Abstract
Linezolid is a promising antimicrobial agent for the treatment of multidrug-resistant tuberculosis (MDR-TB), but its use is limited by toxicity. Therapeutic drug monitoring (TDM) may help to minimize toxicity while adequate drug exposure is maintained. Conventional plasma sampling and monitoring might be hindered in many parts of the world by logistical problems that may be solved by dried blood spot (DBS) sampling. The aim of this study was to develop and validate a novel method for TDM of linezolid in MDR-TB patients using DBS sampling. Plasma, venous DBS, and capillary DBS specimens were obtained simultaneously from eight patients receiving linezolid. A DBS sampling method was developed and clinically validated by comparing DBS with plasma results using Passing-Bablok regression and Bland-Altman analysis. This study showed that DBS analysis was reproducible and robust. Accuracy and between- and within-day precision values from three validations presented as bias and coefficient of variation (CV) were less than 17.2% for the lower limit of quantification and less than 7.8% for other levels. The method showed a high recovery of approximately 95% and a low matrix effect of less than 8.7%. DBS specimens were stable at 37°C for 2 months and at 50°C for 1 week. The ratio of the concentration of linezolid in DBS samples to that in plasma was 1.2 (95% confidence interval [CI], 1.12 to 1.27). Linezolid exposure calculated from concentrations DBS samples and plasma showed good agreement. In conclusion, DBS analysis of linezolid is a promising tool to optimize linezolid treatment in MDR-TB patients. An easy sampling procedure and high sample stability may facilitate TDM, even in underdeveloped countries with limited resources and where conventional plasma sampling is not feasible.
INTRODUCTION
Linezolid is used as a second-line drug in the treatment of multidrug-resistant tuberculosis (MDR-TB) due to its efficacy in vitro (21), in vivo (9), and in patients (1, 2, 14, 17, 34) against Mycobacterium tuberculosis. The World Health Organization (WHO) classifies linezolid as a reserve antituberculosis drug for the treatment of multidrug-resistant/extensively drug-resistant tuberculosis (MDR/XDR-TB) (33). Linezolid is usually added to a treatment regimen consisting of antituberculosis drugs to which M. tuberculosis is still susceptible. However, treatment with linezolid may be limited by toxicity, such as time- and dose-dependent neuropathy or myelosuppression (17, 29), which leads to dose reduction or cessation of treatment with linezolid. Therapeutic drug monitoring (TDM) can be used to implement dose reductions to limit toxicity while preventing inadequate exposure. Efficacy predicting pharmacokinetic/pharmacodynamic (PK/PD) parameters, such as the ratio of the area under the concentration-time curve from 0 to 24 h to the MIC (AUC0–24/MIC), might be helpful in evaluating linezolid dosages (1, 7, 26, 32). The AUC0–24/MIC has been shown to be the best predictive model in a murine model (32), but evidence from human data is lacking. Further PK/PD data from TB programs or large studies are needed for the development of evidence-based PK/PD parameters, such as an AUC0–24/MIC target ratio.
Linezolid treatment has been evaluated for TB treatment in several case series (17, 23). However, neither drug susceptibility tests (DSTs) nor drug exposure assessment was performed for linezolid, making it difficult to draw conclusions about efficacy (5). For instance, drug interactions with other antimicrobial agents might have occurred and might have had an impact on linezolid exposure (6, 15). In addition, conventional drug exposure evaluation for TB drugs using plasma samples might have been hindered in these studies by logistical challenges (30). The use of dried blood spot (DBS) sampling may provide a helpful alternative to conventional plasma sampling through a simplified sampling procedure and increased sample stability. DBS sampling has been applied in the treatment of other infectious diseases like malaria and HIV (30). Other advantages of DBS samples may include the requirement of a lower blood sample volume and lower biohazard risk than for conventional plasma samples (12, 18, 30). Compared to conventional sampling, DBS sampling may be hindered by inter- and intrapatient hematocrit (Hct) variation which leads to different blood viscosity values, yielding a proportional analytical bias with Hct values. Furthermore, Hct may affect the drug blood/plasma partition ratio, complicating the comparison with conventional plasma samples. In the development of a bioanalytical method for linezolid using DBS analysis, important patient-related factors like blood spot volume, Hct value (3, 24), and difference between capillary and venous blood have to be assessed during validation (12, 18, 25, 30). To enable individualized linezolid treatment, the aim of this study was to develop and validate a method for DBS analysis and evaluate it in MDR-TB and XDR-TB patients.
