Abstract
Acinetobacter baumannii is an opportunistic pathogen that is a cause of clinically significant nosocomial infections. Increasingly, clinical isolates of A. baumannii are extensively resistant to numerous antibiotics, and the use of polymyxin antibiotics against these infections is often the final treatment option. Historically, the polymyxins have been thought to kill bacteria through membrane lysis. Here, we present an alternative mechanism based on data demonstrating that polymyxins induce rapid cell death through hydroxyl radical production. Supporting this notion, we found that inhibition of radical production delays the ability of polymyxins to kill A. baumannii. Notably, we demonstrate that this mechanism of killing occurs in multidrug-resistant clinical isolates of A. baumannii and that this response is not induced in a polymyxin-resistant isolate. This study is the first to demonstrate that polymyxins induce rapid killing of A. baumannii and other Gram-negatives through hydroxyl radical production. This significantly augments our understanding of the mechanism of polymyxin action, which is critical knowledge toward the development of adjunctive therapies, particularly given the increasing necessity for treatment with these antibiotics in the clinical setting.
INTRODUCTION
Acinetobacter baumannii is an increasingly prevalent opportunistic pathogen that causes nosocomial infections (5, 6, 11, 34, 43, 47). This Gram-negative, aerobic, coccobacillus is responsible for a significant number of hospital-acquired infections, including those of the skin and bloodstream, as well as pneumonia and meningitis (5, 6, 18, 34, 47). Importantly, A. baumannii is able to persist on hospital surfaces for weeks to months, providing an environmental reservoir for its transmission (44–46). Compounding this problem, multidrug-resistant (MDR) strains of A. baumannii have been isolated with increasing frequency, and strains with pan-drug resistance (PDR) have been described as well, particularly among vulnerable patients within intensive care units or military hospitals (3, 11, 15, 33, 36, 39, 41). The polymyxin class of antibiotics is generally considered a final option of antibiotic therapy against MDR strains of A. baumannii, in large part due to the high potential for nephrotoxicity. Nonetheless, clinical use of the polymyxins, including polymyxin B and polymyxin E (colistin), to treat A. baumannii infection is increasing out of necessity due to antibiotic resistance (25, 26, 50).
Polymyxins are non-ribosomally synthesized, cationic antimicrobial peptides that bind to lipid A in the outer leaflet of the Gram-negative outer membrane (10, 32). Positively charged amino acid residues in the polymyxins form a ring that associates with negatively charged residues within lipid A through electrostatic interactions, causing membrane perturbations (10). In addition, polymyxins contain a string of hydrophobic amino acids which insert into the outer membrane, increasing bacterial membrane permeability (10). It has often been assumed that these membrane disruptions cause bacterial cell death directly through membrane lysis. However, reports from as far back as the late 1970s indicate that under certain conditions, polymyxins are capable of killing bacteria without lysis, suggesting that another mechanism of bacterial cell death may also be induced by treatment with these antibiotics (8, 21).
Recently, it has been demonstrated that a number of classes of antibiotics induce the production of lethal hydroxyl radicals within bacteria through the Fenton reaction (12, 22). Briefly, this reaction occurs when superoxides are converted to peroxides by superoxide dismutases present in the cell. Peroxides are capable of interacting with ferric iron associated with a number of biological molecules within the bacterial cell, oxidizing the iron and forming hydroxyl radicals in the process (16, 17, 22, 49). Ultimately, the concentration of hydroxyl radicals reaches levels that cannot be controlled, and the subsequent oxidative damage to DNA, lipids, and proteins eventually causes cell death (12, 22). Although hydroxyl radical-mediated cell death has been demonstrated with antibiotics that target intracellular proteins (12, 22), it is not known whether the classes of antibiotics that directly target the outer membrane (such as the polymyxins) cause cell death through a similar mechanism.
Here, we demonstrate that polymyxin B and colistin initiate rapid killing of both sensitive and MDR isolates of A. baumannii, as well as other Gram-negative species, through hydroxyl radical production. Treatment of A. baumannii with these antibiotics caused an increase in hydroxyl radicals and, furthermore, killing of A. baumannii by the polymyxins was delayed in the presence of inhibitors that both directly and indirectly block the production of oxygen radicals through the Fenton reaction. To our knowledge, this is the first demonstration of how the polymyxin family induces rapid killing of A. baumannii and provides a rationale for previous observations of polymyxin-induced death without lysis observed in other species. With an increasing number of A. baumannii isolates demonstrating multidrug resistance, this study may provide clues as to how to exploit hydroxyl radical-mediated cell death to combat drug resistance in this and other drug-resistant bacterial pathogens.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All A. baumannii strains (ATCC 17978, CI-2, CI-3, and CI-4), as well as Escherichia coli DH5α (Invitrogen, Grand Island, NY), were routinely grown from frozen stock in Mueller-Hinton (MH) broth (BD Biosciences, Sparks, MD) at 37°C with aeration. For Francisella novicida U112 (a gift from Denise Monack, Stanford University), 0.2% l-cysteine was added to the growth medium. CI-2, CI-3, and CI-4 were kindly provided by Brandi Limbago, Division of Health Care Quality Promotion, Centers for Disease Control and Prevention, Atlanta, GA. CI-2 was isolated in the District of Columbia in 2005, CI-3 was isolated in Ohio in 2006 by endotrachial aspirate, and CI-4 was isolated in Mississippi in 2010 from sputum.
