Abstract
There are an increasing number of indications for trimethoprim-sulfamethoxazole use, including skin and soft tissue infections due to community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Assessing the relationship between rates of use and antibiotic resistance is important for maintaining the expected efficacy of this drug for guideline-recommended conditions. Using interrupted time series analysis, we aimed to determine whether the 2005 emergence of CA-MRSA and recommendations of trimethoprim-sulfamethoxazole as the preferred therapy were associated with changes in trimethoprim-sulfamethoxazole use and susceptibility rates. The data from all VA Boston Health Care System facilities, including 118,863 inpatient admissions, 6,272,661 outpatient clinic visits, and 10,138 isolates were collected over a 10-year period. There was a significant (P = 0.02) increase in trimethoprim-sulfamethoxazole prescriptions in the post-CA-MRSA period (1,605/year) compared to the pre-CA-MRSA period (1,538/year). Although the overall susceptibility of Escherichia coli and Proteus spp. to trimethoprim-sulfamethoxazole decreased over the study period, the rate of change in the pre- versus the post-CA-MRSA period was not significantly different. The changes in susceptibility rates of S. aureus to trimethoprim-sulfamethoxazole and to methicillin were also not significantly different. The CA-MRSA period is associated with a significant increase in use of trimethoprim-sulfamethoxazole but not with significant changes in the rates of susceptibilities among clinical isolates. There is also no evidence for selection of organisms with increased resistance to other antimicrobials in relation to increased trimethoprim-sulfamethoxazole use.
INTRODUCTION
Trimethoprim-sulfamethoxazole (TMP/SMX) is a combination sulfonamide antibiotic recommended as first-line treatment of uncomplicated urinary tract infections (UTI), skin and soft tissue infections, and community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infections by clinical practice guidelines (14, 21, 30). This broad-spectrum agent is also used for treatment of respiratory infections, as well as for prophylaxis in immunocompromised patients at risk for Pneumocystis jiroveci-associated pneumonia (11, 12, 19). Given these multiple potential uses, it is likely that the prescribing of TMP/SMX has increased over the past decade (9).
Although increasing utilization of this drug could be viewed as a positive performance outcome, it is also possible that use is contributing to increasing resistance rates (8). As with many antimicrobials, the development of in vitro resistance to TMP/SMX is considered to be, at least in part, a function of use (4, 5, 10, 20, 26). If so, the benefit of this drug for clinical practice guideline recommended conditions could potentially be compromised over time. Already, national surveys of clinical isolates estimate that >20% of outpatient urinary Escherichia coli isolates from women are resistant to TMP/SMX (4, 16, 27). These rates vary by geographic region and may not reflect the true resistance prevalence in uncomplicated cystitis but nonetheless support the notion that resistance is increasing among Gram-negative clinical isolates (13). Resistance among Gram-positive strains is less prevalent, with most CA-MRSA strains remaining susceptible to TMP/SMX (2). Thus, the associations between use and resistance rates are not clearly understood and may vary by organism (26).
Another impact of increases in TMP/SMX use could be selection or propagation of organisms with resistance to other antimicrobials, also known as collateral damage (24). Children receiving TMP/SMX were found to have higher rates of multidrug-resistant Enterobacteriaceae in their intestinal flora compared to placebo-treated children, possibly through selection of an integron carrying multiple resistance genes (33). However, a recent systematic review did not find a relationship between TMP/SMX prophylaxis and other resistant microbial flora in patients with HIV infection (29). The collateral effects of antibiotic use on the human microbiome are increasingly being recognized as important contributors to multidrug resistance, and studies evaluating the impact of various drugs on the susceptibility rates of clinical isolates over extended periods are needed to promote stewardship and optimize guidelines (32).
If TMP/SMX use is linearly related to increasing resistance, the risks of the drug may outweigh the benefits, at least in certain populations and certain conditions for which alternative agents are available. Thus, the relationship between the use of TMP/SMX and resistance to itself as well as other antimicrobial agents has potential significance for clinicians as well as for treatment guidelines and stewardship policies. The goal of this study was to evaluate the susceptibility rates to TMP/SMX and to other antimicrobials over 10 years spanning the 2005 period when the recognition of CA-MRSA was increasing and recommendations to front-line clinicians from guidelines and other publications advocated TMP/SMX use in patients with skin and soft tissue infections (7, 15, 18). We hypothesized that TMP/SMX use increased after this CA-MRSA emergence period and that susceptibility rates changed but not necessarily in direct temporal correlation to use.
(Presented in part at the American Society of Health System Pharmacists Midyear Clinical Meeting on 7 December 2011, in New Orleans, LA.)
MATERIALS AND METHODS
Study population.
All patients receiving care from the VA Boston Health Care System (VABHS) during the years 2001 through 2010 were eligible to be included in the database. VABHS is an integrated health care system consisting of a 250-bed inpatient acute care hospital, a long-term care hospital, and six community-based outpatient clinics (CBOCs) throughout Massachusetts. VABHS receives more than 10,000 inpatient admissions annually and serves as one of the major medical centers for veterans in New England.
Data collection.
Aggregate data for inpatient and outpatient oral TMP/SMX orders at VABHS from 2001 to 2010 were collected using the Veterans Health Information Systems and Technology Architecture (VISTA) database. Intravenous orders for TMP/SMX were also available but represented a minor fraction of total orders and thus were not included in usage rates. The inpatient order data were adjusted for annual inpatient admissions. The outpatient prescription data were not adjusted for outpatient visit frequency because a large proportion of outpatient visits are nonmedical (e.g., mental health visits and lab draws) and could not be separated from medical visits.
The clinical microbiology laboratory provided annual aggregate data on the rates of susceptibility of commonly encountered pathogens to a panel of Gram-positive and Gram-negative spectrum agents, including TMP/SMX. All isolates are routinely tested for susceptibility to TMP/SMX by an automated MIC system (Microscan) and are interpreted based on Clinical and Laboratory Standards Institute (CLSI) criteria. The data for years 2001 through 2010 were collected from the antibiogram published yearly at VABHS. The antibiogram is compiled from pooled clinical isolates from the three main campuses, Jamaica Plain (outpatient), West Roxbury (acute care), and Brockton (long-term care), as well as a smaller fourth campus in Bedford. MA, and the six CBOCs. In the years 2005 to 2006, aggregate rate calculations for the antibiogram were modified so that only one isolate per patient was included per year, which is consistent with current CLSI recommendations.
To evaluate whether TMP/SMX use increased specifically for treatment of skin and soft tissue infections, a manual chart review was conducted on 240 inpatients (96 before and 144 after the CA-MRSA emergence period) using the Computerized Patient Record System (CPRS) database within VABHS. All study procedures were approved by the VA Boston Institutional Review Board.
Statistical methods.
Because annual observations are not independent, interrupted-time series analysis, which factors in autocorrelations, was used to compare trends in TMP/SMX susceptibility rates and utilization rates pre- and postintervention (25, 28). The intervention was defined as the emergence of CA-MRSA and the publication of expert recommendations for TMP/SMX use as the preferred therapy for skin and soft tissue infections in 2005 (30). The percentage of susceptible clinical isolates was plotted out from 2001 to 2010. Given the small number of data points, in order to obtain a stable index and a reliable estimate of standard error, we used a double-bootstrap procedure recommended by McKnight et al. (23). This permitted a reliable comparison of both pre- and postintervention means (intercepts) and changes in slopes (rates).
RESULTS
TMP/SMX orders from 118,863 inpatient admissions and 6,272,661 outpatient clinic visits over a 10-year period were captured. Resistance data were calculated from a total of 10,138 Gram-positive and Gram-negative clinical isolates from the same time period.
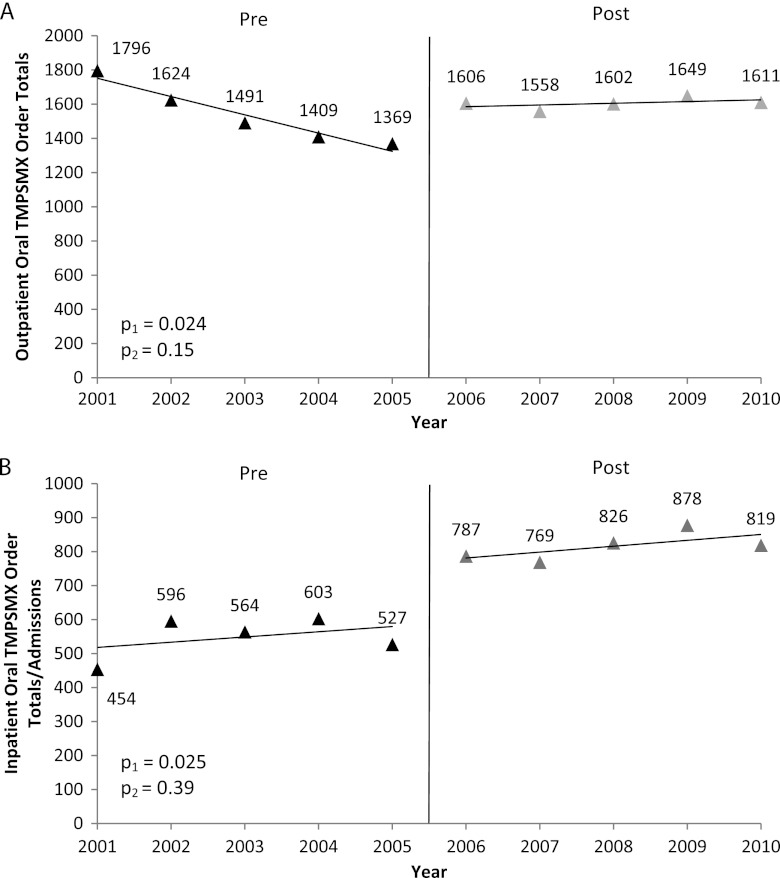
A total of 15,715 orders for TMP/SMX were written in the outpatient setting. The average number of outpatient orders from 2001 to 2005 was 1,538/year and increased to 1,605/year from 2006 to 2010. The averages were significantly different before and after the intervention period (P = 0.024), but the slopes did not differ (Fig. 1A). A total of 6,823 inpatient orders for TMP/SMX were written for patients while admitted to VABHS from 2001 to 2010. A comparison of the preintervention average to the postintervention average of TMP/SMX orders revealed that inpatient use had significantly increased since 2005 (P = 0.025) (Fig. 1B). When TMP/SMX orders were adjusted for the annual VABHS admission rates, the increase in TMP/SMX utilization was still apparent (P = 0.014), with a rate of 35 orders per 1,000 admissions in 2001 rising to 70 orders per 1,000 admissions in 2010.
Fig 1.
Annual outpatient and inpatient TMP/SMX orders. The average number of outpatient orders for TMP/SMX (A) and the average number of inpatient orders (B) at VABHS increased significantly (p1 [comparison of pre- and postintervention intercepts {means}]) after the CA-MRSA emergence period compared to before the CA-MRSA emergence period (interrupted time series analyses) but did not differ in slope before and after the event (p2 [comparison of pre- and postintervention slopes {trends}]). The vertical line indicates the emergence of CA-MRSA.
In the subset of patients evaluated by manual chart review, skin and soft tissue infections were the reason for the TMP/SMX order in 10.4% of patients in the pre-CA-MRSA period compared to 28.5% of patients in the post-CA-MRSA period (P = 0.004). UTI was the other major reason for TMP/SMX use, and the incidence of UTI decreasing as the indication for use from 66.7% to 53.5% in the pre versus post period.
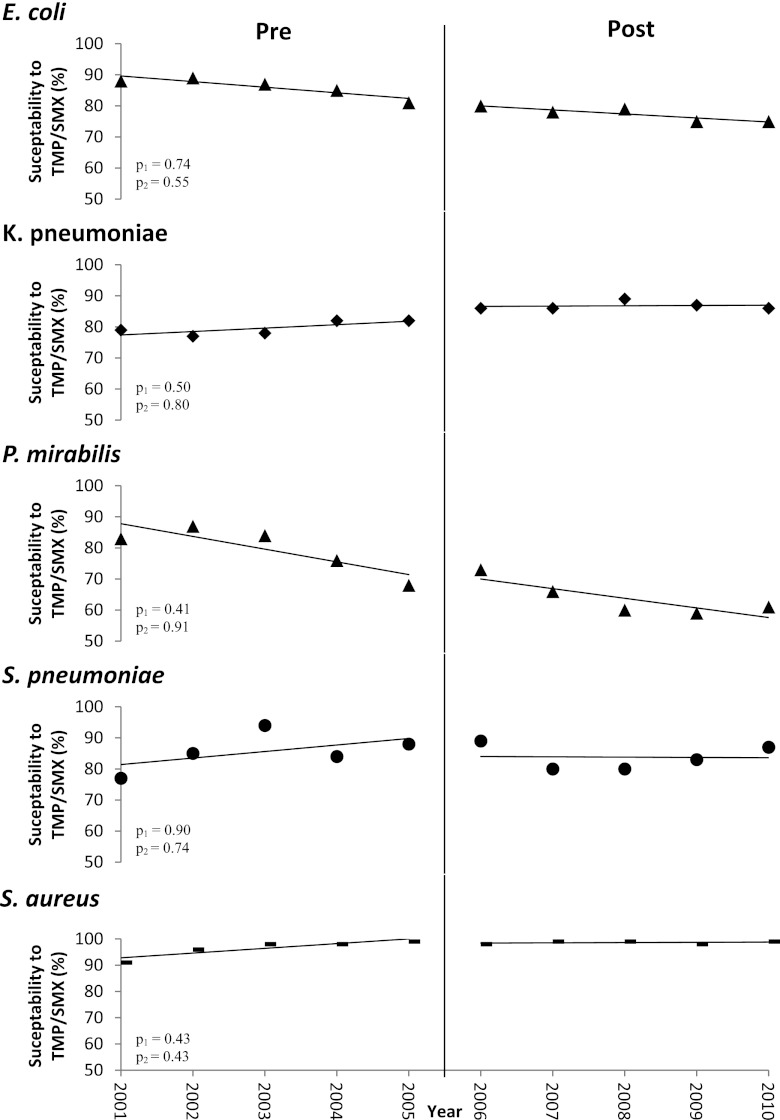
The rates of susceptibility to TMP/SMX varied among Gram-negative and Gram-positive organisms (Fig. 2). We examined the differences in means, adjusted for autocorrelation, as well as the differences in slopes related to the guideline intervention. Over the 10-year study period, the mean TMP/SMX susceptibility decreased from 88 to 75% for E. coli (P = 0.55) and from 65 to 61% for Proteus mirabilis (P = 0.91). The mean TMP/SMX susceptibility increased from 79 to 86% in Klebsiella pneumoniae (P = 0.80) and from 77 to 87% in Streptococcus pneumoniae (P = 0.74). S. aureus remained highly susceptible to TMP/SMX (P = 0.43). However, when the harmonic means were adjusted for autocorrelations, none of these differences were significant. In addition, none of the differences in the trends in susceptibility rates of the isolates evaluated changed significantly in the pre- versus post-CA-MRSA emergence period: E. coli (P = 0.74), P. mirabilis (P = 0.41), K. pneumoniae (P = 0.50), S. pneumonia (P = 0.90), and S. aureus (P = 0.43) (Fig. 2).
Fig 2.
Annual percentage of clinical isolates susceptible to TMP/SMX. The susceptibility rates of clinical isolates to TMP/SMX within VABHS changed over the 10-year period but were not significant (p1 [comparison of pre- and postintervention intercepts]). The trends in change (p2 [comparison of pre- and postintervention slopes]) also were not significantly different after the emergence of CA-MRSA compared to before the emergence of CA-MRSA (interrupted time series analyses). The vertical line indicates the emergence of CA-MRSA.
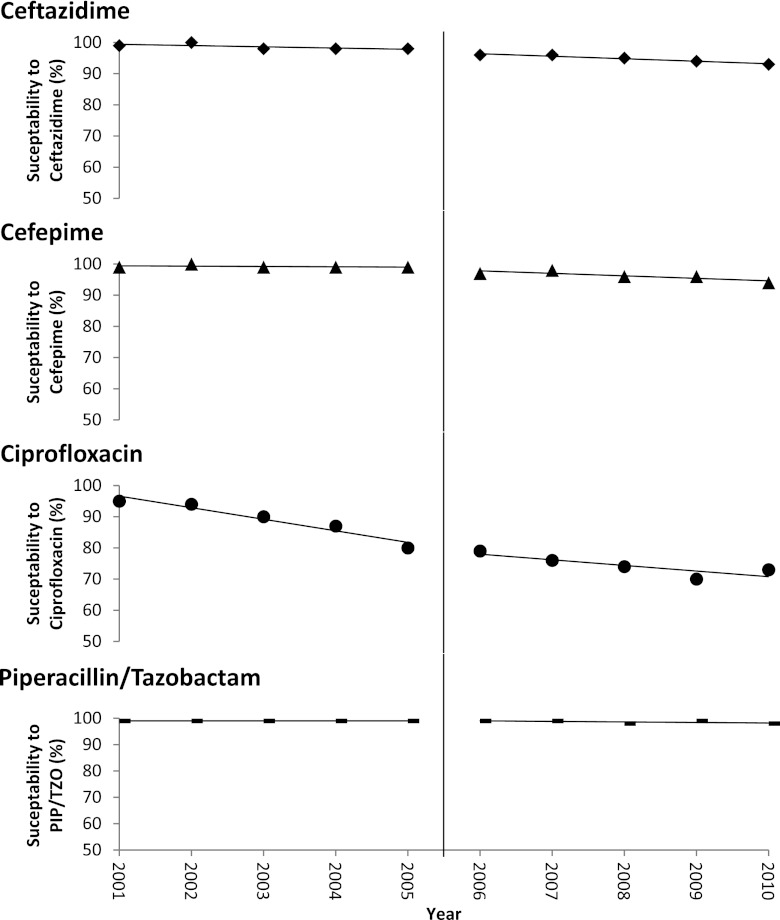
The rates of bacterial susceptibility to other agents were also examined in relation to the CA-MRSA-related recommendations for TMP/SMX use. E. coli susceptibility rates to ceftazidime, cefepime, piperacillin-tazobactam, and ciprofloxacin each changed over the 10-year period, but the changes were not significantly different in the preintervention period compared to the postintervention period for any of the drugs (Fig. 3). A similar evaluation for methicillin susceptibility among S. aureus also did not reveal a significant change before and after the intervention period (data not shown).
Fig 3.
Annual percentage of E. coli clinical isolates susceptible to selected antibiotics. The susceptibility rates of E. coli clinical isolates to TMP/SMX within VABHS changed over the 10-year period, but the trends in change were not significantly different after the emergence of CA-MRSA compared to before the emergence of CA-MRSA (interrupted time series analyses). The vertical line indicates the emergence of CA-MRSA. PIP/TZO, piperacillin-tazobactam.
DISCUSSION
The relationship between TMP/SMX use and resistance is complex, but it is important to understand given increased opportunities for use of this drug. The results of the present study demonstrate that the rates of TMP/SMX use within the VABHS were higher in the 5-year period following the recognition of TMP/SMX as a preferred agent for skin and soft tissue infections due to emergence of CA-MRSA. Most importantly, we did not find a corresponding statistically significant change in the rates of susceptibility to TMP/SMX in temporal correlation to CA-MRSA emergence, suggesting that the changes in susceptibility in the post-CA-MRSA period were just the continuation of changes in susceptibility that were already occurring prior to that event. Previous studies have demonstrated that the use of TMP/SMX is associated with increasing antibiotic resistance, but few have used interrupted time series analyses to correlate a specific event such as a emergence of a new pathogen to changing trends in use and resistance. Our study has the advantages of including data from an integrated health care system (most veterans using VABHS inpatient facilities return for subsequent inpatient as well as outpatient care) and a 10-year study period.
The development of resistance to TMP/SMX, as with most antibiotics, is likely a multifactorial process and is not completely understood (20, 26, 32). Previous observational studies have reported an association between recent TMP/SMX use and the isolation of a resistant uropathogen in individual outpatients with UTI (4). Confounding of the results caused by including patients that have recently failed TMP/SMX therapy due to a resistant uropathogen has been a consideration, since not all studies have been consistent in this finding (3, 4, 31). Prolonged TMP/SMX use as prophylaxis in patients with HIV has been associated with the development of TMP/SMX-resistant flora, including E. coli and S. aureus (22). Exposure to other antimicrobials may also contribute to TMP/SMX resistance through selection for plasmids containing multiple antibiotic resistance genes. This has been the explanation for the observation that restrictions on the use of TMP/SMX are not correlated with temporally associated reductions in resistance rates (1, 6, 34). These studies suggest that the use of other antibiotics in place of TMP/SMX (or trimethoprim alone) may have continued to select for TMP/SMX-resistant organisms.
TMP/SMX is also considered to potentially have significant collateral damage, namely, the selection or propagation of resistant microorganisms on a population level (24, 32). The mechanisms are not fully elucidated, but at least one study found that the sulfamethoxazole component selected for a class 1 integron, resulting in multidrug-resistant Enterobacteriaceae in the intestinal flora of children (33). Integrons are transferable genetic elements capable of expressing multiple resistance genes in cassette structures and are strongly associated with multidrug resistance (33). Concerns over increased resistance and the collateral effects of TMP/SMX use has led to the removal of TMP/SMX as a recommended agent from some guidelines (35). However, a recent systematic review did not find an increase in bacterial resistance to other antimicrobial classes with the use of TMP/SMX as prophylaxis in patients with HIV (29). In fact, TMP/SMX prophylaxis was found to be potentially protective against methicillin resistance in S. aureus and penicillin resistance in S. pneumoniae (29). Our data support the lack of association between increased TMP/SMX use and increased resistance to other drugs, including β-lactams and fluoroquinolones among E. coli and methicillin in S. aureus.
TMP/SMX is effective for many different types of infections (14, 21). Our findings suggest that increases in TMP/SMX use temporally related to CA-MRSA emergence and guideline recommendations do not correlate with the development of antibiotic resistance in our study population. The data suggest that, at baseline, resistance rates to TMP/SMX were already increasing, and the further increase is not statistically significantly linked to the further increase in TMP/SMX use. A major strength is the use of interrupted time series analysis with bootstrapping to adjust for autocorrelation and generate reliable error estimates. If we had simply used a paired t test to compare the before and after mean rates of resistance, we would have erroneously concluded that resistance had significantly changed in association with the increased use. However, the apparent difference in means is not significant when properly adjusting for autocorrelation and comparing slopes before and after the guideline.
Since our study was conducted in a predominantly adult male veteran population, the findings may not be applicable in children or women, although we are unaware of gender differences in the mechanisms of TMP/SMX resistance development. Our population represents a wide geographic region, and thus it is possible that some patients received care that included a TMP/SMX order or isolation of a resistant pathogen outside the VABHS. This is minimized by capturing both prescription and microbiology data from the outlying community clinics. Our measure of use was collected as the number of TMP/SMX prescriptions (and orders for inpatients) rather than the defined daily dose in unique patients. Although infrequent, if a patient was transferred between units, any reordered TMP/SMX would be counted as a new order. We reduced such redundancy by excluding intravenous orders since they most often are followed by an oral stepdown order. Our microbiology data were determined by the existing antibiograms, and thus changes in policies in the laboratory, such as the use of unique isolates or breakpoints for interpreting resistance, were not factors that we could account for. In general, breakpoints for TMP/SMX susceptibility did not change in this study period, and the antibiograms were consistent with concurrent CLSI guidelines.
Conclusion.
Although the use of TMP/SMX has increased significantly at VABHS since the emergence of CA-MRSA and recommendations for TMP/SMX as first-line therapy, the resistance trends of clinical pathogens do not appear to correlate temporally with these recommendations. Our findings support use of TMP/SMX as indicated by expert-based guidelines, while maintaining caution against misuse and overuse, and continuing to assess resistance rates, particularly among Gram-negative flora (17).
ACKNOWLEDGMENTS
We thank Daniel Leavitt and John Roefaro for their help and guidance throughout this study.
K.G. chaired one of the guidelines (uncomplicated UTI) recommending TMP/SMX as a first-line agent. She did not receive any financial compensation for the work and has no financial relationship with the sponsoring foundation (Infectious Diseases Society of America). K.G. has received honoraria for presentation of a CME-approved lecture on ID-related guidelines to the American College of Physicians Primary Care Conference (Pri-Med). K.G. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Published ahead of print 20 August 2012
REFERENCES
- 1. Bean DC, Livermore DM, Hall LM. 2009. Plasmids imparting sulfonamide resistance in Escherichia coli: implications for persistence. Antimicrob. Agents Chemother. 53:1088–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chua K, Laurent F, Coombs G, Grayson ML, Howden BP. 2011. Antimicrobial resistance: not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician's guide to community MRSA—its evolving antimicrobial resistance and implications for therapy. Clin. Infect. Dis. 52:99–114 [DOI] [PubMed] [Google Scholar]
- 3. Colgan R, Johnson JR, Kuskowski M, Gupta K. 2008. Risk factors for trimethoprim-sulfamethoxazole resistance in patients with acute uncomplicated cystitis. Antimicrob. Agents Chemother. 52:846–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. 2010. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 340:c2096. [DOI] [PubMed] [Google Scholar]
- 5. Cunha BA. 2012. Prophylaxis for recurrent urinary tract infections: nitrofurantoin, not trimethoprim-sulfamethoxazole or cranberry juice. Arch. Intern. Med. 172:82–83 [DOI] [PubMed] [Google Scholar]
- 6. Enne VI, Livermore DM, Stephens P, Hall LM. 2001. Persistence of sulphonamide resistance in Escherichia coli in the UK despite national prescribing restriction. Lancet 357:1325–1328 [DOI] [PubMed] [Google Scholar]
- 7. Fridkin SK, et al. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436–1444 [DOI] [PubMed] [Google Scholar]
- 8. Gaynes RP. 2010. Preserving the effectiveness of antibiotics. JAMA 303:2293–2294 [DOI] [PubMed] [Google Scholar]
- 9. Goldberg E, Bishara J. 2012. Contemporary unconventional clinical use of co-trimoxazole. Clin. Microbiol. Infect. 18:8–17 [DOI] [PubMed] [Google Scholar]
- 10. Gonzales R, Steiner JF, Lum A, Barrett PH., Jr 1999. Decreasing antibiotic use in ambulatory practice: impact of a multidimensional intervention on the treatment of uncomplicated acute bronchitis in adults. JAMA 281:1512–1519 [DOI] [PubMed] [Google Scholar]
- 11. Green H, Paul M, Vidal L, Leibovici L. 2007. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst. Rev:CD005590. [DOI] [PubMed] [Google Scholar]
- 12. Grimwade K, Swingler 2003. Cotrimoxazole prophylaxis for opportunistic infections in adults with HIV. Cochrane Database Syst. Rev:CD003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta K, Hooton TM, Miller L. 2011. Managing uncomplicated urinary tract infection: making sense out of resistance data. Clin. Infect. Dis. 53:1041–1042 [DOI] [PubMed] [Google Scholar]
- 14. Gupta K, et al. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 52:e103–120 [DOI] [PubMed] [Google Scholar]
- 15. Gupta K, Macintyre A, Vanasse G, Dembry LM. 2007. Trends in prescribing beta-lactam antibiotics for treatment of community-associated methicillin-resistant Staphylococcus aureus infections. J. Clin. Microbiol. 45:3930–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta K, Scholes D, Stamm WE. 1999. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA 281:736–738 [DOI] [PubMed] [Google Scholar]
- 17. Hughes JM. 2011. Preserving the lifesaving power of antimicrobial agents. JAMA 305:1027–1028 [DOI] [PubMed] [Google Scholar]
- 18. King MD, et al. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA300 clone as the predominant cause of skin and soft-tissue infections. Ann. Intern. Med. 144:309–317 [DOI] [PubMed] [Google Scholar]
- 19. Korbila IP, Manta KG, Siempos II, Dimopoulos G, Falagas ME. 2009. Penicillins versus trimethoprim-based regimens for acute bacterial exacerbations of chronic bronchitis: meta-analysis of randomized controlled trials. Can. Family Physician 55:60–67 [PMC free article] [PubMed] [Google Scholar]
- 20. Levy SB. 2008. Genome size and antibiotic resistance. Antimicrob. Agents Chemother. 52:2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu C, et al. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin. Infect. Dis. 52:285–292 [DOI] [PubMed] [Google Scholar]
- 22. Martin JN, et al. 1999. Emergence of trimethoprim-sulfamethoxazole resistance in the AIDS era. J. Infect. Dis. 180:1809–1818 [DOI] [PubMed] [Google Scholar]
- 23. McKnight SD, McKean JW, Huitema BE. 2000. A double bootstrap method to analyze linear models with autoregressive error terms. Psychol. Methods 5:87–101 [DOI] [PubMed] [Google Scholar]
- 24. Paterson DL. 2004. “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin. Infect. Dis. 38(Suppl 4):S341–S345 [DOI] [PubMed] [Google Scholar]
- 25. Perencevich EN, Lautenbach E. 2011. Infection prevention and comparative effectiveness research. JAMA 305:1482–1483 [DOI] [PubMed] [Google Scholar]
- 26. Projan SJ. 2007. (Genome) size matters. Antimicrob. Agents Chemother. 51:1133–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanchez GV, Master RN, Bordon J. 2011. Trimethoprim-sulfamethoxazole may no longer be acceptable for the treatment of acute uncomplicated cystitis in the United States. Clin. Infect. Dis. 53:316–317 [DOI] [PubMed] [Google Scholar]
- 28. Shardell M, et al. 2007. Statistical analysis and application of quasi experiments to antimicrobial resistance intervention studies. Clin. Infect. Dis. 45:901–907 [DOI] [PubMed] [Google Scholar]
- 29. Sibanda EL, Weller IV, Hakim JG, Cowan FM. 2011. Does trimethoprim-sulfamethoxazole prophylaxis for HIV induce bacterial resistance to other antibiotic classes? Results of a systematic review. Clin. Infect. Dis. 52:1184–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stevens DL, et al. 2005. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin. Infect. Dis. 41:1373–1406 [DOI] [PubMed] [Google Scholar]
- 31. Talan DA, Krishnadasan A, Abrahamian FM, Stamm WE, Moran GJ. 2008. Prevalence and risk factor analysis of trimethoprim-sulfamethoxazole- and fluoroquinolone-resistant Escherichia coli infection among emergency department patients with pyelonephritis. Clin. Infect. Dis. 47:1150–1158 [DOI] [PubMed] [Google Scholar]
- 32. Tosh PK, McDonald LC. 2012. Infection control in the multidrug-resistant era: tending the human microbiome. Clin. Infect. Dis. 54:707–713 [DOI] [PubMed] [Google Scholar]
- 33. van der Veen EL, et al. 2009. Effect of long-term trimethoprim/sulfamethoxazole treatment on resistance and integron prevalence in the intestinal flora: a randomized, double-blind, placebo-controlled trial in children. J. Antimicrob. Chemother. 63:1011–1016 [DOI] [PubMed] [Google Scholar]
- 34. Vernaz N, et al. 2011. Modelling the impact of antibiotic use on antibiotic-resistant Escherichia coli using population-based data from a large hospital and its surrounding community. J. Antimicrob. Chemother. 66:928–935 [DOI] [PubMed] [Google Scholar]
- 35. Wagenlehner FM, et al. 2011. Uncomplicated urinary tract infections. Dtsch. Arztebl. Int. 108:415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]