MATERIALS AND METHODS
Patients.
From September 2010 to March 2012, MDR-TB patients (≥18 years old) were recruited from the Tuberculosis Centre Beatrixoord, University Medical Center Groningen (Haren, The Netherlands). Eligible for inclusion were patients receiving treatment with antituberculosis drugs for which routine therapeutic drug monitoring was scheduled. Patients with bleeding disorders were excluded from the study. The study procedures were reviewed and approved by the local ethics committee. Patients receiving linezolid were included after providing written informed consent.
Sampling was performed at least 1 week after the start of linezolid treatment to ensure that the steady state was achieved. Venous blood samples were obtained before drug intake and at 1, 2, 3, 4, and 8 h after dosing according to a previous study (2) and to local procedures for TDM of TB drugs to be able to calculate drug exposure and other PK parameters. Venous dried blood spot (VDBS) specimens were prepared by pipetting 50 μl of venous blood onto Whatman 31 ET CHR paper. The remaining venous blood was centrifuged at 3,000 rpm for 5 min at room temperature to obtain plasma, which was stored at −20°C until analysis. DBS specimens were obtained through a finger prick by dropping the blood directly on dried blood spot paper. DBS samples were obtained before drug intake and at 2 and 8 h after dosing, representing low, high, and medium linezolid blood levels, respectively. Both the VDBS and DBS samples were left to dry at room temperature and stored in sealed plastic bags with desiccant sachets at −20°C until analysis.
DBS analysis.
To quantify DBS samples, an 8-mm-diameter disc was punched out of each blood spot. Extraction of these discs was performed by sonication with a frequency of 47 kHz for a period of 20 min using 500 μl of extracting solvent consisting of 0.3 mg/liter cyanoimipramine (internal standard) and 1g/liter EDTA in water. From this solution, a volume of 200 μl was added to 750 μl of acetonitrile. The samples were vortexed for 1 min and subsequently centrifuged at 11,000 rpm for 5 min. An injection volume of 5 μl was analyzed using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis method (16). The plasma samples were prepared and analyzed using the same method.
DBS analytical method validation.
The DBS analytical method was validated in accordance with the U.S. Food and Drug Administration (FDA) guidance for bioanalytical method validation (27). For the validation, blood was prepared by mixing plasma and red blood cell and linezolid stock solution to achieve blood at the desired concentration and Hct level. Subsequently, the validation DBS samples were prepared by pipetting 50 μl of blood onto the paper. Linearity was assessed with 1/x2 weighting over a linezolid concentration range of 0.05 to 40 mg/liter. Clinical relevant concentrations were well within the range of the assay standards (2). The within-day and between-day accuracy and precision were evaluated on four validation levels, that is, lower limit of quantification (LLOQ), low, medium, and high, at linezolid concentrations of 0.05, 0.25, 15, and 30 mg/liter, respectively. Each validation level was analyzed in five replicates on three consecutive days. The matrix effect and the recovery of linezolid from DBS samples were determined using a common method (18, 31). The stability of DBS specimens was assessed by storing validation DBS samples under ambient conditions and at 37°C after 1 week, 2 weeks, and 2 months. As a worst-case scenario, the stability of DBS specimens was also assessed at 50°C after 1 day, 2 days, and 1 week. The stability was evaluated at low and high levels of linezolid in five replicates by comparing the analytical results with the nominal concentrations. In addition to the criteria suggested in the FDA guideline (27), the impact on assay accuracy and precision due to the variations of Hct values and blood spot volumes were evaluated. For these purposes, Hct values of 20, 25, 30, 35, 40, 45, and 50% and blood spot volumes of 30, 50, 70, and 90 μl were assessed. During the method validation, blood spot volume and Hct values were standardized at 50 μl and 35%, respectively. Setting the Hct value at 35% reflects the Hct in tuberculosis patients (3).
Pharmacokinetic and pharmacodynamic evaluation.