Determination of MICs.
Identification and antimicrobial susceptibility testing were performed using the MicroScan WalkAway Plus automated system (Siemens Medical Solutions Diagnostics, Deerfield, IL). Organism suspensions were prepared using the Prompt system (Siemens Medical Solutions Diagnostics) to inoculate Neg Combo 41 conventional identification susceptibility panels (Siemens Medical Solutions Diagnostics). Manual susceptibility testing using Etest strips (bioMérieux, Durham, NC) on MH agar was performed for colistin since it is not included on the MicroScan panel. Multidrug resistance (MDR) was defined as resistance to at least one member of three or more classes of antibiotic: aminoglycosides, beta-lactams, cephalosporins, or fluoroquinolones (29). Pan-drug resistance (PDR) was defined as resistance to all antibiotic therapies tested, including colistin (29).
Detection of hydroxyl radicals.
Overnight cultures were subcultured 1:50 into MH broth for 2 h to an optical density at 600 nm of ∼2. Cultures were centrifuged at 5,000 × g for 10 min, washed twice in phosphate-buffered saline (PBS; Lonza, Walkersville, MD), and diluted to 107 CFU/ml in PBS. The cells were subsequently treated with polymyxin B (USB, Cleveland, OH) or polymyxin E (colistin; Sigma-Aldrich, St. Louis, MO) at a final concentration of 2 μg/ml or with hydrogen peroxide at a final concentration of 0.15%, followed by incubation at 37°C with gentle shaking for 30 min. After treatment, the hydroxyl radical-specific fluorescent dye 3′-(p-hydroxyphenyl) fluorescein (HPF; Life Technologies, Grand Island, NY) was added to treated or untreated cultures at a final concentration of 5 μM. Fluorescence was immediately measured in a BioTek Synergy MX plate reader (BioTek, Winooski, VT) with an excitation setting of 490 nm and an emission setting of 515 nm, both with a 9-nm bandwidth.
Time-kill assays.
To determine the levels of killing by antimicrobial compounds, time-kill experiments were performed as previously described (28). Overnight cultures were subcultured as described above and then diluted to a final concentration of 107 CFU/ml in MH broth. Samples were treated with 2 μg of either polymyxin B or colistin/ml (or 400 μg of colistin/ml for the F. novicida experiments) and incubated with aeration at 37°C. At the indicated time points, aliquots of treated cells were harvested, suitable dilutions were performed, and then the cells were plated onto MH agar plates. After overnight incubation of the plates at 37°C, CFU were enumerated. Thiourea (Sigma-Aldrich) was added to cultures concurrently at the indicated doses. When 2,2′-dipyridyl (MP Biomedical, Solon, OH) was utilized, the cells were pretreated for 20 min at 37°C with the indicated doses, before treatment with polymyxins. The addition of thiourea or 2,2′-dipyridyl did not alter growth kinetics in broth (see Fig. S1A and B in the supplemental material).
Statistics.
All experiments were analyzed using a two-tailed, unpaired Student t test.
RESULTS
Polymyxins induce hydroxyl radical production.
Polymyxins are thought to kill Gram-negative bacteria by binding to lipid A in the outer membrane and subsequently disrupting the stability of both the outer and inner membranes, ultimately leading to cell lysis (10, 32). Due to the observations that bactericidal antibiotic treatment induces the production of hydroxyl radicals within bacteria and that these radicals play a significant role in causing bacterial cell death through oxidative damage to DNA, lipids, and proteins (22), we sought to determine whether the last line polymyxin antibiotics induced hydroxyl radical-mediated cell death in A. baumannii.
In order to first establish a baseline of A. baumannii sensitivities to polymyxins, MICs were determined for the type strain, ATCC 17978 (Table 1). We next determined the kinetics of polymyxin-mediated killing of this strain. Time-kill assays revealed that polymyxin B-mediated killing of A. baumannii occurred quickly, with a >3-log reduction of viable cells within 30 min (Fig. 1). Furthermore, similar rapid killing kinetics occurred upon treatment with colistin (polymyxin E) at an identical dose (Fig. 1).
Table 1.
MICs for strains utilized in this study
| Antibiotic | MICs (μg/ml)a |
|||
|---|---|---|---|---|
| ATCC 17978 | CI-2 | CI-3 | CI-4 | |
| Amikacin | ≤16 (S) | ≤16 (S) | >32 (R) | >32 (R) |
| Ampicillin-sulbactam | ≤8/4 (S) | 16/8 (I) | >16/8 (R) | 16/8 (I) |
| Cefepime | ≤8 (S) | >16 (R) | >16 (R) | >16 (R) |
| Ceftazidime | 4 (S) | >16 (R) | >16 (R) | >16 (R) |
| Gentamicin | ≤4 (S) | >8 (R) | >8 (R) | >8 (R) |
| Levofloxacin | ≤2 (S) | ≤2 (S) | >4 (R) | >4 (R) |
| Meropenem | ≤4 (S) | ≤4 (S) | >8 (R) | 8 (I) |
| Ticarcillin-clavulanate | ≤16 (S) | ≤16 (S) | >64 (R) | >64 (R) |
| Tobramycin | ≤4 (S) | 8 (I) | >8 (R) | >8 (R) |
| Trimethoprim-sulfamethoxazole | >2/38 (R) | >2/38 (R) | >2/38 (R) | >2/38 (R) |
| Colistin | 0.19 (S) | 0.5 (S) | 0.19 (S) | >256 (R) |
R, resistant; I, intermediate resistance; S, sensitive. For drug combinations, the two respective MICs are separated by a slash (“/”).
