer side effects.
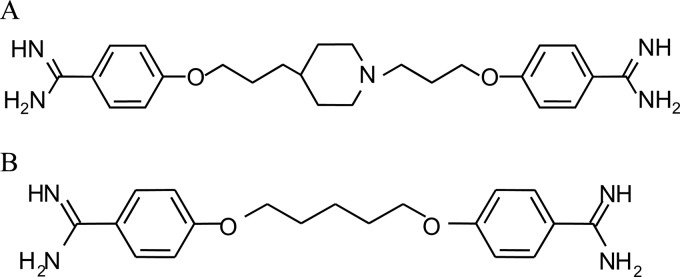
T-2307 (Fig. 1A) is a novel arylamidine and is now undergoing clinical trials. We have previously reported that T-2307 had broad-spectrum potent in vitro and in vivo activities against the majority of fungal pathogens, including Candida albicans, Aspergillus fumigatus, and Cryptococcus neoformans, which is not susceptible to candins (17). T-2307 exhibits excellent antifungal activity against fluconazole-resistant Candida spp. Since T-2307 has a novel arylamidine structure and an antifungal spectrum different from those of azoles, candins, and polyenes, we hypothesized that T-2307 possesses a novel mechanism of action.
Fig 1.
Chemical structure of T-2307 (A) or pentamidine (B).
Pentamidine (Fig. 1B) and furamidine (DB75) are known as compounds with a diamidine structure like that of T-2307. Pentamidine is used mainly to treat AIDS-related Pneumocystis jirovecii pneumonia, the early stage of human African trypanosomiasis, and antimony-resistant leishmaniasis (7, 22, 26). It was reported that the mechanisms of action of pentamidine include inhibition of topoisomerase (2), binding to an AT-rich DNA minor groove (26), inhibition of splicing of group I and II introns (14, 16, 30), and collapse of mitochondrial function (13, 15, 18, 30). Pafuramidine (DB289) has been developed as a prodrug of DB75 and has been demonstrated to have anti-Pneumocystis activity and antiparasitic activity against Plasmodium falciparum and Trypanosoma spp. (29). It has been reported that DB75 is concentrated in the cells of trypanosomes and localizes at nuclear and kinetoplast DNA (26). Reported mechanisms of action of DB75 include binding to an AT-rich DNA minor groove (26), as described for pentamidine, and collapse of mitochondrial function in Saccharomyces cerevisiae (13).
In the present study, we investigated the mechanism of action of T-2307, which has a moiety different from those of pentamidine and DB75 between the amidine functional group. Since susceptibility of Candida glabrata to T-2307 was strongly influenced by the carbon source concentration in the medium (28), we hypothesized that T-2307 may inhibit fungal mitochondrial function. Selectivity of the mechanism of action of T-2307 between fungi and mammalian cells was also investigated.
(A part of this study was presented at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], Chicago, IL, 17 to 20 September 2007.)
MATERIALS AND METHODS
Materials.
T-2307 was synthesized by Toyama Chemical Co., Ltd. (Tokyo, Japan). Pentamidine isethionate and amphotericin B were commercially obtained from Sanofi-Aventis K. K. (Tokyo, Japan) and Bristol-Myers K. K. (Tokyo, Japan), respectively. Carbonyl cyanide m-chlorophenylhydrazone (CCCP) was purchased from Sigma-Aldrich Co. (St. Louis, MO).
Strains.
The C. albicans, S. cerevisiae, and C. neoformans strains used in this study were obtained from American Type Culture Collection (ATCC 90028, 24657 (D273-10B), and 90112, respectively).
Animals.
Male Wistar/ST rats (Japan SLC Inc., Shizuoka, Japan) weighing 200 to 300 g were used.
MIC determination.
MICs were determined by the broth microdilution method based on the Clinical and Laboratory Standards Institute (CLSI)-approved standard M27-A3 for susceptibility testing of yeasts (3); however, media for MIC determination and incubation temperature were based on a report by Ludewig et al. (15). The media used for MIC determination were yeast extract-peptone containing 2% dextrose (YPD) or 2% glycerol (YPG). For the preparation of inocula, fungal cells grown on YPD agar plates were suspended in medium to yield approximately 103 cells/ml. One hundred microliters of each inoculum was added to each well containing 100 μl of 2-fold serial dilutions of the test compounds. The microplates were incubated for 48 h (C. albicans and S. cerevisiae) or 72 h (C. neoformans) at 30°C. The MICs for T-2307 and pentamidine were defined as the lowest concentrations at which a prominent decrease in turbidity was observed, and the MIC for amphotericin B was defined as the lowest concentration at which no visible growth was observed.
MitoTracker analyses.
Mitotracker analyses were performed by modification of the method of Oliver et al. (20). S. cerevisiae and C. albicans were grown overnight in medium containing 1.7 g/liter yeast nitrogen base with amino acids (2.5 mg/liter l-histidine monohydrochloride, 5 mg/liter ld-methionine, and 5 mg/liter ld-tryptophan) (Becton, Dickinson and Company Co., Ltd., Sparks, MD) and 10 g/liter dextrose (YAD) with shaking at 30°C and 35°C, respectively. Cells were diluted in 5 ml YAD plus 0.02 to 1 μM T-2307 or YAD plus 2.5 μM CCCP to yield approximately 106 cells/ml. Cells were incubated for 24 h at 30°C for S. cerevisiae and 35°C for C. albicans. Cells were collected by centrifugation and resuspended in fresh medium with the mitochondrion-specific dye MitoTracker Red CMXRos (MTR; Invitrogen, OR) at a final concentration of 20 nM. Cells were incubated for 15 min at 30°C for S. cerevisiae and 35°C for C. albicans in the dark. Stained cells were washed three times with phosphate-buffered saline in the dark and immediately observed using a biological fluorescence microscope equipped with a Nikon G2A cube. MTR has excitation and emission peaks at 579 and 599 nm, respectively. Images were captured with a ×20 lens and a Moticam 2000 digital camera and analyzed by using the Motic Images Plus 2.1S software program. All images were processed identically. The percentage of cells with intense staining of the mitochondria per approximately 200 cells after exposure to control medium or medium containing T-2307 or CCCP for 24 h was determined by bright-field and fluorescence microscopy. The result represents the average of three independent measurements.
Isolation of yeast and rat liver mitochondria.
Yeast and rat liver mitochondria were extracted from cells and organs by standard methods (4, 6, 27). S. cerevisiae was streaked onto a YPD agar plate and precultured at 30°C overnight. The precultured cells were suspended and diluted in distilled water to yield an inoculum of approximately 1.0 × 108 cells/ml. The inoculum was then added to lactic acid medium containing 3 g/liter Bacto yeast extract, 0.5 g/liter dextrose, 0.5 g/liter CaCl2 · 2H2O, 0.5 g/liter NaCl, 0.6 g/liter MgCl2 · 6H2O, 1 g/liter KH2PO4, 1 g/liter NH4Cl, and 2% lactic acid (the pH of the medium was adjusted to 5.6 with NaOH) in a 1/100 volume. After approximately 21 h at 30°C with shaking, the cells were harvested and incubated in 0.1 mol/liter Tris-HCl, pH 9.3, containing 10 mM dithiothreitol (DTT). After washing in 1.2 mol/liter sorbitol and 20 mM K-PO4, the cells were incubated in the same buffer containing 0.2 mg/ml Zymolyase 100T at 30°C for 30 min to convert the cells to spheroplasts. After harvesting, the spheroplasts were resuspended in 0.6 M mannitol, 20 mM HEPES, pH 7.4, 0.1% fatty acid-free bovine serum albumin (BSA), 1 mM phenylmethylsulfonyl fluoride, and 0.5 M EDTA · 2Na and homogenized in a tight-fitting Dounce homogenizer. Yeast mitochondrial fraction was isolated via differential centrifugation. The pellets were suspended in mannitol buffer containing 0.6 M mannitol, 20 mM HEPES, and 10% BSA by pipetting and kept on ice. This fraction was used as the yeast mitochondrial fraction.
The livers (from 2 rats) were washed in sucrose buffer containing 70 mM sucrose, 210 mM mannitol, 100 μM EDTA · 2Na, 10 mM Tris, and 0.1% BSA. The livers were cut and homogenized in sucrose buffer using a Potter-type homogenizer. Rat liver mitochondrial fraction was isolated from rat liver mitochondria via differential centrifugation. The precipitation was suspended in sucrose buffer without EDTA and quickly placed on ice. This fraction was used as the rat liver mitochondrial fraction. Oxygen consumption rates of yeast and rat liver mitochondria were measured polarographically with an oxygen electrode installed in a closed cell (model 5300; YSI Incorporated, Yellow Springs, OH) at 25°C. The respiratory control ratio (RCR) is one of the indices of the integrity of mitochondrial function. This index was calculated as follows: RCR = (oxygen consumption rate in presence of ADP)/(oxygen consumption rate in absence of ADP) (9, 27). Succinate at 5 mM was used as the substrate. ADP at 1 mM was added to maximize oxygen consumption. Buffer for RCR measurement containing 70 mM sucrose, 210 mM mannitol, 100 μM EDTA · 2Na, 10 mM Tris, 2 mM MgCl2, 10 mM KCl, 10 mM K2HPO4, and 0.1% BSA and ADP solution was dispensed into a sample chamber. One hundred percent air was defined as the oxygen concentration in the buffer stirred in an air atmosphere for 10 min. An aliquot of mitochondrial suspension was dispensed into the buffer. RCR was calculated using the slopes based on the least-squares method in the software program Microsoft Excel 2000. RCRs of yeast and rat liver mitochondria used in the present study were 2.12 to 2.92 and 4.05 to 7.24, respectively.
Measurement of mitochondrial membrane potential.
An appropriate amount of yeast and rat liver mitochondria was added to a reaction buffer in the following experiments to yield the same oxygen consumption rates (5% air/min). The fluorescence intensity of 3,3′-dipropylthiodicarbocyanine iodide [diS-C3-(5); Molecular Probes, Oregon] at 670 nm under excitation light of 620 nm was recorded with a spectrofluorometer (F-2000 fluorescence spectrophotometer; Hitachi Ltd., Tokyo, Japan) (27). Isolated mitochondria were suspended in a reaction buffer containing 0.6 M mannitol, 20 mM potassium phosphate at pH 7.4, 10 mM MgCl2, 0.5 mM EDTA, and 1 mg/ml fatty acid-free BSA at 25°C. The inhibitory activities of T-2307 and pentamidine were measured under the condition that the membrane potential was increased by adding 5 mM succinate to reaction buffer containing 2 μM diS-C3-(5). Increased and decreased fluorescent intensities indicated depolarization and polarization of the mitochondrial membrane potential, respectively. Fluorescence intensities were observed for 300 s after the addition of each test compound. Zero percent (blank) or 100% depolarization were the fluorescence intensities after the addition of sterile water or 10 μM CCCP (protonophore and uncoupler of oxidative phosphorylation), respectively. The values were obtained as the averages of three measurements.
Measurement of oxygen consumption rate with oxygen monitor.
Inhibition and stimulation of respiration by T-2307 or pentamidine were evaluated with 5 mM succinate in the presence and absence of 1 mM ADP, respectively, using rat liver mitochondria. ADP was added to maximize the oxygen consumption of mitochondria. Buffer for RCR measurement was dispensed into a sample chamber. One hundred percent air was defined as the oxygen concentration in the buffer stirred in an air atmosphere for 10 min. After this procedure, mitochondria, succinate, and ADP, if necessary, were added to the buffer. After a few minutes, the test compound solution was added to the buffer for RCR measurement.
RESULTS AND DISCUSSION
Growth-inhibitory effect of T-2307 in yeast cells grown in dextrose and glycerol media.
To investigate the effect of T-2307 on mitochondrial respiratory function, in vitro antifungal activity of T-2307 was determined in comparison with those of pentamidine and amphotericin B using media containing fermentable carbon source dextrose or nonfermentable carbon source glycerol (Table 1). Nonfermentative growth of S. cerevisiae ATCC 24657 and C. albicans ATCC 90028 in YPG, in which yeast relied on mitochondrial respiratory function, was inhibited by T-2307 at 0.001 and 0.002 μg/ml (0.002 to 0.004 μM) and by pentamidine at 0.5 and 1 μg/ml (1.5 to 2.9 μM), respectively. In contrast, fermentative growth of S. cerevisiae ATCC 24657 and C. albicans ATCC 90028 in YPD was not inhibited even at the maximum concentrations of 16 μg/ml (36 μM) T-2307 and 32 μg/ml (94 μM) pentamidine. On the other hand, T-2307 and pentamidine showed almost the same MICs in both media against a nonfermentative fungus, C. neoformans ATCC 90112. Amphotericin B, which attacks the cell membrane (12), showed almost the same MICs in both media against all three strains. The results were consistent with reports showing that antifungal activities of histatin (8), pentamidine (15), and DB75 (13), which inhibit mitochondrial function, are influenced by the carbon source. It has been reported that fungi that lost mitochondrial respiratory chain activity can proliferate on a fermentative carbon source like dextrose but not on a nonfermentative carbon source like lactic acid or acetic acid (10); those facts led to a hypothesis that T-2307 influences fungal mitochondrial function.
Table 1.
MICs of T-2307 and reference agents in YPD or YPG
| Strain | MIC (μg/ml)a |
|||||
|---|---|---|---|---|---|---|
| T-2307 |
Pentamidine |
Amphotericin B |
||||
| YPD | YPG | YPD | YPG | YPD | YPG | |
| Candida albicans ATCC 90028 | >16 | 0.002 | >32 | 1 | 2 | 1 |
| Saccharomyces cerevisiae ATCC 24657 | >16 | 0.001 | >32 | 0.5 | 1 | 1 |
| Cryptococcus neoformans ATCC 90112 | 0.0039 | 0.0039 | 2 | 1 | 1 | 1 |
Cells were incubated at 30°C for 48 h (C. albicans and S. cerevisiae) or for 72 h (C. neoformans). YPD, yeast extract-peptone containing 2% dextrose; YPG, yeast extract-peptone containing 2% glycerol.
Effect of T-2307 on mitochondrial function within yeast cells.
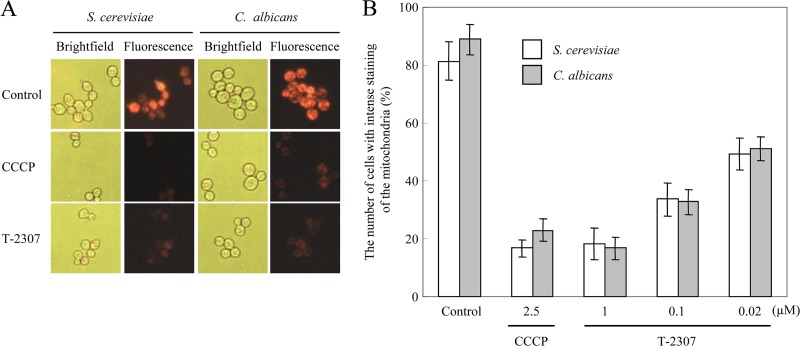
To investigate the effect of T-2307 on mitochondrial function within yeast cells, accumulation of the mitochondrial marker MTR, which diffuses passively across the plasma membrane and concentrates in active mitochondria by membrane potential, after exposure to control medium or medium containing T-2307 or CCCP for 24 h, was tested using bright-field and fluorescence microscopy (Fig. 2). Cells in the untreated control showed intense staining with mitochondria in most cells (Fig. 2A). Cells incubated in the presence of CCCP at 2.5 μM showed almost undetectable MTR staining; thus, CCCP served as a positive control for collapse of mitochondrial membrane potential. Cells incubated in the presence of T-2307 at 1 μM also showed less-intense staining of the mitochondria, indicating that mitochondrial function was collapsed. To quantify these results, we examined the percentage of cells with intense staining of the mitochondria (Fig. 2B). Treatment of cells with T-2307 for 24 h resulted in less-intense staining of the mitochondria in a dose-dependent manner. The proportion of less-stained cells after exposure to 1 μM T-2307 was approximately 80%, which was comparable to the result with 2.5 μM CCCP. The proportion of less-stained cells was approximately 50% after exposure to T-2307 at 0.02 μM, which was near the MIC (0.001 to 0.002 μg/ml; 0.002 to 0.004 μM) in YPG (Table. 1).
Fig 2.
Effect of T-2307 on mitochondrial function within live yeast cells. Cells of S. cerevisiae and C. albicans were incubated in the presence or absence of T-2307 for 24 h prior to mitochondrial staining with MTR. CCCP at 2.5 μM was used as a positive control for disruption of mitochondrial membrane potential. Cells were observed by bright-field and fluorescence microscopy and photographed. (A) The concentration of T-2307 was 1 μM. (B) The concentrations of T-2307 were 0.02, 0.1, and 1 μM. Cells with intense staining of the mitochondria were counted. The values are the average for three independent experiments. Error bars represent SD (n = 3).
Effect of T-2307 and pentamidine on mitochondrial membrane potential in isolated yeast mitochondria.
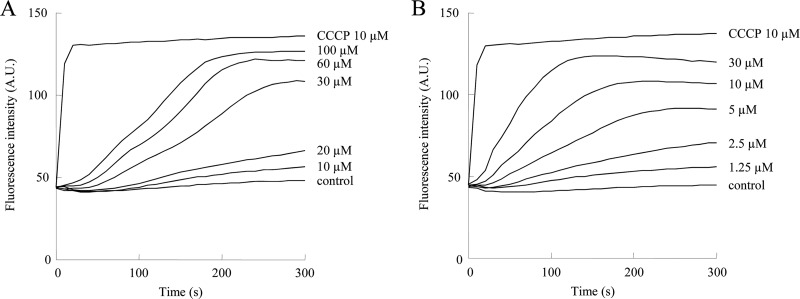
We investigated whether T-2307 and pentamidine disrupt mitochondrial function by collapsing the mitochondrial membrane potential (Fig. 3). Treatment of isolated S. cerevisiae mitochondria with T-2307 or pentamidine caused a gradual and dose-dependent collapse of the mitochondrial membrane potential (Fig. 3A and B). Since inhibition by T-2307 was not rapid, unlike the case with the uncoupler CCCP, we consider the possibility that T-2307 collapses mitochondrial membrane potential in isolated yeast mitochondria by a mode of action unlike that of the uncoupler CCCP. T-2307 at 20 μM and 2.5 μM pentamidine showed a slight collapse of mitochondrial membrane potential. T-2307 at 30 μM and 5 μM pentamidine led to the collapse of mitochondrial membrane potential by more than 50%. The concentrations of T-2307 required to cause an inhibitory effect were far higher than the measured MICs. We considered that this disconnect may be because T-2307 is actively accumulated by cells. It has been reported that T-2307 is concentrated approximately 5,000-fold from the extracellular medium by C. albicans cells (19). In our preliminary experiments, T-2307 at 10 μM significantly collapsed the membrane potential of mitochondria isolated from C. albicans. These findings suggested that T-2307 exhibited antifungal activity mainly by inhibiting fungal mitochondrial activity. The structural similarity between T-2307 and pentamidine is a diamidine structure, suggesting that the cationic property of diamidine may be involved in the action on fungal mitochondria.
Fig 3.
Membrane potential in isolated S. cerevisiae mitochondria after addition of T-2307 or pentamidine. The mitochondrial membrane potential depolarization achieved by T-2307 (A) or pentamidine (B) was monitored by the increase of diS-C3-(5) fluorescence intensity. Wavelengths used for diS-C3-(5) were as follows: excitation (λEX) = 620 nm, and emission (λEM) = 670 nm. Fully depolarized mitochondria were obtained by incubation with 10 μM CCCP. The values are the averages for three independent experiments. A.U., arbitrary units.
Effect of T-2307 and pentamidine on mitochondrial membrane potential in isolated rat liver mitochondria.
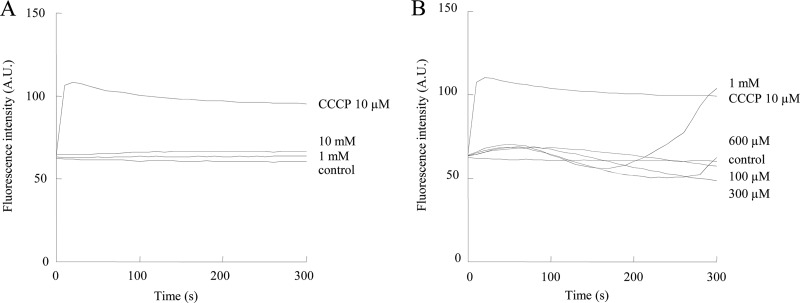
We investigated whether T-2307 disrupts mitochondrial function by collapsing mitochondrial membrane potential in isolated rat liver mitochondria (Fig. 4). T-2307 did not have any inhibitory effect on mitochondrial membrane potential at concentrations of 1 mM and 10 mM in isolated rat liver mitochondria (Fig. 4A). Pentamidine had little inhibitory effect on mitochondrial membrane potential at concentrations of 100, 300, and 600 μM; however, it showed inhibition at 1 mM (Fig. 4B). The inhibitory activity of T-2307 on mitochondria isolated from the rat liver was more than 10 times weaker than that of pentamidine. The selectivity of T-2307 for fungal mitochondria over mammalian cell mitochondria was more than 500 times, which was superior to that (400 times) of pentamidine.
Fig 4.
Membrane potential in isolated rat liver mitochondria after addition of T-2307 or pentamidine. The mitochondrial membrane potential depolarization achieved by T-2307 (A) or pentamidine (B) was monitored by the increase of diS-C3-(5) fluorescence intensity. Wavelengths used were as follows: λEXC = 620 nm, and λEM = 670 nm [for diS-C3-(5)]. Fully depolarized mitochondria were obtained by incubation with 10 μM CCCP. The values are the average for three independent experiments. A.U., arbitrary units.
Effect of T-2307 and pentamidine on isolated rat liver mitochondrial respiration.
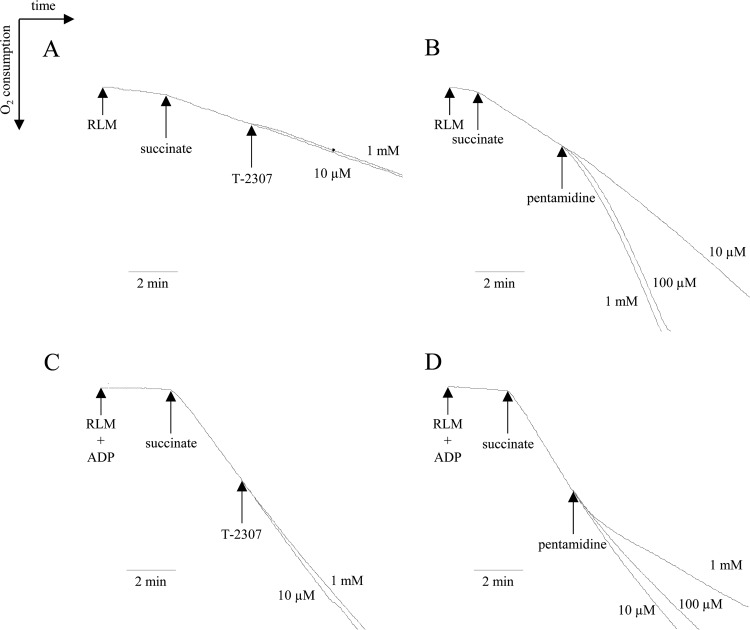
For a more detailed analysis, the influence of T-2307 on mitochondrial oxygen consumption was investigated. Since rat liver mitochondria had more tightly coupled respiratory functions than yeast mitochondria under the conditions of this experiment, as previously reported (13), analysis to distinguish between the stimulation and inhibition of respiration was performed using only rat liver mitochondria. In the absence of ADP, negligible stimulation of respiration was observed with 1 mM T-2307 (Fig. 5A). On the other hand, addition of pentamidine at 10 μM slightly stimulated mitochondrial respiration (Fig. 5B). Pentamidine at 100 μM and 1 mM potently stimulated respiration. Stimulation of respiration in rat liver mitochondria by pentamidine has been reported in a similar concentration range (18). The effects of T-2307 and pentamidine on ADP-stimulated respiration were also investigated in isolated rat liver mitochondria. In the presence of ADP, T-2307 at 10 μM and 1 mM had little inhibitory effect on the oxygen consumption rate (Fig. 5C); however, pentamidine at 10 μM to 1 mM inhibited ADP-stimulated respiration, as described by Lanteri et al. (13) (Fig. 5D). A diamidine compound, DB75, also acts on mitochondria but shows little selectivity for fungal over mammalian cell mitochondria (13). These compounds containing the diamidine structure act on fungal mitochondria; however, the selectivity for fungal mitochondria over mammalian cell mitochondria varied.
Fig 5.
Effect of T-2307 and pentamidine on oxygen consumption in isolated rat liver mitochondria (RLM). Stimulation and inhibition of respiration by T-2307 or pentamidine were evaluated with 5 mM succinate in the absence (A and B) or presence (C and D) of ADP. T-2307 (10 μM and 1 mM) or pentamidine (10 μM, 100 μM, and 1 mM) was added to buffer for RCR measurement as indicated by arrows.
Several studies of the difference between fungal and mammalian cell mitochondria have been described. For example, the constituents of the electron transport system differ between some fungi and mammalian cell mitochondria. In S. cerevisiae, the small and large supercomplexes containing dimer complex III and one or two copies of monomer complex IV, respectively, are formed. In contrast, in mammals, a multiple supercomplex containing monomer complex I, dimer complex III, and multiple (up to four) copies of complex IV is formed (23). In C. albicans, complex I is present (11), but the details of supercomplex formation are unclear, and it may differ from those in rats and humans. Detailed analysis of the interaction between these constituents of mitochondria and T-2307 may clarify the mechanism of the high selectivity of T-2307 for fungal mitochondria.
T-2307 possesses inhibitory activity for fungal mitochondria similarly to that of pentamidine, but the in vitro antifungal activity was approximately 100 times higher than that of pentamidine. We previously confirmed that T-2307 was actively incorporated into C. albicans through two specific transporters (19). Since the affinity of pentamidine for these transporters is low, the difference in in vitro antifungal activity between T-2307 and pentamidine may be due to the difference in the uptake rate. We also confirmed that T-2307 is incorporated by C. albicans with approximately 130 times higher concentration than that by mammalian cells (19). T-2307 showed marked selectivity for C. albicans over mammalian cells regarding in vitro growth inhibition (data not shown). This may be a reflection of the combination (more than 130,000 times) of the high selectivity of T-2307 for C. albicans mitochondria (more than 1,000 times) and selective incorporation by C. albicans (approximately 130 times).
In addition to the inhibition of mitochondrial function (13, 15, 18, 30), pentamidine exhibits various actions, such as inhibition of Pneumocystis and Trypanosoma topoisomerases (2), kinetoplast DNA (26), binding to AT-rich DNA minor grooves (26), and inhibition of splicing of group I and II introns (14, 16, 30). We consider it necessary to confirm these actions for T-2307.
In summary, we demonstrated the mechanism of action of T-2307 and its high selectivity for fungal mitochondria. It was also demonstrated that fungal mitochondria might be an attractive antifungal target. We are planning to further analyze the mechanism of the high selectivity of T-2307 for fungal mitochondria, and this may contribute to clarification of the differences in mitochondrial functions between fungal and mammalian cells.
Footnotes
Published ahead of print 4 September 2012
REFERENCES
- 1. Abi-Said D, et al. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122–1128 [DOI] [PubMed] [Google Scholar]
- 2. Bakshi RP, Shapiro TA. 2003. DNA topoisomerases as targets for antiprotozoal therapy. Mini Rev. Med. Chem. 3:597–608 [DOI] [PubMed] [Google Scholar]
- 3. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 3rd ed M27-A3 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 4. Daum G, Böhni PC, Schatz G. 1982. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J. Biol. Chem. 257:13028–13033 [PubMed] [Google Scholar]
- 5. Denning DW. 2003. Echinocandin antifungal drugs. Lancet 362:1142–1151 [DOI] [PubMed] [Google Scholar]
- 6. Fleischer S. 1979. Long-term storage of mitochondria to preserve energy-linked functions. Methods Enzymol. 55:28–32 [DOI] [PubMed] [Google Scholar]
- 7. Goa KL, Campoli-Richards DM. 1987. Pentamidine isethionate. A review of its antiprotozoal activity, pharmacokinetic properties and therapeutic use in Pneumocystis carinii pneumonia. Drugs 33:242–258 [DOI] [PubMed] [Google Scholar]
- 8. Gyurko C, Lendenmann U, Troxler RF, Oppenheim FG. 2000. Candida albicans mutants deficient in respiration are resistant to the small cationic salivary antimicrobial peptide histatin 5. Antimicrob. Agents Chemother. 44:348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hagihara B. 1965. Methods of experiments on mitochondria. 2. Measurement of oxygenation with the oxygen electrode method. Tanpakushitsu Kakusan Koso 10:1689–1702 [PubMed] [Google Scholar]
- 10. Hatab MA, Whittaker PA. 1992. Isolation and characterization of respiration-deficient mutants from the pathogenic yeast Candida albicans. Antonie Van Leeuwenhoek 61:207–219 [DOI] [PubMed] [Google Scholar]
- 11. Joseph-Horne T, Hollomon DW, Wood PM. 2001. Fungal respiration: a fusion of standard and alternative components. Biochim. Biophys. Acta 1504:179–195 [DOI] [PubMed] [Google Scholar]
- 12. Laniado-Laborín R, Cabrales-Vargas MN. 2009. Amphotericin B: side effects and toxicity. Rev. Iberoam. Micol. 26:223–227 [DOI] [PubMed] [Google Scholar]
- 13. Lanteri CA, Trumpower BL, Tidwell RR, Meshnick SR. 2004. DB75, a novel trypanocidal agent, disrupts mitochondrial function in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 48:3968–3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y, Tidwell RR, Leibowitz MJ. 1994. Inhibition of in vitro splicing of a group I intron of Pneumocystis carinii. J. Eukaryot. Microbiol. 41:31–38 [DOI] [PubMed] [Google Scholar]
- 15. Ludewig G, Williams JM, Li Y, Staben C. 1994. Effects of pentamidine isethionate on Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 38:1123–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miletti KE, Leibowitz MJ. 2000. Pentamidine inhibition of group I intron splicing in Candida albicans correlates with growth inhibition. Antimicrob. Agents Chemother. 44:958–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitsuyama J, et al. 2008. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob. Agents Chemother. 52:1318–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moreno SN. 1996. Pentamidine is an uncoupler of oxidative phosphorylation in rat liver mitochondria. Arch. Biochem. Biophys. 326:15–20 [DOI] [PubMed] [Google Scholar]
- 19. Nishikawa H, et al. 2010. Uptake of T-2307, a novel arylamidine, in Candida albicans. J. Antimicrob. Chemother. 65:1681–1687 [DOI] [PubMed] [Google Scholar]
- 20. Oliver BG, et al. 2008. Tetracycline alters drug susceptibility in Candida albicans and other pathogenic fungi. Microbiology 154:960–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfaller MA, et al. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J. Clin. Microbiol. 39:3254–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sands M, Kron MA, Brown RB. 1985. Pentamidine: a review. Rev. Infect. Dis. 7:625–634 [DOI] [PubMed] [Google Scholar]
- 23. Schägger H, Pfeiffer K. 2000. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 19:1777–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Viscoli C, et al. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071–1079 [DOI] [PubMed] [Google Scholar]
- 25. White TC, Marr KA, Bowden RA. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wilson WD, et al. 2008. Antiparasitic compounds that target DNA. Biochimie 90:999–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yaffe MP. 1991. Analysis of mitochondrial function and assembly. Methods Enzymol. 194:627–643 [DOI] [PubMed] [Google Scholar]
- 28. Yamada E, Nishikawa H, Nomura N, Mitsuyama J. 2010. T-2307 shows efficacy in murine model of Candida glabrata infection despite in vitro trailing growth phenomena. Antimicrob. Agents Chemother. 54:3630–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yeates C. 2003. DB-289 Immtech International. IDrugs 6:1086–1093 [PubMed] [Google Scholar]
- 30. Zhang Y, Bell A, Perlman PS, Leibowitz MJ. 2000. Pentamidine inhibits mitochondrial intron splicing and translation in Saccharomyces cerevisiae. RNA 6:937–951 [DOI] [PMC free article] [PubMed] [Google Scholar]