Pharmacokinetic parameters were evaluated using a noncompartmental model of the KINFIT module of MW Pharm (version 3.9; Mediware, The Netherlands). The AUC from 0 to 12 h (AUC0–12) was calculated using the trapezoidal rule from 0 up to 12 h, and the AUC0–24 was calculated by doubling the AUC0–12. The maximum concentration (Cmax) was defined as the highest observed linezolid concentration, and the corresponding time at which this value was reached was designated Tmax. The elimination half-life (t1/2) was calculated by dividing the natural logarithm of 2 (ln2) by the elimination constant (ke), as calculated by MW Pharm. The apparent clearance (Cl) of linezolid was calculated by dose/AUC0-12. The volume of distribution (Vd) was calculated by dividing Cl by ke.
The drug susceptibility testing of the Mycobacterium tuberculosis isolates was performed at the Dutch National Mycobacteria Reference Laboratory (National Institute for Public Health and the Environment [RIVM]) using the Middlebrook 7H10 agar dilution method (28). The AUC0-24/MIC ratio, often used as a predictive pharmacodynamic parameter for efficacy, was calculated (32).
Statistics.
In the method validation, the bias was defined as the difference (in percentage) between the analytical result and the nominal concentration. The method was clinically validated by comparing the linezolid concentrations in DBS and VDBS samples with the concentration in plasma using Passing-Bablok regressions and Bland-Altman analysis by applying the software tool Analyze-it, version 2.20 (Analyze-it Software, Ltd.). Conversion factors, calculated from geometric mean (V)DBS/plasma concentration ratios, were used to calculate converted DBS and VDBS concentrations (4). Subsequently, the converted concentrations were used to calculate the AUC0-12 for linezolid in DBS and VDBS samples. The agreement between AUC0-12 values for converted linezolid concentrations in DBS and plasma was evaluated using Bland-Altman analysis. The Spearman correlation and Wilcoxon signed-rank test was applied to other comparisons.
RESULTS
Patients.
Eight patients with a median age of 29 years (interquartile range [IQR], 24 to 33 years) were included in this study. The baseline characteristics are presented in Table 1. The median Hct value was 37.4% (IQR, 33.0 to 41.4%). During the study, three of eight patients received linezolid at 300 mg twice a day and five patients received a dose of 600 mg twice daily. Isolates of seven patients showed resistance to the first-line drugs isoniazid, rifampin, ethambutol, pyrazinamide, and streptomycin. The isolate of one patient showed resistance to all first-line drugs except pyrazinamide. All DSTs revealed resistance to rifabutin, whereas one isolate showed fluoroquinolone resistance, and three showed protionamide resistance. None of the patients experienced significant discomfort from the finger pricks during DBS sampling, which was supported by the fact that all completed the three consecutive samples in this study.
Table 1.
Baseline demographics of study patients (n = 8)
| Parameter | Value |
|---|---|
| Age (yr) | 29 (24–33)a |
| Sex (no. of patients) | |
| Male | 2 |
| Female | 6 |
| Bodyweight (kg) | 60.5 (55.8–61.5)a |
| Height (m) | 1.69 (1.67–1.76)a |
| Body mass index (kg/m2) | 20.1 (19.0–21.4)a |
| Ethnicity (no. of patients) | |
| Caucasian | 4 |
| African | 3 |
| Asian | 1 |
| Comorbidity (n/N)d | |
| HIV | 1/8 |
| Hemoglobin (mmol/liter) | 7.0 (6.4–8.8)a,b |
| Hematocrit (%) | 37.4 (33.0–41.4)a,c |
| TB treatment other than linezolid (no. of patients) | |
| Moxifloxacin | 7 |
| Amikacin/kanamycin | 6 |
| Ethambutol | 5 |
| Cotrimoxazole | 3 |
| Clofazimine | 2 |
| Ertapenem | 2 |
| Pyrazinamide | 1 |
Median value (IQR).
Reference values for healthy subjects, 7.5 to 9.9 mmol/liter (female) and 8.7 to 10.6 mmol/liter (male).
Reference values for healthy subjects, 37.0 to 47.0% (female) and 42.0 to 52.0% (male).
n/N, number of TB patients with other infections/total number of TB patients.
DBS method validation.
The DBS assay method showed linearity over the analytical concentration range. The pooled correlation coefficient, r2, was 0.9947. The regression equation is as follows: concentration = (0.1635 ± 0.0025) × response + (0.0001 ± 0.0003). Within-day and between-day accuracy and precision showed coefficients of variation (CVs) within accepted ranges. Within-day CVs ranged from 1.6% to 13.8%, and between-day CVs were from 3.5% to 10.2%. The mean measured concentration was within 98.7% to 106.3% of the nominal concentration. The bias caused by variable matrices, i.e., DBS and EDTA matrices, was less than 8.7%. The recovery of DBS extraction was between 94.1% and 97.2%. No significant linezolid degradation was observed after DBS samples were stored at 50°C for at least 1 week and at 37°C or ambient temperature for 2 months as biases were less than 15%.