Fig 1.
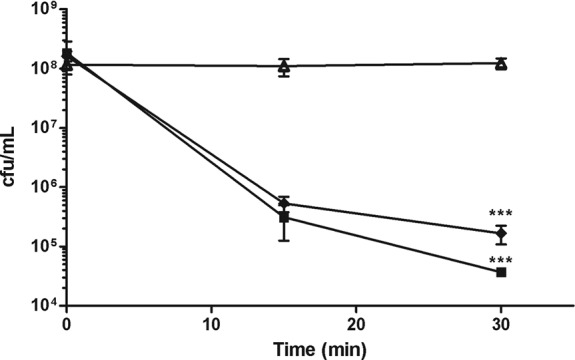
Polymyxins induce rapid killing of A. baumannii. A. baumannii cultures were treated with 2 μg of polymyxin B/ml (■) or colistin/ml (⧫) or left untreated (△). At 0, 15, and 30 min, cultures were plated, and the CFU were enumerated. The data are representative of three independent experiments. Points represent the means and bars represent the standard deviation of triplicate samples. ***, P < 0.0001.
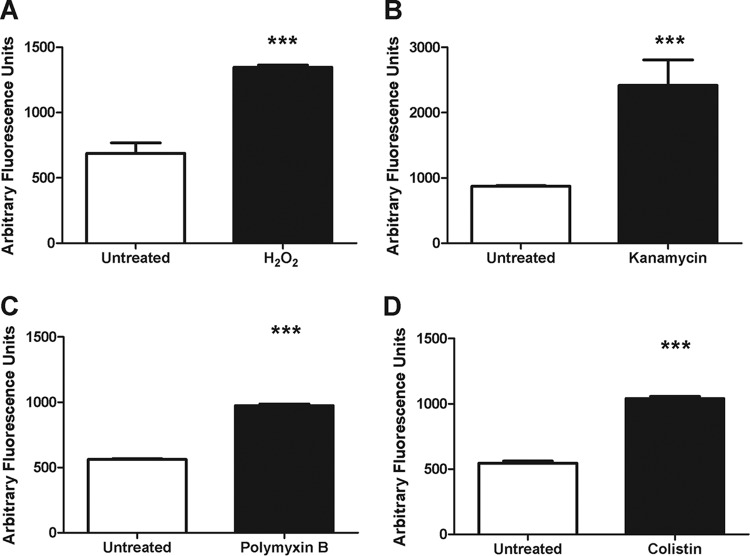
In order to determine whether hydroxyl radicals were induced during the observed rapid polymyxin killing, we utilized the cell permeable, hydroxyl radical-specific fluorescent dye, 3′-(p-hydroxyphenyl) fluorescein (HPF) (12). As positive controls, we treated cultures with hydrogen peroxide or kanamycin (previously demonstrated to induce hydroxyl radicals [22]) for 30 min and measured HPF fluorescence compared to untreated cultures (Fig. 2A and B). We observed an ∼2-fold increase in fluorescence, indicating an increase in hydroxyl radical production. We next assayed the A. baumannii cultures treated with either polymyxin B or colistin and observed that both treatments induced an ∼2-fold increase in fluorescence compared to the untreated control (Fig. 2C and D). To demonstrate that the increased fluorescence was not simply due to bacterial lysis, we sonicated A. baumannii and observed that the resulting lysates did not produce increased HPF fluorescence above the untreated control (see Fig. S2 in the supplemental material). These data indicate that lysis is not sufficient to cause an induction of HPF fluorescence and, furthermore, that hydroxyl radicals are induced by treatment with the polymyxins.
Fig 2.
Polymyxins induce hydroxyl radical production. A. baumannii cultures were left untreated or were treated with 0.15% hydrogen peroxide (H2O2) (A), 5 μg of kanamycin/ml (B), 2 μg of polymyxin B/ml (C), or 2 μg of colistin/ml (D) for 30 min. After treatment, the hydroxyl radical specific fluorescent dye 3′-(p-hydroxyphenyl) fluorescein was added, and the fluorescence was measured (490 nm/515 nm). The data are representative of three independent experiments. Bars represent the means and standard deviations of triplicate samples. ***, P < 0.0001.
To determine whether polymyxin-mediated radical production was limited to its action on A. baumannii, we utilized the Gram-negative species Escherichia coli and Francisella novicida, a model intracellular pathogen (19). We first determined the kinetics of colistin-mediated killing in these species (see Fig. S3A and B in the supplemental material) and observed that both species were killed with similar kinetics as A. baumannii at the doses utilized. We subsequently measured hydroxyl radical production and observed a significant increase in HPF fluorescence following both kanamycin and colistin treatment of these strains (see Fig. S4A to D in the supplemental material). Together, these data indicate that treatment with polymyxins induces the production of hydroxyl radicals, and that radical production is concurrent with rapid killing of these Gram-negative species by polymyxins.
Rapid polymyxin killing of A. baumannii is mediated by hydroxyl radicals.
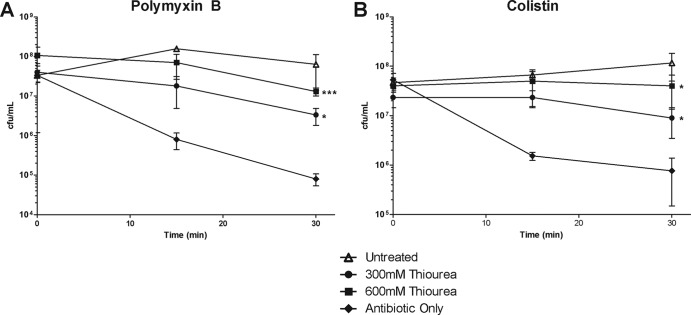
Due to our observations that polymyxin B and colistin treatment induced hydroxyl radical production in A. baumannii, we sought to determine whether hydroxyl radicals were required for the rapid killing of A. baumannii. We therefore utilized a hydroxyl radical scavenging compound, thiourea (35), and assessed its ability to prevent polymyxin killing of A. baumannii. If hydroxyl radicals do indeed mediate the rapid killing of A. baumannii, then concurrent treatment with thiourea would be hypothesized to prevent cell death. In fact, when we performed time-kill assays in the presence of thiourea, we found a striking decrease in the ability of both polymyxin B and colistin to kill A. baumannii compared to treatment with either antibiotic alone (Fig. 3A and B). Notably, treatment with thiourea rescued survival to nearly the level observed in bacteria not treated with either antibiotic and markedly inhibited the rapid decrease in viability. We further examined the ability of thiourea to rescue colistin-mediated killing in E. coli and F. novicida and observed similar magnitudes of rescue in these organisms as well (see Fig. S3A and B in the supplemental material). As an additional control, we directly assayed the ability of thiourea to prevent colistin-mediated radical formation. We observed that thiourea significantly dampened the amount of colistin-mediated radical formation in A. baumannii, as well as in E. coli and F. novicida (see Fig. S5A to C in the supplemental material). Since quenching hydroxyl radicals delays killing, these data indicate that the radicals induced by the polymyxins play a significant role in mediating rapid cell death in A. baumannii and other Gram-negative species.
Fig 3.
Polymyxin killing is delayed by hydroxyl radical quenching. A. baumannii cultures were treated with either 2 μg of polymyxin B/ml (A) or 2 μg of colistin/ml (B) alone (⧫) or in combination with either 300 mM thiourea (●) or 600 mM thiourea (■), or they were left untreated in culture medium (△). At 0, 15, and 30 min, the cultures were plated, and the CFU were enumerated. The data are representative of three independent experiments. Points represent the means and bars represent the standard deviations of triplicate samples. ***, P < 0.0001; *, P < 0.05.
Polymyxin killing of A. baumannii is delayed by inhibition of the Fenton reaction.
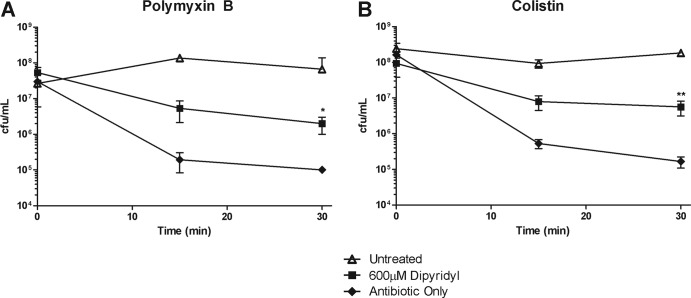
Previous studies have indicated that reactive oxygen species initiate an exponential increase in hydroxyl radical production through an intracellular Fenton reaction (12, 16, 22). The iron chelator, 2,2′-dipyridyl (dipyridyl), has previously been shown to be a potent inhibitor of the Fenton reaction by sequestering available iron, thereby preventing its interaction with peroxides (16). In order to determine whether Fenton chemistry was indeed playing a role in the killing of A. baumannii during polymyxin action, we assayed the ability of dipyridyl to prevent polymyxin-mediated killing. Treatment of A. baumannii with dipyridyl significantly inhibited killing by both polymyxin B and colistin (Fig. 4A and B). Furthermore, we determined that dipyridyl was also capable of rescuing colistin-mediated killing of E. coli and F. novicida (see Fig. S3A and B in the supplemental material). We further examined whether dipyridyl prevented colistin-mediated radical production, in A. baumannii, E. coli, and F. novicida. Similar to our results with thiourea, we observed a significant decrease in colistin-mediated hydroxyl radical production following treatment with dipyridyl (see Fig. S5A to C in the supplemental material). Since dipyridyl is capable of inhibiting the rapid loss of viability observed during polymyxin-mediated death, as well as the induction of hydroxyl radicals, these data implicate the involvement of the Fenton reaction in the rapid killing of A. baumannii, as well as other Gram-negative organisms, by the polymyxins. Together with the ability of the hydroxyl radical scavenger thiourea to inhibit polymyxin-mediated death, these iron depletion data strongly suggest that polymyxins induce rapid killing of Gram-negative bacteria through Fenton chemistry-mediated hydroxyl radical production.
Fig 4.
Polymyxin killing is mediated by iron. A. baumannii cultures were treated with either 2 μg of polymyxin B/ml (A) or 2 μg of colistin (B) alone (⧫) or in combination with 600 μM dipyridyl (■), or they were left untreated in culture medium (△). At 0, 15, and 30 min, the cultures were plated, and the CFU were enumerated. The data are representative of three independent experiments. Points represent the means and bars the standard deviations of triplicate samples. **, P < 0.005; *, P < 0.05.
Drug-resistant clinical isolates of A. baumannii are killed through hydroxyl radicals after polymyxin treatment.
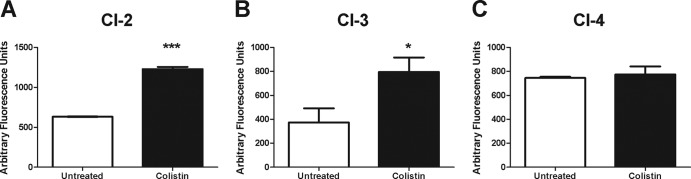
MDR and PDR clinical isolates of A. baumannii have been identified with increasing frequency and present a significant problem in health care settings (3, 33, 36, 39, 41). We therefore sought to determine whether the hydroxyl radical-mediated cell death pathway was induced in recent clinical isolates recalcitrant to the majority of current therapies. These isolates would therefore represent potential candidates for treatment with the polymyxins, including colistin. We first examined the ability of colistin to induce hydroxyl radical production in two MDR clinical isolates of A. baumannii, CI-2 and CI-3, both of which are colistin sensitive (Table 1). Consistent with our previous results, following 30 min of treatment with colistin, we observed a significant increase in hydroxyl radical production (Fig. 5A and B). Furthermore, we examined whether treatment of a PDR isolate, CI-4, which has significant resistance to colistin (Table 1), would induce the production of hydroxyl radicals. Interestingly, colistin treatment did not cause an induction of hydroxyl radicals in this strain (Fig. 5C), indicating that sublethal levels of colistin are not able to induce hydroxyl radicals, which is consistent with data on other antibiotic treatments (22). Together, these data suggest that the induction of hydroxyl radicals following colistin treatment is conserved in recent clinical isolates, and that colistin resistance prevents their induction.
Fig 5.
Colistin induces hydroxyl radical production in MDR clinical isolates. Cultures of colistin-sensitive MDR strain CI-2 (A) or CI-3 (B) or a colistin-resistant PDR A. baumannii strain CI-4 (C) were treated with 2 μg of colistin/ml or left untreated for 30 min. After treatment, the hydroxyl radical specific fluorescent dye 3′-(p-hydroxyphenyl) fluorescein was added, and fluorescence was measured (490 nm/515 nm). The data are representative of two independent experiments. Bars represent the means and standard deviations of triplicate samples. ***, P < 0.0001; *, P < 0.05.
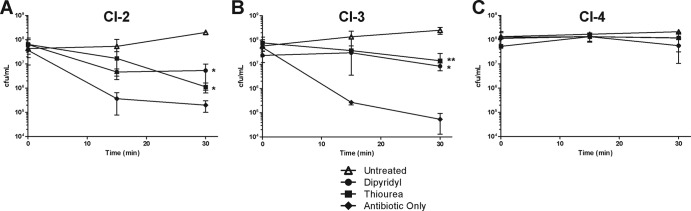
To determine whether these hydroxyl radicals were participating in rapid killing by colistin, we utilized time-kill assays and assessed the ability of thiourea and dipyridyl to prevent colistin-mediated death in these clinical isolates of A. baumannii. We observed that by quenching radical oxygen species or depleting iron to suppress the Fenton reaction, the rapid killing of CI-2 and CI-3 by colistin was significantly inhibited (Fig. 6A and B). Furthermore, the PDR/colistin-resistant isolate CI-4 did not demonstrate a significant change in viability following treatment with colistin, alone or in combination with thiourea or dipyridyl (Fig. 6C). In total, these data demonstrate that the induction of hydroxyl radicals by colistin is responsible for the rapid killing of sensitive A. baumannii isolates, including clinically important MDR strains, and that resistance to the polymyxins prevents hydroxyl radical production.
Fig 6.
Clinical isolates are killed through hydroxyl radical production during polymyxin treatment. The colistin-sensitive MDR clinical isolates CI-2 (A) and CI-3 (B) or the colistin-resistant PDR A. baumannii strain CI-4 (C) were treated with 2 μg of colistin/ml (⧫) alone or in combination with either 600 μM dipyridyl (●) or 600 mM thiourea (■), or they were left untreated in culture medium (△). At 0, 15, and 30 min, the cultures were plated, and the CFU were enumerated. The data are representative of two independent experiments. Points represent the means and bars represent the standard deviation of triplicate samples. **, P < 0.005; *, P < 0.05.
DISCUSSION
Classically, the polymyxins have been thought to kill bacteria through membrane disruptions and, ultimately, cell lysis (10). However, there exist historical reports of polymyxins killing bacteria without actively lysing those cells (8, 21). Here, we demonstrated that treatment with polymyxins induces hydroxyl radical production through the Fenton reaction and that this radical production mediates the rapid killing of A. baumannii, as well as E. coli and F. novicida.
Other bactericidal antibiotics, including the quinolones, β-lactams, and aminoglycosides, have previously been shown to induce the production of hydroxyl radicals, which ultimately mediate killing of Escherichia coli and Staphylococcus aureus (12, 22). Notably, each of these antibiotic classes interact directly with enzymes involved in different aspects of bacterial physiology: DNA replication, cell wall synthesis, and translation, respectively. Conversely, the polymyxins are not known to interact with bacterial enzymes and instead target lipid A in the outer membrane of Gram-negative bacteria (10, 32). Thus, their ability to both induce hydroxyl radicals and kill A. baumannii through hydroxyl radical production is somewhat surprising. To our knowledge, this is the first demonstration that the polymyxins induce an oxidative cell death pathway.
The mechanism by which polymyxin treatment induces the production of hydroxyl radicals in A. baumannii is not completely clear. Other bactericidal antibiotics were shown to induce a stress response in E. coli, disrupting the production of NADH by inhibiting the tricarboxylic acid cycle and thus ultimately causing aberrant respiration in the electron transport chain (12, 22). Disruptions in the electron transport chain promote the production of superoxide that can participate in the Fenton reaction and induce production of hydroxyl radicals (12, 16, 22). In addition, it has been demonstrated that the mistranslation of membrane proteins following treatment with aminoglycosides or the mammalian peptidoglycan recognition proteins activates the CpxAR two-component system in E. coli, which is involved in the bacterial envelope stress response, and subsequently triggers the depletion of NADH and production of hydroxyl radicals through the process described above (20, 23). It is therefore tempting to posit that the polymyxins may induce hydroxyl radicals in a similar fashion, by activating an envelope stress response in the bacterial cell, which shifts the metabolic state and causes aberrant electron transport. In fact, Vibrio cholerae treated with polymyxin B exhibits an increase in the transcriptional levels of rpoE, the sigma factor involved in envelope and oxidative stress responses (37). Interestingly, previous studies have indicated that polymyxin B treatment can induce aberrant oxidative respiration (31, 40, 42). In organisms with no experimentally identified CpxAR envelope stress-sensing two-component system, such as A. baumannii and F. novicida, this envelope stress response may be triggered by other, as-yet-unidentified, sensory systems. In total, these past observations are consistent with the data presented here and together suggest that killing of Gram-negative bacteria, including A. baumannii, by the polymyxins may follow the proposed conserved pathway of hydroxyl radical-mediated cell death. It is also interesting to consider the possibility that other membrane-targeting antibiotics, such as daptomycin, which depolarizes the bacterial membrane and also kills bacteria without causing lysis, induce this cell death pathway as well (7, 13).
Since polymyxin treatment is increasingly the last line therapeutic option for patients infected with MDR strains of A. baumannii, we further elucidated the conserved nature of hydroxyl radical-mediated killing in MDR clinical isolates. Not only did colistin treatment induce hydroxyl radical production in colistin-sensitive strains, but both thiourea and dipyridyl were able to inhibit the ability of colistin to kill these strains, indicating that hydroxyl radical-mediated cell death can occur in MDR nosocomial isolates of A. baumannii (Fig. 5 and 6). We also note that colistin resistance prevented the induction of hydroxyl radicals in A. baumannii.
Colistin resistance in A. baumannii has primarily been linked to changes in its lipid A, dampening or preventing the initial interaction between colistin and the bacterial envelope. These changes include complete loss of lipid A or additions of phosphoethanolamine to mask negatively charged phosphate moieties (2, 4, 14, 30, 38). A. baumannii can become resistant through mutations in genes necessary to produce lipid A or through mutations in the PmrAB two-component system, which signals for lipid A alterations (1, 2, 4). The PmrAB system is activated by ferric iron, and growth in iron replete conditions has been shown to provide a slight increase in the A. baumannii MIC for colistin, likely due to PmrAB activation of lipid A alterations (1, 2, 4). This is not contrary to our data, which suggests that when colistin interacts with the bacterial envelope, intracellular iron potentiates killing through the Fenton reaction and the induction of hydroxyl radicals (16). Thus, as we demonstrate, iron depletion prevents Fenton chemistry from potentiating the production of hydroxyl radicals following polymyxin treatment. Notably, it has been demonstrated that some pathogenic bacterial species limit intracellular iron, and those with lower levels have increased resistance to oxidative killing (27). It is interesting to consider the possibility that this intracellular iron limitation may provide resistance to a broad range of host defenses, as well as the polymyxins.
With the increasing frequency of A. baumannii as a nosocomial pathogen, a rising percentage of these infections requiring treatment with polymyxins, as well as the growing cost of treatment of infections with this pathogen, understanding the precise mechanism of action of these last line therapeutics is imperative (3, 5, 6, 24, 34, 36, 43, 47). Our findings not only augment our knowledge of the mechanism of polymyxin action but also provide support to current concepts of utilizing hydroxyl radical-inducing agents as therapies against extensively drug-resistant pathogens (9, 48).
Supplementary Material
ACKNOWLEDGMENTS
We thank William Shafer, Emory University, for critical review of the manuscript; Philip Rather, Emory University, for critical review, reagents, and helpful discussion; Brandi Limbago, Division of Health Care Quality Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia, for acquisition of the clinical isolates used in this study; and Crystal Jones, Emory University, for superb technical assistance.
This study was supported by National Institutes of Health (NIH) grants U54-AI057157 from the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense and R21-AI098800. T.R.S. was supported by a National Science Foundation Graduate Research Fellowship, as well as the ARCS Foundation. C.S.K. was supported by KL2 RR025009.
The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Published ahead of print 20 August 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Adams MD, et al. 2009. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob. Agents Chemother. 53:3628–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arroyo LA, et al. 2011. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob. Agents Chemother. 55:3743–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baang JH, et al. 2012. Longitudinal epidemiology of multidrug-resistant (MDR) Acinetobacter species in a tertiary care hospital. Am. J. Infect. Control 40:134–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beceiro A, et al. 2011. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob. Agents Chemother. 55:3370–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bergogne-Berezin E. 2001. The increasing role of Acinetobacter species as nosocomial pathogens. Curr. Infect. Dis. Rep. 3:440–444 [PubMed] [Google Scholar]
- 6. Bergogne-Berezin E, Towner KJ. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cotroneo N, Harris R, Perlmutter N, Beveridge T, Silverman JA. 2008. Daptomycin exerts bactericidal activity without lysis of Staphylococcus aureus. Antimicrob. Agents Chemother. 52:2223–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. David HL, Rastogi N. 1985. Antibacterial action of colistin (polymyxin E) against Mycobacterium aurum. Antimicrob. Agents Chemother. 27:701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies BW, et al. 2009. Hydroxyurea induces hydroxyl radical-mediated cell death in Escherichia coli. Mol. Cell 36:845–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dixon RA, Chopra I. 1986. Polymyxin B and polymyxin B nonapeptide alter cytoplasmic membrane permeability in Escherichia coli. J. Antimicrob. Chemother. 18:557–563 [DOI] [PubMed] [Google Scholar]
- 11. Durante-Mangoni E, Zarrilli R. 2011. Global spread of drug-resistant Acinetobacter baumannii: molecular epidemiology and management of antimicrobial resistance. Future Microbiol. 6:407–422 [DOI] [PubMed] [Google Scholar]
- 12. Dwyer DJ, Kohanski MA, Hayete B, Collins JJ. 2007. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol. Syst. Biol. 3:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eisenstein BI. 2008. Treatment of staphylococcal infections with cyclic lipopeptides. Clin. Microbiol. Infect. 14(Suppl 2):10–16 [DOI] [PubMed] [Google Scholar]
- 14. Henry R, et al. 2012. Colistin-resistant, lipopolysaccharide-deficient Acinetobacter baumannii responds to lipopolysaccharide loss through increased expression of genes involved in the synthesis and transport of lipoproteins, phospholipids, and poly-β-1,6-N-acetylglucosamine. Antimicrob. Agents Chemother. 56:59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hong KB, et al. 2012. Investigation and control of an outbreak of imipenem-resistant Acinetobacter baumannii infection in a pediatric intensive care unit. Pediatr. Infect. Dis. J. 31:685–690 [DOI] [PubMed] [Google Scholar]
- 16. Imlay JA, Chin SM, Linn S. 1988. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240:640–642 [DOI] [PubMed] [Google Scholar]
- 17. Imlay JA, Linn S. 1988. DNA damage and oxygen radical toxicity. Science 240:1302–1309 [DOI] [PubMed] [Google Scholar]
- 18. Jimenez-Mejias ME, et al. 1997. Treatment of multidrug-resistant Acinetobacter baumannii meningitis with ampicillin/sulbactam. Clin. Infect. Dis. 24:932–935 [DOI] [PubMed] [Google Scholar]
- 19. Jones CL, et al. 2012. Subversion of host recognition and defense systems by Francisella spp. Microbiol. Mol. Biol. Rev. 76:383–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kashyap DR, et al. 2011. Peptidoglycan recognition proteins kill bacteria by activating protein-sensing two-component systems. Nat. Med. 17:676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klemperer RM, Gilbert P, Meier AM, Cozens RM, Brown MR. 1979. Influence of suspending media upon the susceptibility of Pseudomonas aeruginosa NCTC 6750 and its spheroplasts to polymyxin B. Antimicrob. Agents Chemother. 15:147–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810 [DOI] [PubMed] [Google Scholar]
- 23. Kohanski MA, Dwyer DJ, Wierzbowski J, Cottarel G, Collins JJ. 2008. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell 135:679–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee BY, McGlone SM, Doi Y, Bailey RR, Harrison LH. 2010. Economic impact of Acinetobacter baumannii infection in the intensive care unit. Infect. Control Hosp. Epidemiol. 31:1087–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li J, et al. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 6:589–601 [DOI] [PubMed] [Google Scholar]
- 26. Lim LM, et al. 2010. Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy 30:1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lindgren H, et al. 2011. Iron content differs between Francisella tularensis subspecies tularensis and subspecies holarctica strains and correlates to their susceptibility to H2O2-induced killing. Infect. Immun. 79:1218–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mai J, et al. 2011. A novel target-specific, salt-resistant antimicrobial peptide against the cariogenic pathogen Streptococcus mutans. Antimicrob. Agents Chemother. 55:5205–5213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manchanda V, Sanchaita S, Singh N. 2010. Multidrug resistant acinetobacter. J. Global Infect. Dis. 2:291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moffatt JH, et al. 2010. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 54:4971–4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mogi T, et al. 2009. Polymyxin B identified as an inhibitor of alternative NADH dehydrogenase and malate: quinone oxidoreductase from the Gram-positive bacterium Mycobacterium smegmatis. J. Biochem. 146:491–499 [DOI] [PubMed] [Google Scholar]
- 32. Morrison DC, Jacobs DM. 1976. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 13:813–818 [DOI] [PubMed] [Google Scholar]
- 33. Park YK, et al. 2009. Extreme drug resistance in Acinetobacter baumannii infections in intensive care units, South Korea. Emerg. Infect. Dis. 15:1325–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Repine JE, Fox RB, Berger EM. 1981. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J. Biol. Chem. 256:7094–7096 [PubMed] [Google Scholar]
- 36. Sengstock DM, et al. 2010. Multidrug-resistant Acinetobacter baumannii: an emerging pathogen among older adults in community hospitals and nursing homes. Clin. Infect. Dis. 50:1611–1616 [DOI] [PubMed] [Google Scholar]
- 37. Sikora AE, Beyhan S, Bagdasarian M, Yildiz FH, Sandkvist M. 2009. Cell envelope perturbation induces oxidative stress and changes in iron homeostasis in Vibrio cholerae. J. Bacteriol. 191:5398–5408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soon RL, et al. 2011. Different surface charge of colistin-susceptible and -resistant Acinetobacter baumannii cells measured with zeta potential as a function of growth phase and colistin treatment. J. Antimicrob. Chemother. 66:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sutter DE, et al. 2011. High incidence of multidrug-resistant gram-negative bacteria recovered from Afghan patients at a deployed US military hospital. Infect. Control Hosp. Epidemiol. 32:854–860 [DOI] [PubMed] [Google Scholar]
- 40. Terce F, Gillois M, Laneelle G. 1979. Respiratory chain inhibition by polymyxin b in a gram-positive bacterium (Micrococcus luteus). FEMS Microbiol. Lett. 6:357–360 [Google Scholar]
- 41. Tien HC, et al. 2007. Multidrug resistant Acinetobacter infections in critically injured Canadian forces soldiers. BMC Infect. Dis. 7:95 doi:10.1186/1471-2334-7-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tochikubo K, Yasuda Y, Kozuka S. 1986. Decreased particulate NADH oxidase activity in Bacillus subtilis spores after polymyxin B treatment. J. Gen. Microbiol. 132:277–287 [DOI] [PubMed] [Google Scholar]
- 43. Villers D, et al. 1998. Nosocomial Acinetobacter baumannii infections: microbiological and clinical epidemiology. Ann. Intern. Med. 129:182–189 [DOI] [PubMed] [Google Scholar]
- 44. Weber DJ, Rutala WA, Miller MB, Huslage K, Sickbert-Bennett E. 2010. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am. J. Infect. Control. 38:S25–S33 [DOI] [PubMed] [Google Scholar]
- 45. Webster C, Towner KJ, Humphreys H. 2000. Survival of Acinetobacter on three clinically related inanimate surfaces. Infect. Control Hosp. Epidemiol. 21:246. [DOI] [PubMed] [Google Scholar]
- 46. Wendt C, Dietze B, Dietz E, Ruden H. 1997. Survival of Acinetobacter baumannii on dry surfaces. J. Clin. Microbiol. 35:1394–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wolff M, et al. 1997. The changing epidemiology of severe infections in the ICU. Clin. Microbiol. Infect. 3:s36–s47 [Google Scholar]
- 48. Wright GD. 2007. On the road to bacterial cell death. Cell 130:781–783 [DOI] [PubMed] [Google Scholar]
- 49. Yeom J, Imlay JA, Park W. 2010. Iron homeostasis affects antibiotic-mediated cell death in Pseudomonas species. J. Biol. Chem. 285:22689–22695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zavascki AP, Goldani LZ, Li J, Nation RL. 2007. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J. Antimicrob. Chemother. 60:1206–1215 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.