Abstract
Herpes simplex viruses 1 and 2 (HSV-1 and HSV-2) are responsible for lifelong latent infections in humans, with periods of viral reactivation associated with recurring ulcerations in the orofacial and genital tracts. In immunosuppressed patients and neonates, HSV infections are associated with severe morbidity and, in some cases, even mortality. Today, acyclovir is the standard therapy for the management of HSV infections. However, the need for novel antiviral agents is apparent, since HSV isolates resistant to acyclovir therapy are frequently isolated in immunosuppressed patients. In this study, we assessed the anti-HSV activity of the antiadenoviral compounds 2-[2-(2-benzoylamino)-benzoylamino]benzoic acid (benzavir-1) and 2-[4,5-difluoro-2-(2-fluorobenzoylamino)-benzoylamino]benzoic acid (benzavir-2) on HSV-1 and HSV-2. Both compounds were active against both viruses. Importantly, benzavir-2 had potency similar to that of acyclovir against both HSV types, and it was active against clinical acyclovir-resistant HSV isolates.
INTRODUCTION
Herpes simplex virus (HSV) is a double-stranded DNA virus that falls into two types, herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2). HSV infection rates are very high; at the age of 60 years, 60 to 85% of adults in the United States are positive for HSV-1, and at age 40 years, 25% have antibodies to HSV-2 (21, 40). The prevalence of HSV, however, is influenced by socioeconomic status and geographic location (55, 66). The primary site of infection with HSV-1 is the orolabial mucosa, whereas HSV-2 mainly infects the genital mucosa. Several recently reported studies have shown that HSV-1 is frequently associated with genital tract infections and that HSV-2 can cause orofacial infections (10, 26, 48). After the primary infection, the HSV-1 virus is transported retrogradely to the trigeminal ganglion and HSV-2 travels to the sacral ganglia. At these locations the viruses are protected from the host immune system and establish a lifelong latent infection that can be reactivated by hormonal changes, UV light, and stress. When reactivated, HSV can be transported to the primary site of infection and cause recurrent ulcerations. During recurrence, HSV-1 can appear in the eye and cause ocular keratitis, resulting in blindness, and in rare cases it can infect the brain. HSV-1 encephalitis has mortality rates of up to 70% if left untreated (11, 29–31, 35, 65). HSV-2 may be even more prone to recur (33) and is frequently shed in asymptomatic individuals (63). Furthermore, there is a strong correlation between increased HIV transmission and HSV-2 seropositivity due to genital ulcer disease (62).
In people with an impaired immune system due to immunosuppressive drugs or AIDS, the incidence of HSV recurrence is increased. In allogeneic bone marrow transplant recipients, HSV-1 and HSV-2 account for as much as 70 to 80% of severe mucocutaneous diseases, and 32 to 53% of solid organ transplant patients show HSV-associated disease (24, 37, 54). HSV reactivation is one of the first opportunistic infections seen in patients with AIDS (49, 53, 58) and can lead to death (14, 39). Likewise, the lack of immunity to HSV in the newborn child can lead to devastating generalized primary infection in the newborn if the mother has a primary or reactivated HSV infection in the genital tract. Neonatal HSV infections are relatively common in the United States (8 to 60 per 100,000), and transmission of the virus most often occurs in the HSV-infected birth canal during vaginal delivery (17). Neonatal HSV infection can cause eye or skin lesions, severe meningoencephalitis, or disseminated disease associated with long-term neurological sequelae and high mortality rates (5).
The standard therapy for management of HSV includes acyclovir and penciclovir and their respective derivates valacyclovir and famciclovir. Acyclovir was discovered in 1977 and is a guanosine analog that must be phosphorylated by the virus-encoded thymidine kinase followed by cellular kinases to serve as a substrate for the viral DNA polymerase. Acyclovir prevents chain elongation and therefore synthesis of the viral DNA (18, 38, 44, 50). Acyclovir has proven to be useful in managing herpesvirus infections and has a favorable safety record (57, 61). However, due to its limited oral bioavailability (15 to 20%) and low solubility in water, derivates of acyclovir have been developed (6). The ester prodrug of acyclovir, valacyclovir, has higher absorption characteristics and a bioavailability of up to 54% (25, 64). The guanine analog, penciclovir, has an activity similar to that of acyclovir, but it is poorly absorbed after oral administration and is therefore not commercially available as an oral agent (4, 9, 56). Famciclovir, the ester prodrug of penciclovir, increases the absolute bioavailability of penciclovir when administered orally (59).
Given that acyclovir and its derivatives are valuable antiherpes agents with low toxicities, emerging drug-resistant HSV isolates are a major threat worldwide. The development of acyclovir resistance is much more common in immunosuppressed patients than in immunocompetent individuals. This is mainly due to long-term prophylactic acyclovir therapy in combination with an impaired host response that enables less virulent viruses to continue to replicate. The rate of isolation of acyclovir-resistant HSV in immunocompetent patients is reported to be between 0.1% and 0.6% (3, 8, 15), whereas drug-resistant HSV is found in 4% to 6% of immunosuppressed patients (13, 15, 19). However, an even higher frequency (36%) has been reported (34). The vast majority of drug-resistant isolates have mutations in the thymidine kinase (TK) gene (23), but alterations in the DNA polymerase gene have also been observed (16, 36). All TK-negative HSV isolates show cross-resistance between acyclovir and penciclovir, although some isolates with an altered TK gene are susceptible to penciclovir (9). When acyclovir or penciclovir therapy fails, the second line of defense is most often foscarnet followed by cidofovir therapy. Foscarnet is a phosphonic acid derivative that selectively binds and inhibits the pyrophosphate site on the herpesvirus DNA polymerase at concentrations that do not affect the cellular DNA polymerases. Foscarnet has been used successfully in managing acyclovir-resistant HSV infections (12, 41). However, emerging foscarnet-resistant isolates have been reported (46, 47). Cidofovir, a monophosphate form of an acyclic nucleoside analog that is TK independent, has been used in acyclovir- and foscarnet-resistant HSV infections (7, 13) but is known to be nephrotoxic and can be used only in patients with normal renal function.
Thus, there is a great need for the development of new antiviral drugs with novel targets. We have previously addressed this need by developing a cell-based viral replication assay and identifying several compounds that are active against human adenovirus (HAdV) (2). Similar to HSV infections, HAdV infections in immunocompetent individuals are mild and self limited, whereas in immunosuppressed patients they can cause life-threatening diseases (32, 51). HAdV infections have been reported in 5 to 21% of allogeneic bone marrow transplant recipients (22, 52). In addition, pediatric transplantation patients are at higher risk of developing disseminated HAdV infections, which are associated with high mortality rates (19 to 82%) (28). One of the compounds with promising results for screening of antiviral activity, 2-[2-benzoylamino)benzoylamino]-benzoic acid (benzavir-1), proved to be a potent anti-HAdV compound with low toxicity for cells (2). Furthermore, the potency of this compound against HAdV was improved by iterative design, synthesis, and biological evaluation to furnish structure-activity relationships to the potent compound 2-[4,5-difluoro-2-(2-fluorobenzoylamino)-benzoylamino]benzoic acid (benzavir-2) (42) (Fig. 1).
Fig 1.
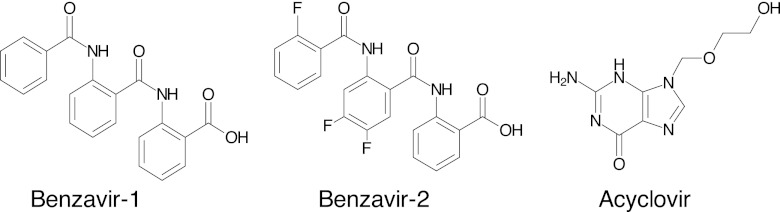
The compounds assessed for anti-HSV activities in this study. Benzavir-1 was reported previously to have anti-HAdV activity (2) and has been optimized, using structure-activity relationship analysis, to give benzavir-2 (42).
We addressed the activity of benzavir-1 and benzavir-2, with previously reported anti-HAdV activity, on HSV-1 and HSV-2. In addition, we assessed the activities on acyclovir-resistant isolates of both HSV-1 and HSV-2.
MATERIALS AND METHODS
HSV isolates and cells.
The HSV isolates used in this study were from clinical samples collected from patients in Sweden (Table 1). The two acyclovir-sensitive isolates (of HSV-1 and HSV-2) were derived from separate patients and had been passaged 12 to 13 times in African green monkey kidney (GMK) cells at our clinical laboratory. The acyclovir-resistant isolates had been collected from five patients with recurrent herpetic lesions and had been sent to the virology laboratory of Sahlgrens University Hospital, Gothenburg, Sweden, due to suspected resistance to acyclovir. All five were found by plaque assay to be fully resistant to acyclovir. Four of these isolates were also tested by plaque assay for resistance to foscarnet and were found to be foscarnet sensitive. All HSV isolates were grown in GMK cells to produce viral stocks. These stocks were later quantified using quantitative PCR (qPCR) to determine the number of viral genomes per milliliter.
Table 1.
HSV isolates assessed in this studya
| Isolate | Type | Gender/age (yr) | Anatomical location of lesion | EC50 of acyclovir | EC50 of foscarnet |
|---|---|---|---|---|---|
| Acyclovir-sensitive HSV | |||||
| HSV-1 | 1 | M/22 | Orolabial | 1.6 μMb | NDc |
| HSV-2 | 2 | F/24 | Genital | 1.6 μMb | ND |
| Acyclovir-resistant HSV | |||||
| DE-5016 | 1 | M/61 | Corneal | >80 μM | 80 μM |
| DE-625 | 2 | M/60 | Knee pit | >80 μM | ND |
| DE-3657 | 2 | F/52 | Genital | >80 μM | 30 μM |
| DE-6820 | 2 | F/83 | Genital | 80 μM | 50 μM |
| DE-14574 | 2 | F/82 | Genital | >80 μM | 125 μM |
The acyclovir-resistant isolates were analyzed with a plaque assay at Sahlgrenska University Hospital, Gothenburg, Sweden. The reference values for acyclovir and foscarnet resistance were >4 μM and >160 μM, respectively.
EC50s as presented in Fig. 3, analyzed by qPCR.
ND, not determined.
GMK cells were used throughout the study to assess HSV infection and the toxicities of the compounds. The cells were grown in Dulbecco's modified Eagle medium (DMEM) (Sigma-Aldrich, St. Louis, MO) containing 0.75 mg/ml NaHCO3, 20 mM HEPES (EuroClone, Milan, Italy), 1× PEST (100 IU/ml penicillin G, 100 μg/ml streptomycin sulfate; Gibco, Carlsbad, CA) and 5% fetal bovine serum (FBS) (Gibco) at 37°C.
Compounds.
Benzavir-1 and benzavir-2 were synthesized as described previously (42). Acyclovir and cidofovir were purchased from Sigma-Aldrich (Schnelldorf, Germany) as a powder. The compounds were dissolved in dimethyl sulfoxide (DMSO) in 20 mM stock solutions (benzavir-1, benzavir-2, and cidofovir) or 200 mM stock solution (acyclovir) and were stored at room temperature in the dark and in a dry atmosphere.
Quantitative real-time PCR for assessment of antiviral activity.
On the day before infection, approximately 7.5 × 104 GMK cells were seeded in 24-well plates (Nunc) in DMEM with 5% FBS. On the day of infection, the cells in one well were counted in a Bürker chamber to establish the number of viral genomes (virions) per cell to be added. Amounts of the acyclovir-sensitive HSV-1 and HSV-2 viral stocks corresponding to 75 viral genomes/cell were added to the cells simultaneously with the addition of test compound in DMEM containing 2% FBS. For the acyclovir-resistant isolates, the numbers of viral genomes added per cell were normalized so that the replicated numbers of viral genomes were similar to the qPCR readouts of the acyclovir-sensitive HSV-1 and HSV-2 isolates (approximately 3,000 viral copies/cell). For the acyclovir-resistant isolates DE-625 and DE-6820, 20 copies per cell were added, for DE-3657, 25 copies per cell were added, for DE-14574, 50 copies per cell were added, and for DE-5016, 30 copies per cell were added to achieve a final number of 3,000 replicated viral copies/cell. The compounds were added simultaneously in concentrations ranging from 0.020 μM to 20 μM per well, and the plate was incubated at 37°C in 5% CO2 for 24 h. The final concentration of DMSO was less than 0.5% in all samples.
Twenty-four hours later, the medium was collected, and the cells were harvested (using 0.05% EDTA–phosphate-buffered saline [PBS] and 0.05% trypsin in 0.05% EDTA-PBS), and the total DNA in the medium and cells was extracted using the QIAamp DNA blood minikit (Qiagen, Solna, Sweden) according to the manufacturer's instructions. The principle of qPCR has been described elsewhere (27), as has the design of primers and probes for HSV-1 and HSV-2 detection (20). In brief, the qPCR was carried out using a degenerate primer pair specific for the glycoprotein G gene (HSV-2) and both the G glycoprotein gene and the J glycoprotein gene (HSV-1): forward primer (HSV-1, 5′-GGC CTG GCT ATC CGG AGA-3′; HSV-2, 5′-AGA TAT CCT CTT TAT CAT CAG CAC CA-3′) and reverse primer (HSV-1, 5′-GCG CAG AGA CAT CGC GA-3′; HSV-2, 5′-TTG TGC TGC CAA GGC GA-3′). 6-Carboxyfluorescein (FAM)–6-carboxytetramethylrhodamine (TAMRA) probes were used for signal detection (HSV-1, 5′-CAG CAC ACG ACT TGG CGT TCT GTG T-3′; HSV-2, 5′-CGG CGG CGT TCG TTT GTC TG-3′). To quantify the number of viral genomes, a standard curve ranging from 5 to 5 × 105 genomes was run separately but in parallel to the HSV samples in each experiment according to the standard procedure in our clinical laboratory. The standard curve was generated by serial dilution of known amounts of full-length DNA from HAdV type 5. The origin of the standard curve, HAdV type 5, did not influence the quantification of HSV genomes, since different primers and probes were used for HSV and HAdV quantification. The design and analysis of primers and probes for the detection of HAdV have been described (1). The forward primer used for HAdV detection was Kadgen 1 (5′-CWT ACA TGC ACA TCK CSG G-3′), and the reverse primer was Kadgen 2 (5′-CRC GGG CRA AYT GCA CCA G-3′). Furthermore, the FAM-TAMRA-labeled probe for HAdV detection was AdC (5′-AGG ACG CCT CGG AGT ACC TGA GCC CCG-3′). The amplification step was performed in a 25-μl reaction mixture containing 10 μl DNA prepared from HSV-infected samples or 10 μl DNA for the standard curve, 12.5 μl master mix (TaqMan; Applied Biosystems), 0.3 μl of a 25 μM concentration of each respective primer, and 0.22 μl of a 20 μM concentration of each respective probe. Distilled water (1.68 μl) was added to give a total volume of 25 μl.
Real-time PCR was performed in an ABI Prism 7900HT sequence detector (Applied Biosystems) and analyzed with Sequence Detector software version 2.4 (Applied Biosystems). The program for the real-time PCR was 2 min at 50°C followed by amplification (10 min at 95°C and then 40 cycles of 15 s at 95°C and 1 min at 60°C). To normalize the number of viral genome copies to the number of cells, real-time qPCR analysis was performed, in parallel, on the same samples with the cellular RNaseP gene used as a reference gene. This analysis was performed using the TaqMan RNaseP detection kit (20× mix containing primers and a FAM-TAMRA probe) (Applied Biosystems, Foster City, CA).
Evaluation of cellular viability in the presence of a test compound.
To determine the effect of the compounds on cellular viability, a colorimetric assay, the 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT)-based toxicity test (Sigma-Aldrich), was used. The assay is based on the reduction of the tetrazolium salt XTT (2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide) to a formazan dye by metabolically active cells. Due to solubility issues of benzavir at ≥150 μM, its toxicity was analyzed at two concentrations, 30 and 60 μM. Approximately 15,000 GMK cells were seeded in 96-well plates (Nunc) on the day before addition of the compounds. The next day, the medium with 5% FBS was removed and replaced with 30 μM or 60 μM test compound in 100 μl phenol red-free DMEM with 2% FBS. In parallel, amounts of DMSO corresponding to 30 or 60 μM test compound were added to the wells as controls, and the plate was incubated at 37°C for 24 h. The percentage of DMSO used to correspond to 30 μM was 0.15% and to 60 μM was 0.3%. Four hours before the measurement of toxicity, 50 μl of XTT solution was added per well, and the plate was again incubated at 37°C. Twenty-four hours after addition of the test compound, the intensity of the formazan dye was measured by spectrophotometry at a wavelength of 490 nm (45).
Statistical analysis.
Determination of the half-maximum effective concentrations (EC50s) was performed with nonlinear regression analysis with a variable slope using GraphPad Prism software version 5.0 (GraphPad Software, San Diego, CA).
RESULTS
Benzavir-1 and its analog benzavir-2 at 15 and 5 μM have potent antiviral activities against HSV-1 and HSV-2.
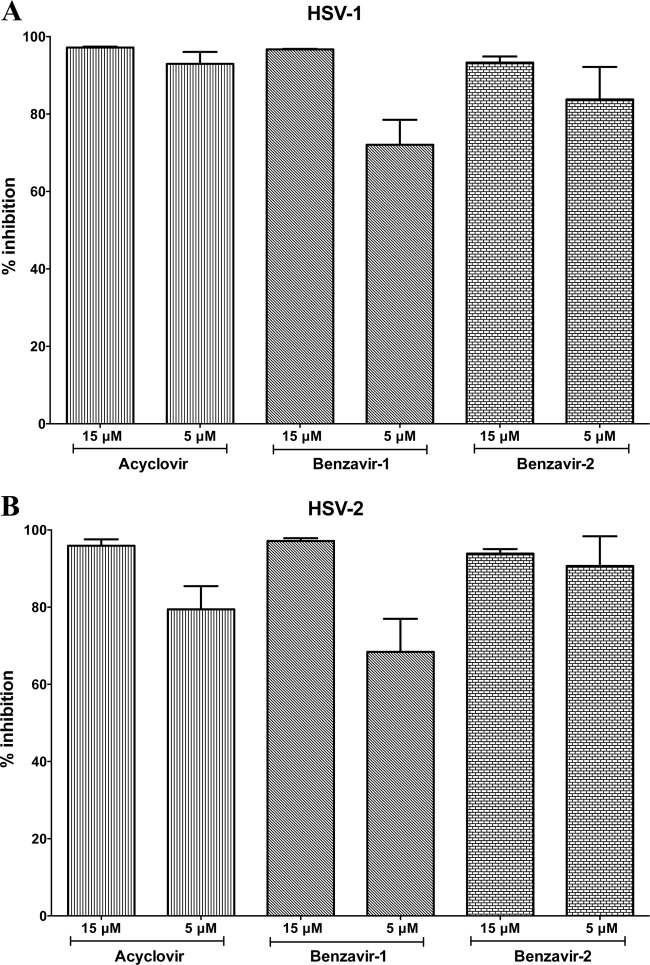
The antiviral activities of 2-[2-(2-benzoylamino)-benzoylamino]benzoic acid (benzavir-1) and 2-[4,5-difluoro-2-(2-fluorobenzoylamino)-benzoylamino]benzoic acid (benzavir-2) were initially screened at 15 and 5 μM and compared with the activities of acyclovir on acyclovir-sensitive isolates of both HSV-1 and HSV-2. The antiviral efficacy of the compounds was determined by quantitative PCR (qPCR) throughout the study. The percent inhibition of replication of viral genomes per cell treated with 15 or 5 μM acyclovir, benzavir-1, or benzavir-2, compared to infected but untreated GMK cells, is presented (Fig. 2). For HSV-1, a nearly complete inhibition (>93%) of the viral replication was observed for acyclovir at both 15 and 5 μM (Fig. 2A). However, for HSV-2, acyclovir appeared to be slightly less potent, with 80% inhibition at 5 μM (Fig. 2B). Both benzavir-1 and benzavir-2 had similar activities against HSV, regardless of type, and the overall results indicate that benzavir-2 is more potent than benzavir-1, which is in line with the activity against HAdV (42).
Fig 2.
Activities of benzavir-1 and benzavir-2, in comparison to acyclovir, against replication of acyclovir-sensitive clinical isolates of HSV-1 (A) and HSV-2 (B). The percent inhibitions at 15 and 5 μM were evaluated using qPCR, and the data are based on the results of three independent experiments. The error bars show the standard deviations.
Acyclovir and benzavir-2 have similar EC50s but different inhibitory profiles.
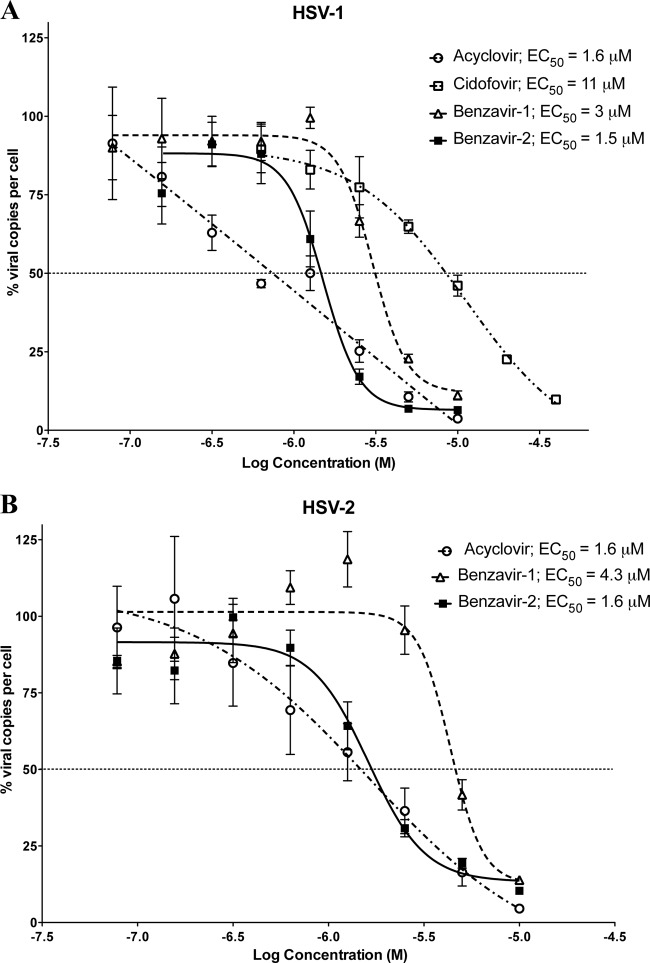
Since acyclovir and both benzavir-1 and benzavir-2 almost completely abolished the replication of HSV at 15 μM and only minor differences were observed at 5 μM (Fig. 2), the efficacies were assessed by determining the half-maximum effective concentrations (EC50s) on HSV-1 and HSV-2 (Fig. 3). The dose-response curves for acyclovir, cidofovir, benzavir-1, and benzavir-2 with HSV-1 and HSV-2 are shown in Fig. 3. The efficacies of acyclovir and benzavir-2 were the same irrespective of HSV type (acyclovir, EC50 = 1.6 μM for both HSV-1 and HSV-2; benzavir-2, EC50 = 1.5 μM for HSV-1 and EC50 = 1.6 μM for HSV-2). However, the efficacy of benzavir-1 was lower, and a slight difference was seen between HSV types (HSV-1, EC50 = 3 μM; HSV-2, EC50 = 4.3 μM). The efficacy of cidofovir was low against HSV-1 (EC50 = 11 μM). Although the dose-response curves for benzavir-1 and benzavir-2 followed a sigmoidal shape and the curves for acyclovir were straighter, the EC50s were similar for acyclovir and benzavir-2.
Fig 3.
Shown are the dose-response curves of acyclovir, cidofovir, benzavir-1, and benzavir-2 with acyclovir-sensitive HSV-1 (A) and of acyclovir, benzavir-1, and benzavir-2 with acyclovir-sensitive HSV-2 (B). The effects on the ratios of the numbers of viral copies per cell in treated and untreated cells, expressed in percentages with increasing concentrations of test compounds, are shown. The concentrations ranged from 0.078 to 10 μM for acyclovir, benzavir-1, and benzavir-2 and from 0.625 to 40 μM for cidofovir. Based on three independent experiments, the EC50s were calculated from the dose-response curve for each compound with GraphPad Prism.
Benzavir-1 and benzavir-2 have antiviral activity against acyclovir-resistant clinical isolates of HSV-1 and HSV-2.
In view of the facts that benzavir-1 and benzavir-2 had potent antiviral effects against both HSV-1 and HSV-2, and that benzavir-2 and acyclovir had similar EC50s (Fig. 3), we investigated their activities against acyclovir-resistant clinical isolates (Table 2). The initial assessment was performed at concentrations of 15 and 5 μM. We assessed four HSV-2 isolates and one HSV-1 isolate, all of which were acyclovir resistant (Table 1). As expected, acyclovir had no or little effect on the replication of these isolates. A modest antiviral activity was seen against the HSV-1 isolate DE-5016 and HSV-2 isolates DE-14574 and DE-6820 (with approximately 20% inhibition at both 15 and 5 μM). On the other hand, benzavir-1 and benzavir-2 had potent activities against all isolates. However, the activity of benzavir-1 on DE-625 was low (43% inhibition at 15 μM and 13% at 5 μM). Benzavir-1 showed the highest overall activity against isolates DE-3657 and DE-6820 at 15 μM (97% inhibition for both). Benzavir-2 was more potent than benzavir-1. Benzavir-2 showed an overall inhibition of >82% at 15 μM and >72% at 5 μM for all 5 isolates.
Table 2.
The antiviral activities of benzavir-1 and benzavir-2 against acyclovir-resistant isolates of HSV-1 and HSV-2a
| Acyclovir-resistant HSV |
Acyclovir inhibition (%) at: |
Benzavir-1 inhibition (%) at: |
Benzavir-2 inhibition (%) at: |
||||
|---|---|---|---|---|---|---|---|
| Isolate | Type | 15 μM | 5 μM | 15 μM | 5μ M | 15 μM | 5 μM |
| DE-5016 | 1 | 24 ± 10 | 20 ± 1 | 67 ± 3 | 14 ± 2 | 87 ± 1 | 83 ± 2 |
| DE-625 | 2 | 0 ± 2 | 3 ± 4 | 43 ± 17 | 13 ± 12 | 82 ± 0 | 72 ± 1 |
| DE-3657 | 2 | 6 ± 9 | 5 ± 7 | 97 ± 0 | 67 ± 8 | 95 ± 1 | 85 ± 3 |
| DE-6820 | 2 | 11 ± 16 | 20 ± 11 | 97 ± 0 | 75 ± 6 | 89 ± 1 | 81 ± 3 |
| DE-14574 | 2 | 28 ± 5 | 18 ± 7 | 92 ± 1 | 70 ± 2 | 87 ± 2 | 73 ± 0 |
Based on three independent experiments, the percent inhibition values at 15 or 5 μM are given, along with standard deviations.
The efficacy of benzavir-2 against acyclovir-resistant isolates is in the low micromolar range.
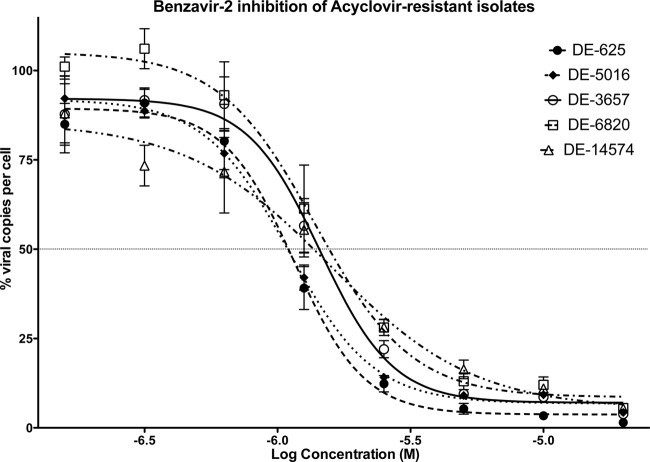
All the previous results indicated that benzavir-1 and benzavir-2 were potent inhibitors of HSV replication and that they were active against acyclovir-resistant isolates of HSV. In addition, our previous structure-activity relationship analysis of benzavir-1 had indicated that benzavir-2 was a more potent inhibitor of HAdV replication than benzavir-1 (42). In the present study, on HSV replication, the results also indicated that benzavir-2 is a more potent antiviral agent than benzavir-1 (Fig. 2 and 3), and interestingly, it was also more effective against acyclovir-resistant isolates (Table 2). We therefore concentrated on benzavir-2 and established its efficacy on acyclovir-resistant isolates. As seen in Table 3 and Fig. 4, benzavir-2 showed high potency against all acyclovir-resistant isolates, with an average EC50 of ∼1.3 μM for all isolates.
Table 3.
The efficacy of benzavir-2 against acyclovir-resistant HSV isolatesa
| Acyclovir-resistant HSV |
Benzavir-2 EC50 | |
|---|---|---|
| Isolate | Type | |
| DE-5016 | 1 | 1.1 μM |
| DE-625 | 2 | 1.2 μM |
| DE-3657 | 2 | 1.4 μM |
| DE-6820 | 2 | 1.4 μM |
| DE-14574 | 2 | 1.6 μM |
The EC50 values of benzavir-2 are based on three independent experiments with each concentration point in duplicate. Concentrations ranging from 20 μM to 0.156 μM, in 2-fold dilution steps (Fig. 4), were tested, and the EC50 values were calculated with nonlinear regression (log concentration of the test compound against response) with variable slopes.
Fig 4.
Dose-response curves for benzavir-2 with acyclovir-resistant isolates. Only small differences in efficacy were observed for benzavir-2 between the acyclovir-resistant isolates. The effects on the ratios of the numbers of viral copies per cell in treated and untreated cells, expressed in percentages with increasing concentrations of the test compounds, are shown. The EC50s for each isolate are given in Table 3. Each concentration point was assessed in duplicate and was analyzed by qPCR with three independent repetitions.
The ratio between the antiviral activity and the cellular toxicity of benzavir-2 is high.
When the antiviral activity in cell-based systems is evaluated, a very important factor to be considered is the cellular toxicity. Compounds that interfere with essential cellular pathways or processes will eventually cause cell death. Such compounds will give false-positive results when inhibition of intracellular replication of viruses is analyzed. To ensure that the observed antiviral activities of benzavir-1 and benzavir-2 were not due to toxicity, XTT toxicity tests were performed (45). The XTT toxicity test quantifies the ability of mitochondrial enzymes to reduce 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) to a colored formazan dye. After 24 h of treatment with acyclovir, benzavir-1, and benzavir-2, the percentages of viable GMK cells were assessed (Table 4). We decided to analyze the cellular viability at 20 times (30 μM) and 40 times (60 μM) the EC50 concentration of benzavir-2 due to observed compound precipitation when the concentration reached 150 μM (unpublished data). Acyclovir did not have any negative effect on cell viability at these concentrations. However, benzavir-1 reduced the viability to 87% of DMSO controls at 30 μM and to 63% at 60 μM. Benzavir-2 showed low toxicity and reduced the viability to approximately 90% at 20 times the EC50 concentration and 83% at 40 times the EC50 concentration. This gives a favorable ratio between antiviral activity and toxicity for benzavir-2.
Table 4.
The effect of acyclovir, benzavir-1, and benzavir-2 on cell viabilitya
| Concn | Viability (%; mean ± SD) of: |
||
|---|---|---|---|
| Acyclovir | Benzavir-1 | Benzavir-2 | |
| 30 μM | 99.9 ± 2.5 | 87.3 ± 1.9 | 89.7 ± 2 |
| 60 μM | 99.7 ± 5.7 | 62.6 ± 1.8 | 83.2 ± 2.8 |
Cell viability was assessed in GMK cells using the XTT-based toxicity test at 20 and 40 times the EC50 of benzavir-2. The percent viability values given were normalized against DMSO controls with the same amount of DMSO as used for the compounds.
DISCUSSION
Despite the fact that acyclovir and its derivatives have been successful drugs in the management of HSV infections, HSV remains a major global health problem. It causes great morbidity in immunocompetent hosts and is associated with fatality in immunosuppressed patients, and it is a contributory factor in the spread of HIV (39, 62). Few other antiviral agents besides acyclovir are available for HSV, and isolates that are resistant to acyclovir therapy are frequently isolated from immunosuppressed patients (13, 34). Thus, there is an obvious need for new antiviral drugs against HSV infections.
In this study, we assessed the antiviral activity of two compounds (Fig. 1) with previously reported anti-HAdV activity against HSV, another DNA virus of medical importance. Using a whole-cell-based small-molecule screening assay, we have identified benzavir-1 as a novel anti-HAdV compound with low cellular toxicity (2). In the screening, benzavir-1 efficiently reduced the expression of green fluorescent protein (GFP) from a replication-competent GFP-expressing vector based on HAdV type 11. Moreover, benzavir-1 was equally effective against representatives of all HAdV species, with a mean EC50 of 3.5 μM, and according to qPCR was approximately five times more efficient than cidofovir. Subsequently we applied iterative design, synthesis, and biological evaluation to generate structure-activity relationships and the improved compound benzavir-2 (42). Benzavir-2 was found to have an EC50 of 0.6 μM against HAdV type 5 and had a better cell viability profile than benzavir-1. In the present study, we found that benzavir-1 and benzavir-2 inhibited the replication of HSV to the same extent as they inhibited HAdV replication. The potential antiviral activity of benzavir-2 against other types of viruses will be assessed in planned studies. Benzavir-2 had an EC50 similar to that of acyclovir for both HSV-1 and HSV-2 and was approximately seven times more potent than cidofovir on HSV-1. However, the profiles of the dose-response curves differed between benzavir-2, acyclovir, and cidofovir (Fig. 3). Benzavir-1 and benzavir-2 inhibited HSV replication in a sigmoidal manner (with average slope parameters of −4.7 and −3.4, respectively) whereas the inhibitory profiles of acyclovir and cidofovir were more linear (slope parameters of −0.9 and −1.3, respectively). This observed difference in the dose-response curves might indicate that the mechanisms of action of benzavir-1 and benzavir-2 are different from those of acyclovir and cidofovir.
Interestingly, both benzavir-1 and benzavir-2 showed antiviral activities against clinical acyclovir-resistant isolates (Table 2) that were comparable to those against acyclovir-sensitive isolates (Fig. 2). Furthermore, the efficacy of benzavir-2 was the same for acyclovir-sensitive isolates (Fig. 3) and acyclovir-resistant isolates (Table 3 and Fig. 4). Acyclovir is a guanosine analog with a 2-hydroxyethyloxymethyl group that is selectively phosphorylated by the viral HSV thymidine kinase to its monophosphate form. This monophosphate form is further converted by cellular kinases to the triphosphate form, which is selectively incorporated into the virus DNA by the viral DNA polymerase. Due to the absence of a 3′ end, the triphosphate form of acyclovir causes chain termination of viral DNA polymerization (18). The structures of benzavir-1 and benzavir-2 are fundamentally different from the structure of the nucleoside analog acyclovir, suggesting that the mechanism of action for benzavir-1 and benzavir-2 may not be chain determination. All drugs currently used clinically against HSV target viral DNA replication, and it is quite possible that benzavir-2 also interferes with the viral DNA replication machinery. However, given the previously reported anti-HAdV activity of benzavir-2, the mechanism of action cannot, as with acyclovir, be specific for HSV infections. Accordingly, the target of benzavir-2 may well be shared or be homologous in HAdV- and HSV-infected cells. The potential development of resistance against benzavir-2, in both HAdV and HSV isolates, will be addressed in planned studies that may contribute to the elucidation of the target of benzavir-2. Due to the fact that both HAdV and HSV replication are also dependent on the host cell machinery, we cannot rule out a possible interference between benzavir-2 and cellular components. This could explain the observed effect on cell viability in the presence of high concentrations of benzavir-2 (Table 4). Ideally, an antiviral agent should be specific for virally encoded targets or, as for acyclovir, be activated by viral proteins. However, the specificity against the target is generally reduced with increasing drug concentrations. The nonnucleoside pyrophosphate analog foscarnet, which does not require virus-specific intracellular phosphorylation, indiscriminately inhibits cellular DNA polymerases at a 100-fold-greater concentration than viral polymerases (60). Foscarnet has been associated with serious side effects, most commonly nephrotoxicity, which affects 30% of patients (43). The favorable property of acyclovir, the requirement for an initial phosphorylation by the viral thymidine kinase to be activated, increases the specificity of acyclovir dramatically. A 3,000-fold-higher concentration of acyclovir is needed to inhibit the growth of the host cell compared to the inhibition of viral multiplication. However, in its triphosphate form, the selectivity between inhibition of the viral and cellular DNA polymerases is only 10 to 30 times (18). With this in mind, the low effect on the cellular viability of acyclovir (Table 3) can be explained by the absence of initial phosphorylation by the viral thymidine kinase that triggers further phosphorylations and results in the biologically active triphosphate form that terminates the DNA polymerization.
To conclude, both benzavir-1 and benzavir-2 are potent inhibitors of HSV replication, and they are active against acyclovir-sensitive and acyclovir-resistant isolates of both HSV-1 and HSV-2. Benzavir-2 is more potent than benzavir-1, with an efficacy similar to that of acyclovir, and this compound should be assessed further as a prelude to determining potential use in the possible management of acyclovir-resistant HSV infections.
ACKNOWLEDGMENTS
This work was supported by the Swedish Cancer Society (grant 100356), the Swedish Research Council (grant 621-2010-4746), the UCMR Linnaeus program (grant 313-48-09), and the Kempe Foundation (grant SMK-2859).
Footnotes
Published ahead of print 20 August 2012
REFERENCES
- 1. Allard A, Albinsson B, Wadell G. 2001. Rapid typing of human adenoviruses by a general PCR combined with restriction endonuclease analysis. J. Clin. Microbiol. 39:498–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andersson EK, et al. 2010. Small-molecule screening using a whole-cell viral replication reporter gene assay identifies 2-{[2-(benzoylamino)benzoyl]amino}-benzoic acid as a novel antiadenoviral compound. Antimicrob. Agents Chemother. 54:3871–3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bacon TH, Boon RJ, Schultz M, Hodges-Savola C. 2002. Surveillance for antiviral-agent-resistant herpes simplex virus in the general population with recurrent herpes labialis. Antimicrob. Agents Chemother. 46:3042–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bacon TH, Howard BA, Spender LC, Boyd MR. 1996. Activity of penciclovir in antiviral assays against herpes simplex virus. J. Antimicrob. Chemother. 37:303–313 [DOI] [PubMed] [Google Scholar]
- 5. Berardi A, et al. 2011. Neonatal herpes simplex virus. J. Matern. Fetal Neonatal Med. 1(Suppl 24):88–90 [DOI] [PubMed] [Google Scholar]
- 6. Beutner KR. 1995. Valacyclovir: a review of its antiviral activity, pharmacokinetic properties, and clinical efficacy. Antiviral Res. 28:281–290 [DOI] [PubMed] [Google Scholar]
- 7. Blot N, et al. 2000. Treatment of an acyclovir and foscarnet-resistant herpes simplex virus infection with cidofovir in a child after an unrelated bone marrow transplant. Bone Marrow Transplant. 26:903–905 [DOI] [PubMed] [Google Scholar]
- 8. Boon RJ, et al. 2000. Antiviral susceptibilities of herpes simplex virus from immunocompetent subjects with recurrent herpes labialie: a UK-based survey. J. Antimicrob. Chemother. 46:324–325 [DOI] [PubMed] [Google Scholar]
- 9. Boyd MR, Safrin S, Kern ER. 1993. Penciclovir—a review of its spectrum of activity, selectivity, and cross-resistance pattern. Antiviral Chem. Chemother. 4:3–11 [Google Scholar]
- 10. Buxbaum S, et al. 2003. Epidemiology of herpes simplex virus types 1 and 2 in Germany: what has changed? Med. Microbiol. Immunol. 192:177–181 [DOI] [PubMed] [Google Scholar]
- 11. Cantin EM, Chen J, McNeill J, Willey DE, Openshaw H. 1991. Detection of herpes simplex virus DNA sequences in corneal transplant recipients by polymerase chain reaction assays. Curr. Eye Res. 10(Suppl):15–21 [DOI] [PubMed] [Google Scholar]
- 12. Chatis PA, Miller CH, Schrager LE, Crumpacker CS. 1989. Successful treatment with foscarnet of an acyclovir-resistant mucocutaneous infection with herpes simplex virus in a patient with acquired immunodeficiency syndrome. N. Engl. J. Med. 320:297–300 [DOI] [PubMed] [Google Scholar]
- 13. Chen Y, et al. 2000. Resistant herpes simplex virus type 1 infection: an emerging concern after allogeneic stem cell transplantation. Clin. Infect. Dis. 31:927–935 [DOI] [PubMed] [Google Scholar]
- 14. Chretien F, et al. 1996. Herpes simplex virus type 1 encephalitis in acquired immunodeficiency syndrome. Neuropathol. Appl. Neurobiol. 22:394–404 [DOI] [PubMed] [Google Scholar]
- 15. Christophers J, et al. 1998. Survey of resistance of herpes simplex virus to acyclovir in northwest England. Antimicrob. Agents Chemother. 42:868–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coen DM, Schaffer PA. 1980. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc. Natl. Acad. Sci. U. S. A. 77:2265–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corey L, Wald A. 2009. Maternal and neonatal herpes simplex virus infections. N. Engl. J. Med. 361:1376–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elion GB, et al. 1977. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc. Natl. Acad. Sci. U. S. A. 74:5716–5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Englund JA, et al. 1990. Herpes simplex virus resistant to acyclovir. A study in a tertiary care center. Ann. Intern. Med. 112:416–422 [DOI] [PubMed] [Google Scholar]
- 20. Filen F, Strand A, Allard A, Blomberg J, Herrmann B. 2004. Duplex real-time polymerase chain reaction assay for detection and quantification of herpes simplex virus type 1 and herpes simplex virus type 2 in genital and cutaneous lesions. Sex. Transm. Dis. 31:331–336 [DOI] [PubMed] [Google Scholar]
- 21. Fleming DT, et al. 1997. Herpes simplex virus type 2 in the United States, 1976 to 1994. N. Engl. J. Med. 337:1105–1111 [DOI] [PubMed] [Google Scholar]
- 22. Flomenberg P, et al. 1994. Increasing incidence of adenovirus disease in bone marrow transplant recipients. J. Infect. Dis. 169:775–781 [DOI] [PubMed] [Google Scholar]
- 23. Gaudreau A, Hill E, Balfour HH, Jr, Erice A, Boivin G. 1998. Phenotypic and genotypic characterization of acyclovir-resistant herpes simplex viruses from immunocompromised patients. J. Infect. Dis. 178:297–303 [DOI] [PubMed] [Google Scholar]
- 24. Greenberg MS, et al. 1987. A comparative study of herpes simplex infections in renal transplant and leukemic patients. J. Infect. Dis. 156:280–287 [DOI] [PubMed] [Google Scholar]
- 25. Guo A, Hu P, Balimane PV, Leibach FH, Sinko PJ. 1999. Interactions of a nonpeptidic drug, valacyclovir, with the human intestinal peptide transporter (hPEPT1) expressed in a mammalian cell line. J. Pharmacol. Exp. Ther. 289:448–454 [PubMed] [Google Scholar]
- 26. Haddow LJ, et al. 2006. Increase in rates of herpes simplex virus type 1 as a cause of anogenital herpes in western Sydney, Australia, between 1979 and 2003. Sex. Transm. Infect. 82:255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heid CA, Stevens J, Livak KJ, Williams PM. 1996. Real time quantitative PCR. Genome Res. 6:986–994 [DOI] [PubMed] [Google Scholar]
- 28. Heim A. 2011. Advances in the management of disseminated adenovirus disease in stem cell transplant recipients: impact of adenovirus load (DNAemia) testing. Expert Rev. Anti Infect. Ther. 9:943–945 [DOI] [PubMed] [Google Scholar]
- 29. Hjalmarsson A, Blomqvist P, Skoldenberg B. 2007. Herpes simplex encephalitis in Sweden, 1990–2001: incidence, morbidity, and mortality. Clin. Infect. Dis. 45:875–880 [DOI] [PubMed] [Google Scholar]
- 30. Khetsuriani N, Holman RC, Anderson LJ. 2002. Burden of encephalitis-associated hospitalizations in the United States, 1988–1997. Clin. Infect. Dis. 35:175–182 [DOI] [PubMed] [Google Scholar]
- 31. Kimberlin DW. 2007. Management of HSV encephalitis in adults and neonates: diagnosis, prognosis and treatment. Herpes 14:11–16 [PubMed] [Google Scholar]
- 32. La Rosa AM, et al. 2001. Adenovirus infections in adult recipients of blood and marrow transplants. Clin. Infect. Dis. 32:871–876 [DOI] [PubMed] [Google Scholar]
- 33. Lafferty WE, Coombs RW, Benedetti J, Critchlow C, Corey L. 1987. Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N. Engl. J. Med. 316:1444–1449 [DOI] [PubMed] [Google Scholar]
- 34. Langston AA, et al. 2002. Development of drug-resistant herpes simplex virus infection after haploidentical hematopoietic progenitor cell transplantation. Blood 99:1085–1088 [DOI] [PubMed] [Google Scholar]
- 35. Liesegang TJ. 2001. Herpes simplex virus epidemiology and ocular importance. Cornea 20:1–13 [DOI] [PubMed] [Google Scholar]
- 36. Matthews JT, Carroll RD, Stevens JT, Haffey ML. 1989. In vitro mutagenesis of the herpes simplex virus type 1 DNA polymerase gene results in altered drug sensitivity of the enzyme. J. Virol. 63:4913–4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meyers JD, Flournoy N, Thomas ED. 1980. Infection with herpes simplex virus and cell-mediated immunity after marrow transplant. J. Infect. Dis. 142:338–346 [DOI] [PubMed] [Google Scholar]
- 38. Miller WH, Miller RL. 1982. Phosphorylation of acyclovir diphosphate by cellular enzymes. Biochem. Pharmacol. 31:3879–3884 [DOI] [PubMed] [Google Scholar]
- 39. Moulignier A, et al. 1996. Fatal brain stem encephalitis due to herpes simplex virus type 1 in AIDS. J. Neurol. 243:491–493 [DOI] [PubMed] [Google Scholar]
- 40. Nahmias AJ, Lee FK, Beckman-Nahmias S. 1990. Sero-epidemiological and -sociological patterns of herpes simplex virus infection in the world. Scand. J. Infect. Dis. Suppl. 69:19–36 [PubMed] [Google Scholar]
- 41. Naik HR, Siddique N, Chandrasekar PH. 1995. Foscarnet therapy for acyclovir-resistant herpes simplex virus 1 infection in allogeneic bone marrow transplant recipients. Clin. Infect. Dis. 21:1514–1515 [DOI] [PubMed] [Google Scholar]
- 42. Oberg CT, et al. 2012. Synthesis, biological evaluation, and structure-activity relationships of 2-[2-(benzoylamino)benzoylamino]-benzoic acid analogs as inhibitors of adenovirus replication. J. Med. Chem. 55:3170–3181 [DOI] [PubMed] [Google Scholar]
- 43. Razonable RR. 2011. Antiviral drugs for viruses other than human immunodeficiency virus. Mayo Clin. Proc. 86:1009–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reardon JE, Spector T. 1989. Herpes simplex virus type 1 DNA polymerase. Mechanism of inhibition by acyclovir triphosphate. J. Biol. Chem. 264:7405–7411 [PubMed] [Google Scholar]
- 45. Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. 1991. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J. Immunol. Methods 142:257–265 [DOI] [PubMed] [Google Scholar]
- 46. Safrin S, et al. 1994. Foscarnet-resistant herpes simplex virus infection in patients with AIDS. J. Infect. Dis. 169:193–196 [DOI] [PubMed] [Google Scholar]
- 47. Saijo M, et al. 2002. Bone marrow transplantation in a child with Wiskott-Aldrich syndrome latently infected with acyclovir-resistant (ACV(r)) herpes simplex virus type 1: emergence of foscarnet-resistant virus originating from the ACV (r) virus. J. Med. Virol. 68:99–104 [DOI] [PubMed] [Google Scholar]
- 48. Samra Z, Scherf E, Dan M. 2003. Herpes simplex virus type 1 is the prevailing cause of genital herpes in the Tel Aviv area, Israel. Sex. Transm. Dis. 30:794–796 [DOI] [PubMed] [Google Scholar]
- 49. Schacker T, Zeh J, Hu HL, Hill E, Corey L. 1998. Frequency of symptomatic and asymptomatic herpes simplex virus type 2 reactivations among human immunodeficiency virus-infected men. J. Infect. Dis. 178:1616–1622 [DOI] [PubMed] [Google Scholar]
- 50. Schaeffer HJ, et al. 1978. 9-(2-Hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature 272:583–585 [DOI] [PubMed] [Google Scholar]
- 51. Seidemann K, et al. 2004. Monitoring of adenovirus infection in pediatric transplant recipients by quantitative PCR: report of six cases and review of the literature. Am. J. Transplant. 4:2102–2108 [DOI] [PubMed] [Google Scholar]
- 52. Shields AF, Hackman RC, Fife KH, Corey L, Meyers JD. 1985. Adenovirus infections in patients undergoing bone-marrow transplantation. N. Engl. J. Med. 312:529–533 [DOI] [PubMed] [Google Scholar]
- 53. Siegal FP, et al. 1981. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N. Engl. J. Med. 305:1439–1444 [DOI] [PubMed] [Google Scholar]
- 54. Singh N, et al. 1988. Infections with cytomegalovirus and other herpesviruses in 121 liver transplant recipients: transmission by donated organ and the effect of OKT3 antibodies. J. Infect. Dis. 158:124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith JS, Robinson NJ. 2002. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J. Infect. Dis. 186(Suppl 1):S3–S28 [DOI] [PubMed] [Google Scholar]
- 56. Sutton D, Boyd MR. 1993. Comparative activity of penciclovir and acyclovir in mice infected intraperitoneally with herpes simplex virus type 1 SC16. Antimicrob. Agents Chemother. 37:642–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tyring SK, Baker D, Snowden W. 2002. Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years' experience with acyclovir. J. Infect. Dis. 186(Suppl 1):S40–S46 [DOI] [PubMed] [Google Scholar]
- 58. Umar SH, Kanth A. 1999. Disseminated cutaneous herpes simplex virus type-1 with interstitial pneumonia as a first presentation of AIDS. J. Natl. Med. Assoc. 91:471–474 [PMC free article] [PubMed] [Google Scholar]
- 59. Vere Hodge RA, Sutton D, Boyd MR, Harnden MR, Jarvest RL. 1989. Selection of an oral prodrug (BRL 42810; famciclovir) for the antiherpesvirus agent BRL 39123 [9-(4-hydroxy-3-hydroxymethylbut-l-yl)guanine; penciclovir]. Antimicrob. Agents Chemother. 33:1765–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wagstaff AJ, Bryson HM. 1994. Foscarnet. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with viral infections. Drugs 48:199–226 [DOI] [PubMed] [Google Scholar]
- 61. Wagstaff AJ, Faulds D, Goa KL. 1994. Aciclovir. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs 47:153–205 [DOI] [PubMed] [Google Scholar]
- 62. Wald A, Link K. 2002. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J. Infect. Dis. 185:45–52 [DOI] [PubMed] [Google Scholar]
- 63. Wald A, et al. 2000. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N. Engl. J. Med. 342:844–850 [DOI] [PubMed] [Google Scholar]
- 64. Weller S, et al. 1993. Pharmacokinetics of the acyclovir pro-drug valaciclovir after escalating single- and multiple-dose administration to normal volunteers. Clin. Pharmacol. Ther. 54:595–605 [DOI] [PubMed] [Google Scholar]
- 65. Whitley RJ. 2006. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 71:141–148 [DOI] [PubMed] [Google Scholar]
- 66. Whitley RJ, Roizman B. 2001. Herpes simplex virus infections. Lancet 357:1513–1518 [DOI] [PubMed] [Google Scholar]