Variation of blood spot volume between 30 μl to 90 μl had a minor impact on the assay accuracy as the bias ranged from −11.6% to 7.1%. The variation of Hct levels from 20% to 50% yielded biases within −7.6% to 6.8% and −12.5% to 5.7% for medium and high linezolid concentration levels. Larger biases of −17.8% to 11.9% were observed at the low concentration level (0.25 mg/liter) (Table 2).
Table 2.
Summarized results of the validation of DBS analysis
| Validation criterion | Validation level (n = 5)e |
|||
|---|---|---|---|---|
| LLOQ | Low | Medium | High | |
| Nominal linezolid concentration (mg/liter) | 0.05 | 0.25 | 15 | 30 |
| Reproducibilitya | ||||
| Accuracy (bias [%]) | 4.5 | 6.3 | 3.2 | −1.3 |
| Within-day precision (CV [%]) | 13.8 | 4.0 | 4.1 | 1.6 |
| Between-day precision (CV [%]) | 10.2 | 3.5 | 6.1 | 7.7 |
| Overall precision (CV [%]) | 17.2 | 5.3 | 7.4 | 7.8 |
| Matrix effect (%) | 2.9 | 8.7 | 1.9 | |
| Recovery (%) | 95.5 | 94.1 | 97.2 | |
| Effect of blood vol (range of bias [%])b | −2.9–4.1 | −11.4–7.1 | −11.6–9 | |
| Effect of hematocrit (range of bias [%])c | −17.8–11.9 | −7.6–6.8 | −12.5–5.7 | |
| Stabilityd | ||||
| 1 week at 50°C (bias [%]) | 6.7 | −3.4 | ||
| 2 mos at 37°C (bias [%]) | −10 | −5.9 | ||
| 2 mos at ambient temp (bias [%]) | −2.5 | −2.0 | ||
Data are from three separate validation days.
Comparison with sample of standardized blood spot volume (35 μl).
Comparison with samples of standardized hematocrit (35%).
Present data are from the last time point of the stability test only.
n, number of replicates.
Comparisons of DBS, VDBS, and plasma analyses.
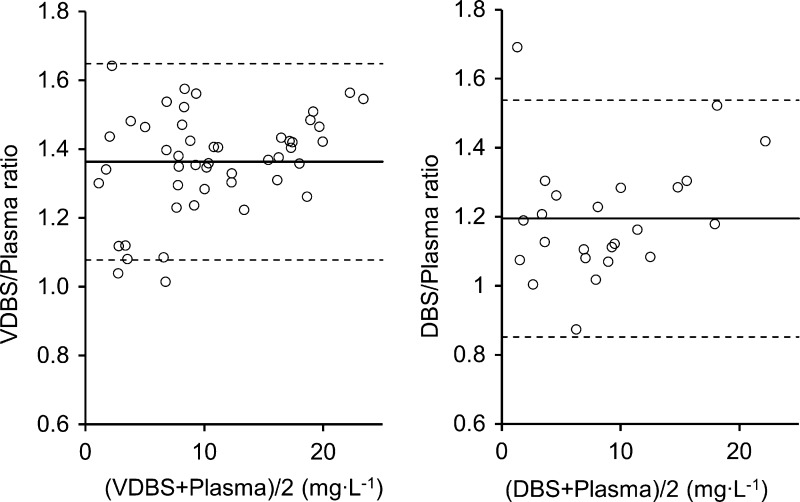
Significant proportional biases were observed in Passing-Bablok regressions in which the slope of regression line for linezolid concentrations between DBS samples and plasma was 1.28 (95% confidence interval [CI], 1.13 to 1.44) and between VDBS samples and plasma was 1.46 (95% CI, 1.40 to 1.54). The intercepts were −0.42 (95% CI, −1.72 to 0.17) and −0.67 (95% CI, −1.36 to −0.09), respectively (Fig. 1). In Bland-Altman analysis, the geometric mean linezolid concentration ratios in DBS and VDBS samples versus plasma were 1.20 (95% CI, 1.12 to 1.27) and 1.36 (95% CI, 1.32 to 1.40), respectively. The ratio of VDBS/plasma was higher than that of DBS/plasma (Wilcoxon signed-rank test, n = 24, P < 0.01). Limits of agreement of 95% were shown, with less than 5% of the values falling out of the ranges (Fig. 2).
Fig 1.
Passing-Bablok regression between measurements in DBS/VDBS samples and plasma: VDBS-plasma regression line (dashed line, +), slope of 1.46 (95% CI, 1.40 to 1.54) and intercept of −0.67 (95% CI, −1.36 to −0.09); DBS-plasma regression line (solid line, ○), slope of 1.28 (95% CI, 1.13 to 1.44) and intercept of −0.42 (95% CI, −1.72 to 0.17).
Fig 2.
Bland-Altman plot of linezolid concentration ratios in DBS and VDBS samples versus plasma: solid line, mean ratio; dashed line, limit of agreement (mean ratio ± 1.96 × standard deviation of the ratio).
Pharmacokinetic and pharmacodynamic evaluation.
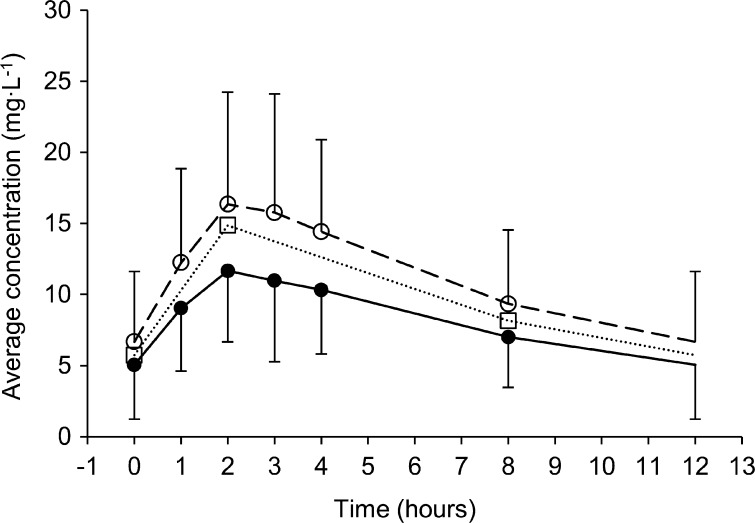
Median AUC0–12 values for linezolid in plasma were 50.9 (IQR, 50.5 to 54.9) mg · h/liter following a dose of 300 mg and 126 (IQR, 121.6 to 127.6) mg · h/liter following a dose of 600 mg. Linezolid pharmacokinetic parameters are shown in Table 3. The concentration-time curves for linezolid in plasma, DBS, and VDBS are presented in Fig. 3.
Table 3.
Steady-state pharmacokinetic parameters of linezolid in plasmaa
| Parameter | Value for the parameter by linezolid dosea |
|
|---|---|---|
| 300 mg (n = 3) | 600 mg (n = 5) | |
| AUC0–12 (mg · h/liter) | 50.9 (50.5–54.9) | 126.9 (121.6–127.6) |
| Cmax (mg/liter) | 8.8 (7.8–8.9) | 16.5 (14.4–16.5) |
| Tmax (h) | 1.9 (1.9–4.8) | 1.9 (1.7–3.0) |
| t1/2 (h) | 4.6 (4.0–6.9) | 7.5 (7.3–7.9) |
| Cl (liters/h) | 4.9 (3.8–5.1) | 3.1 (3.0–3.1) |
| Vd (liters) | 32.6 (29.4–34.4) | 34.8 (32.9–41.6) |
Data are presented as medians (IQR). All patients (n = 8) received linezolid twice daily.
Fig 3.
Concentration-time curves of linezolid in plasma (●, solid line), VDBS samples (○, dashed line), and DBS samples (□, dotted line). Plasma and VDBS data are presented as means and standard deviations. For visual purposes, the DBS data are presented as means without error bars.
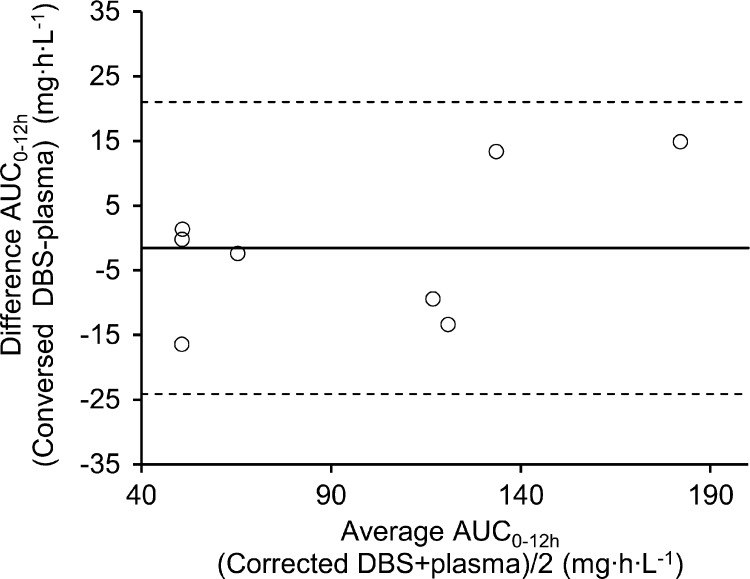
The AUC0–12 values for linezolid in DBS and VDBS were calculated using the conversion factors 1.20 and 1.36 for DBS and VDBS, respectively. The subsequent results showed a good agreement with plasma. All the values were within the 95% limit of agreement (Fig. 4). The individual data for each patient for AUC0–12 attained from plasma and converted (V)DBS concentrations and the respective AUC0–24/MIC values are presented in Table 4. Patients that received a linezolid dose of 300 mg twice daily (n = 3) had a median AUC0–24/MIC ratio in plasma of 236 (IQR, 219 to 322), and patients that received 600 mg twice daily (n = 5) had a median AUC0–24/MIC ratio in plasma of 508 (IQR, 486 to 1,398).
Fig 4.
Bland-Altman plot of AUC0–12 for linezolid from corrected DBS versus AUC0–12 plasma samples. Solid line, mean difference; dashed line, limit of agreement (mean difference ± 1.96 × standard deviation of the difference).
Table 4.
Pharmacokinetic and pharmacodynamic parameters of linezolid using concentrations in plasma, VDBS, and DBS
| Patient | Dose (mg)a | MIC (mg/liter) | AUC0–12 (mg · h/liter) in: |
AUC0–24/MIC in: |
||||
|---|---|---|---|---|---|---|---|---|
| Plasma | VDBSb | DBSb | Plasma | VDBSb | DBSb | |||
| 1 | 300 | 0.5 | 50.1 | 46.7 | 51.5 | 201 | 187 | 206 |
| 2 | 300 | 0.25 | 50.9 | 54.2 | 50.7 | 407 | 433 | 405 |
| 3 | 600 | 0.5 | 121.6 | 118.1 | 112.1 | 486 | 472 | 449 |
| 4 | 600 | <0.125 | 127.6 | 130.9 | 114.2 | >2,042 | >2,094 | >1,827 |
| 5 | 600 | 0.5 | 126.9 | 132.0 | 140.2 | 508 | 528 | 561 |
| 6 | 600 | 0.5 | 66.6 | 69.4 | 64.2 | 266 | 278 | 257 |
| 7 | 300 | 0.5 | 58.9 | 46.1 | 42.4 | 236 | 184 | 170 |
| 8 | 600 | 0.25 | 174.7 | 183.1 | 189.6 | 1,398 | 1,465 | 1,517 |
Twice daily.
Relative AUC0–12 and AUC0–24/MIC calculated using conversion factors (i.e., 1.20 for DBS and 1.36 for VDBS).
DISCUSSION
This study showed that DBS analysis is an easy tool to individualize MDR-TB treatment with linezolid. In addition, this report presents a novel, validated method of analysis of linezolid in dried blood spots, with specimens that proved to be very stable over time.
In previous studies on DBS analysis of other drugs, several technical factors were pointed out that have to be considered when interpreting DBS analysis, such as the effect of Hct and blood spot volume (12, 13, 18, 30). For the analysis of linezolid in DBS samples, the effect of Hct seemed to be of minor concern. In this study, biases fell within accepted ranges for Hct values between 20 and 50%. These Hct values cover an even broader range than clinical Hct values found in TB patients in literature, i.e., 35.4% ± 6.7% (3) and in this study 37.4% ± 4.4%. Based on these findings, the standardization of Hct at 35% during DBS validation is acceptable. Furthermore, variation of blood spot volume between 30 and 90 μl had little effect as biases were within 15%.
Despite the minor influence of technical factors, i.e., Hct value and blood spot volume, physiological factors are also mentioned in literature to possibly limit the applicability and interpretation of DBS analysis (13). Such a factor might be differences between the concentration of linezolid in plasma and that in whole blood. This study shows that concentration of linezolid is higher in blood than in plasma. This is caused by different capacities of binding to plasma proteins and blood cells. Furthermore, concentrations of linezolid were higher in VDBS than in DBS samples. This might be caused by differences between the capillary and venous blood (13, 25, 30). Nevertheless, the linezolid concentrations in both DBS and VDBS specimens showed good correlation with the plasma concentration. To compensate for these differences, we propose conversion factors of 0.83 (1/1.20) for DBS and 0.74 (1/1.36) for VDBS to calculate corresponding plasma values. After the conversion, good agreement between the AUC0–12 h values for linezolid in DBS samples and plasma was observed.
A meta-analysis showed that a ≤600-mg linezolid daily dose resulted in lower frequency of either an adverse event or adverse events necessitating treatment discontinuation than a dose of >600 mg daily (8). Among the published data, the lowest rate of adverse effects was observed with a dose of 300 mg once daily (17). Nevertheless, lowering the dose clearly results in lower exposure to the drug (2, 19). In addition, interpatient variability and possible drug-drug interactions may lead to under- or overexposure. Therefore, treatment with a fixed dose may be questionable (6, 11, 22, 26). The application of TDM for linezolid can help avoid under- or overexposure, which may occur in 30 to 40% of the cases (20).
In this study, all patients had Mycobacterium tuberculosis isolates with a linezolid MIC of ≤0.5 mg/liter. With a dose of 600 mg (n = 5) twice daily, very high AUC0-24/MIC ratios were reached (10), so dose reductions could be implemented to prevent time- and dose-dependent toxicity. Furthermore, a high correlation of AUC0-24/MIC values between converted values for DBS and plasma (Spearman's rho = 0.976, n = 8) was observed. This suggests that TDM using DBS may result in interventions identical to those with conventional plasma sampling. Therefore, adaptive dosing of linezolid to prevent potential toxicity and to ensure therapeutic exposure is feasible using DBS sampling.
The high stability of DBS specimens can minimize the logistical burden of conventional sampling in limited-resource areas. With simple instructions, the DBS samples can be obtained easily and sent to equipped facilities for analysis by mail (12, 30). This could allow TDM in TB programs worldwide, including in resource-limited settings where an MDR/XDR-TB epidemic is a growing problem. TDM using DBS sampling for MDR/XDR-TB should be especially considered in areas where HIV or malaria coinfections are highly prevalent as DBS sampling has been successfully applied to monitor the treatment of such diseases (30).
Since treatment of MDR/XDR-TB is long and complicated by adverse drug reactions, TDM of linezolid with DBS sampling could be used to optimize drug exposure during treatment. In conclusion, this study presents a novel, validated analysis of linezolid in DBS specimens that is suitable for optimization of linezolid treatment of MDR-TB. Advantages include a very simple, low-biohazard-risk sampling method using a finger prick, easy logistics, and very good stability of DBS specimens.
Footnotes
Published ahead of print 27 August 2012
REFERENCES
- 1. Alffenaar JW, et al. 2010. Limited sampling strategies for therapeutic drug monitoring of linezolid in patients with multidrug-resistant tuberculosis. Ther. Drug Monit. 32:97–101 [DOI] [PubMed] [Google Scholar]
- 2. Alffenaar JW, et al. 2010. Comparison of the pharmacokinetics of two dosage regimens of linezolid in multidrug-resistant and extensively drug-resistant tuberculosis patients. Clin. Pharmacokinet. 49:559–565 [DOI] [PubMed] [Google Scholar]
- 3. Antony SJ, Harrell V, Christie JD, Adams HG, Rumley RL. 1995. Clinical differences between pulmonary and extrapulmonary tuberculosis: a 5-year retrospective study. J. Natl. Med. Assoc. 87:187–192 [PMC free article] [PubMed] [Google Scholar]
- 4. Bland JM, Altman DG. 1999. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 8:135–160 [DOI] [PubMed] [Google Scholar]
- 5. Bolhuis MS, Pranger AD, Alffenaar JW. 2012. Linezolid: safety and efficacy monitoring. Eur. