Abstract
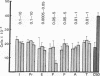
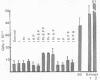
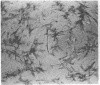
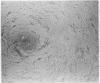
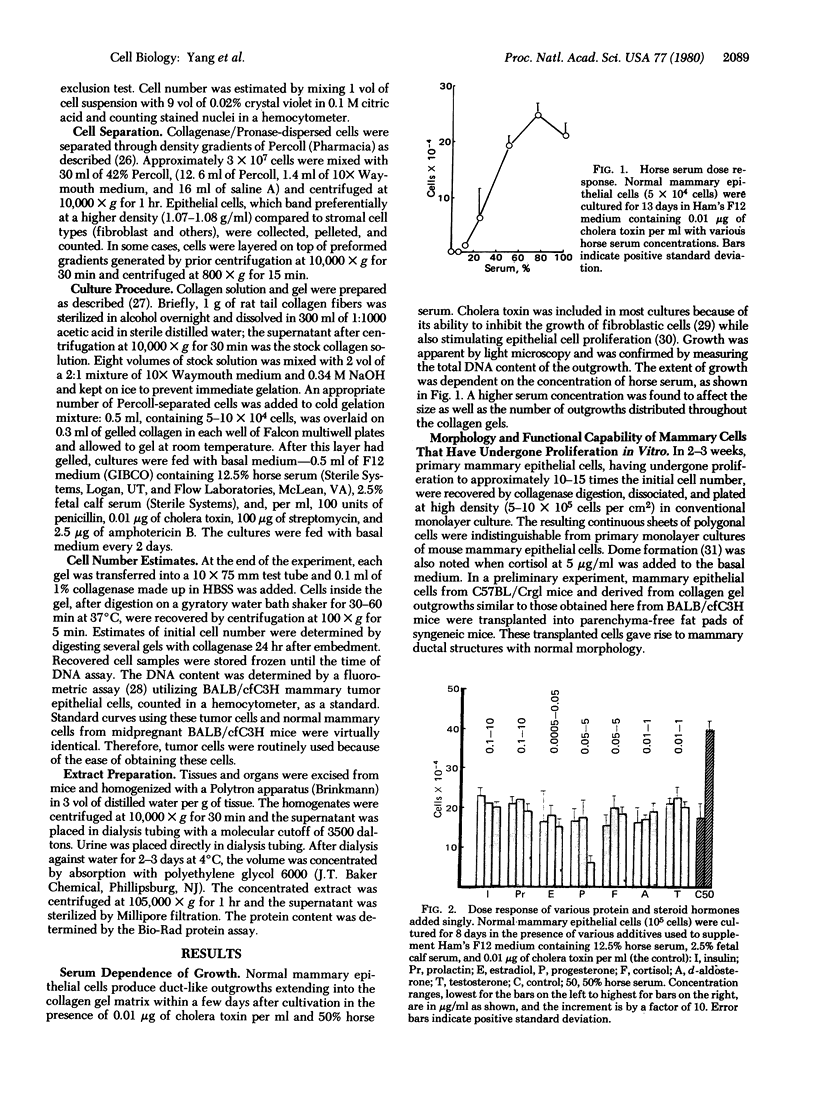
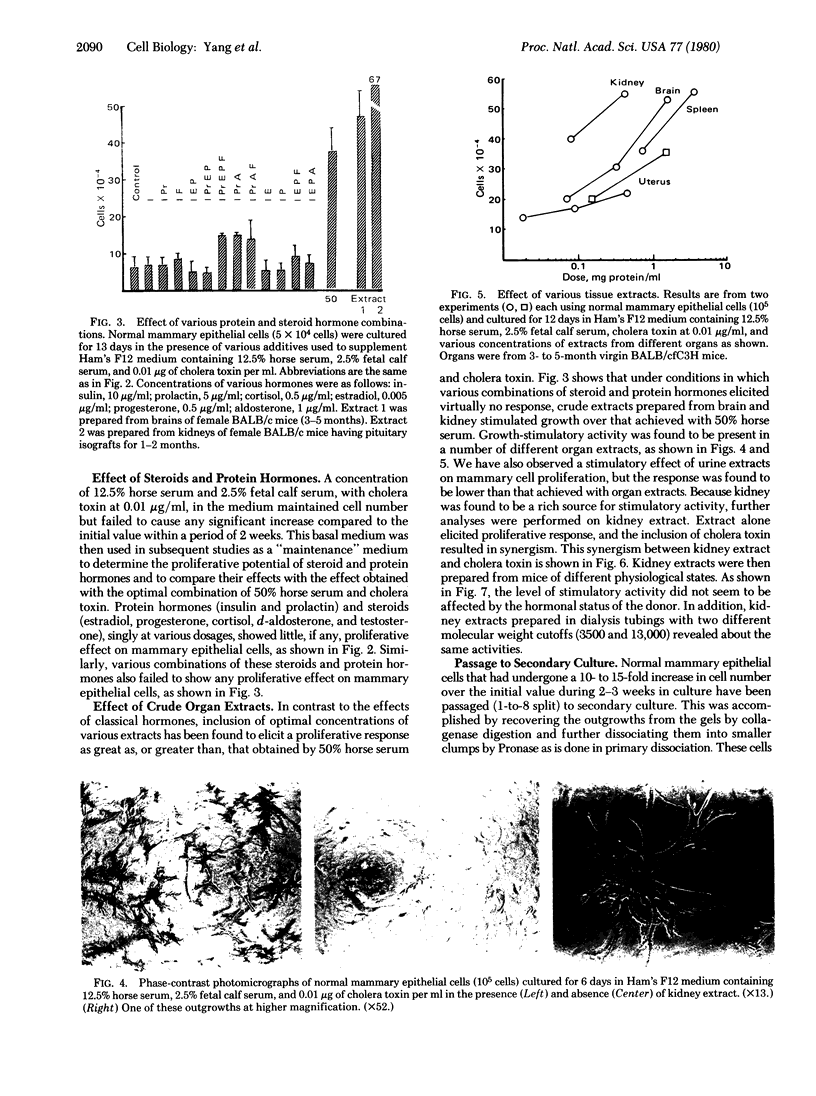
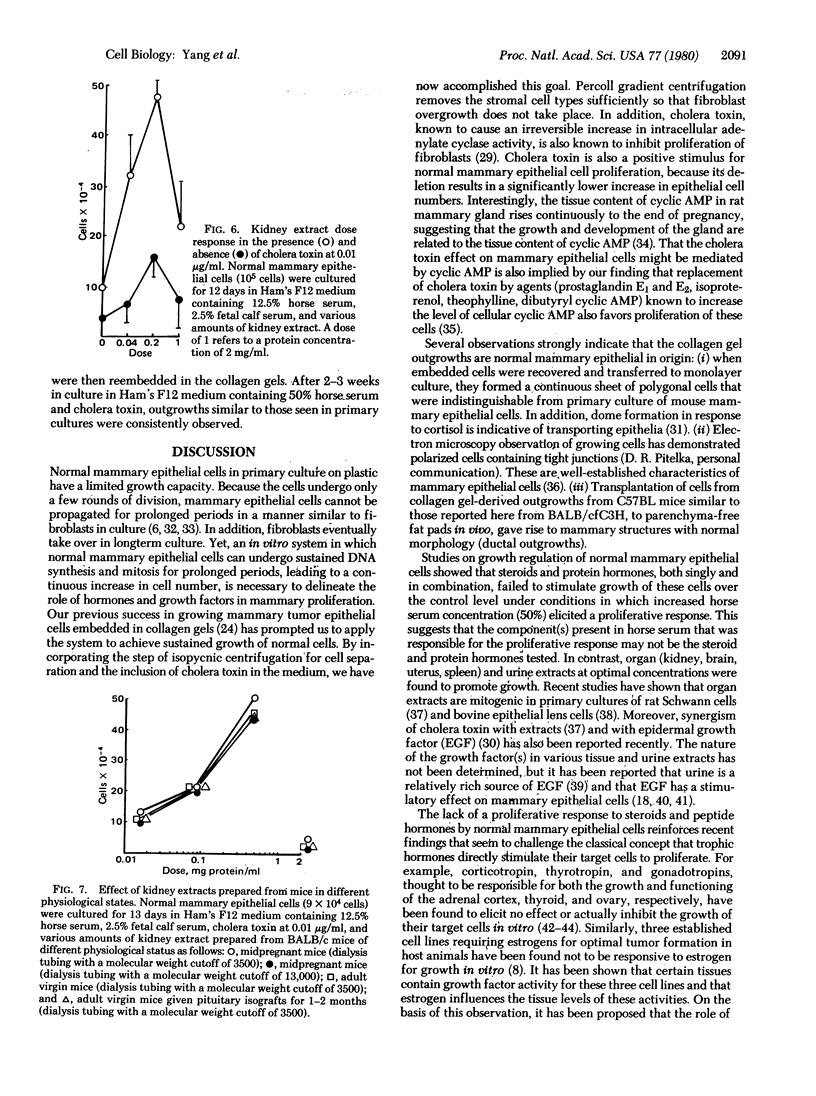
Normal mammary epithelial cells from BALB/cfC3H midpregnant mice were freed from stromal cell types by Percoll density gradient centrifugation after collagenase digestion and were then embedded within collagen gels. Sustained growth leading to an increase in cell number was accomplished in response to cholera toxin and high concentrations of horse serum. The extent of growth was found to be dependent on the horse serum concentration, the maximum growth being attained at 50%. A serum concentration of 12.5% horse serum and 2.5% fetal calf serum, along with cholera toxin at 0.01 μg/ml, allowed maintenance but failed to cause any significant increase in cell number during the experimental period of 2 weeks. This same maintenance medium was used to determine the effects of various exogenously added steroids, protein hormones, and organ extracts on the proliferation of mammary epithelial cells in culture. Hormones failed to elicit any proliferative response, but extracts of kidney, brain, uterus, and spleen produced proliferative responses equal to or greater than the response obtained with 50% horse serum and cholera toxin. Kidney extracts prepared from midpregnant mice, virgin mice, and virgin mice given pituitary isografts all showed comparable activities, suggesting that the concentration of stimulatory factor(s) was not influenced by the hormonal status of the donor. Normal mammary epithelial cells that had undergone a 10- to 15-fold increase in cell number over initial values during 2-3 weeks in culture were passaged to secondary gel cultures. Outgrowth similar to those seen in primary culture were seen again in secondary culture. The present system provides a method for sustaining growth in culture of primary mammary epithelial cells from normal tissues.
Keywords: proliferation, growth factors, cholera toxin, passage
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aidells B. D., Martin L. Growth in short term primary cell cultures derived from pregnancy-dependent mammary tumors. Cell Biol Int Rep. 1979 Jul;3(4):345–357. doi: 10.1016/s0309-1651(79)80005-3. [DOI] [PubMed] [Google Scholar]
- Arruti C., Courtois Y. Morphological changes and growth stimulation of bovine epithelial lens cells by a retinal extract in vitro. Exp Cell Res. 1978 Dec;117(2):283–292. doi: 10.1016/0014-4827(78)90142-8. [DOI] [PubMed] [Google Scholar]
- Buehring G. C., Williams R. R. Growth rates of normal and abnormal human mammary epithelia in cell culture. Cancer Res. 1976 Oct;36(10):3742–3747. [PubMed] [Google Scholar]
- Ceriani R. L., Blank E. W. Response to prolactin and ovarian steroids of normal mammary epithelial cell cultures. Mol Cell Endocrinol. 1977 Aug;8(2):95–103. doi: 10.1016/0303-7207(77)90022-3. [DOI] [PubMed] [Google Scholar]
- Cohen L. A., Tsuang J., Chan P. C. Characteristics of rat normal mammary epithelial cells and dimethylbenzanthracene-induced mammary adenocarcinoma cells grown in monolayer culture. In Vitro. 1974 Jul-Aug;10:51–62. doi: 10.1007/BF02615338. [DOI] [PubMed] [Google Scholar]
- Cohen S., Carpenter G. Human epidermal growth factor: isolation and chemical and biological properties. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1317–1321. doi: 10.1073/pnas.72.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N. K., Hosick H. L., Nandi S. Influence of seeding density on multicellular organization and nuclear events in cultures of normal and neoplastic mouse mammary epithelium. J Natl Cancer Inst. 1974 Mar;52(3):849–861. doi: 10.1093/jnci/52.3.849. [DOI] [PubMed] [Google Scholar]
- Enami J., Yang J., Nandi S. Simultaneous production of casein and mammary tumor virus in mouse mammary epithelial cells grown on floating collagen gels. Cancer Lett. 1979 Feb;6(2):99–105. doi: 10.1016/s0304-3835(79)80007-5. [DOI] [PubMed] [Google Scholar]
- Gaffney E. V., Polanowski F. P., Blackburn S. E., Lambiase J. T., Burke R. E. Cultures of normal human mammary cells. Cell Differ. 1976 Jul;5(2):69–81. doi: 10.1016/0045-6039(76)90001-4. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R., Birdwell C. R. Effects of fibroblast and epidermal growth factors on ovarian cell proliferation in vitro. I. Characterization of the response of granulosa cells to FGF and EGF. Endocrinology. 1977 Apr;100(4):1108–1120. doi: 10.1210/endo-100-4-1108. [DOI] [PubMed] [Google Scholar]
- Green H. Cyclic AMP in relation to proliferation of the epidermal cell: a new view. Cell. 1978 Nov;15(3):801–811. doi: 10.1016/0092-8674(78)90265-9. [DOI] [PubMed] [Google Scholar]
- Hallowes R. C., Rudland P. S., Hawkins R. A., Lewis D. J., Bennet D., Durbin H. Comparison of the effects of hormones on DNA synthesis in cell cultures of nonneoplastic and neoplastic mammary epithelium from rats. Cancer Res. 1977 Aug;37(8 Pt 1):2492–2504. [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Hosick H. L. A note on growth patterns of epithelial tumor cells in primary culture. Cancer Res. 1974 Jan;34(1):259–261. [PubMed] [Google Scholar]
- Hosick H. L., Nandi S. Plating and maintenance of epithelial tumor cells in primary culture: interacting roles of serum and insulin. Exp Cell Res. 1974 Mar 15;84(1):419–425. doi: 10.1016/0014-4827(74)90424-8. [DOI] [PubMed] [Google Scholar]
- Hsueh H. W., Stockdale F. E. Effects of serum on mammary epithelial proliferation in vitro during mammary gland development. J Cell Biol. 1975 Aug;66(2):243–250. doi: 10.1083/jcb.66.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland W. L., Yang N. S., Jorgensen T., Longley C., Furmanski P. Growth of normal and malignant human mammary epithelial cells in culture. J Natl Cancer Inst. 1979 Jul;63(1):29–41. [PubMed] [Google Scholar]
- Lasfargues E. Y., Moore D. H. A method for the continuous cultivation of mammary epithelium. In Vitro. 1971 Jul-Aug;7(1):21–25. doi: 10.1007/BF02619001. [DOI] [PubMed] [Google Scholar]
- Lin F. K., Banerjee M. R., Crump L. R. Cell cycle-related hormone carcinogen interaction during chemical carcinogen induction of nodule-like mammary lesions in organ culture. Cancer Res. 1976 May;36(5):1607–1614. [PubMed] [Google Scholar]
- Linebaugh B. E., Rillema J. A. Actions of insulin and hydrocortisone on macromolecular synthesis in primary epithelial cell cultures from mouse mammary glands. Endocrinology. 1979 Sep;105(3):806–811. doi: 10.1210/endo-105-3-806. [DOI] [PubMed] [Google Scholar]
- Lippman M., Bolan G., Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976 Dec;36(12):4595–4601. [PubMed] [Google Scholar]
- Michalopoulos G., Pitot H. C. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975 Aug;94(1):70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- Misfeldt D. S., Hamamoto S. T., Pitelka D. R. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Perry J. W., Topper Y. J. Changes in insulin responsiveness during development of mammary epithelium. J Cell Biol. 1974 Aug;62(2):550–556. doi: 10.1083/jcb.62.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Hackett A. J. Epithelial cell cultures from normal glandular tissue of mice. J Natl Cancer Inst. 1974 Jul;53(1):261–269. doi: 10.1093/jnci/53.1.261. [DOI] [PubMed] [Google Scholar]
- Pertoft H., Rubin K., Kjellén L., Laurent T. C., Klingeborn B. The viability of cells grown or centrifuged in a new density gradient medium, Percoll(TM). Exp Cell Res. 1977 Dec;110(2):449–457. doi: 10.1016/0014-4827(77)90311-1. [DOI] [PubMed] [Google Scholar]
- Pickett P. B., Pitelka D. R., Hamamoto S. T., Misfeldt D. S. Occluding junctions and cell behavior in primary cultures of normal and neoplastic mammary gland cells. J Cell Biol. 1975 Aug;66(2):316–332. doi: 10.1083/jcb.66.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne K., Hsueh H. W., Stockdale F. E. Partial purification and characterization of mammary stimulating factor, a protein which promotes proliferation of mammary epithelium. Biochemistry. 1979 Aug 7;18(16):3533–3539. doi: 10.1021/bi00583a015. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Abney E., Brockes J. P., Hornby-Smith A. Schwann cell growth factors. Cell. 1978 Nov;15(3):813–822. doi: 10.1016/0092-8674(78)90266-0. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Suyama A. T. Inhibition of replication of normal adrenocortical cells in culture by adrenocorticotropin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):113–117. doi: 10.1073/pnas.72.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J., Nandi S. Neoplastic transformation of rat mammary cells exposed to 7,12-dimethylbenz[alpha]anthracene or N-nitrosomethylurea in cell culture. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3836–3840. doi: 10.1073/pnas.75.8.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J., Nandi S. Primary culture of rat mammary epithelial cells. I. Effect of plating density, hormones, and serum on DNA synthesis. J Natl Cancer Inst. 1978 Sep;61(3):765–771. [PubMed] [Google Scholar]
- Sapag-Hagar M., Greenbaum A. L. The role of cyclic nucleotides in the development and function of rat mammary tissue. FEBS Lett. 1974 Sep 15;46(1):180–183. doi: 10.1016/0014-5793(74)80363-7. [DOI] [PubMed] [Google Scholar]
- Sirbasku D. A. Estrogen induction of growth factors specific for hormone-responsive mammary, pituitary, and kidney tumor cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3786–3790. doi: 10.1073/pnas.75.8.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M. G., Pigott D., Taylor-Papadimitriou J. Response to epidermal growth factors of cultured human mammary epithelial cells from benign tumours. Nature. 1976 Dec 23;264(5588):764–767. doi: 10.1038/264764a0. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Shearer M., Stoker M. G. Growth requirements of human mammary epithelial cells in culture. Int J Cancer. 1977 Dec 15;20(6):903–908. doi: 10.1002/ijc.2910200613. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Shearer M., Tilly R. Some properties of cells cultured from early-lactation human milk. J Natl Cancer Inst. 1977 Jun;58(6):1563–1571. doi: 10.1093/jnci/58.6.1563. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Mitchell W. M., Cohen S. Characterization of the high molecular weight form of epidermal growth factor. J Biol Chem. 1974 May 25;249(10):3198–3203. [PubMed] [Google Scholar]
- Turkington R. W. Hormone-induced synthesis of DNA by mammary gland in vitro. Endocrinology. 1968 Mar;82(3):540–546. doi: 10.1210/endo-82-3-540. [DOI] [PubMed] [Google Scholar]
- Turkington R. W. Stimulation of mammary carcinoma cell proliferation by epithelial growth factor in vitro. Cancer Res. 1969 Jul;29(7):1457–1458. [PubMed] [Google Scholar]
- Westermark B., Karlsson F. A., Wålinder O. Thyrotropin is not a growth factor for human thyroid cells in culture. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2022–2026. doi: 10.1073/pnas.76.4.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Richards J., Bowman P., Guzman R., Enami J., McCormick K., Hamamoto S., Pitelka D., Nandi S. Sustained growth and three-dimensional organization of primary mammary tumor epithelial cells embedded in collagen gels. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3401–3405. doi: 10.1073/pnas.76.7.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]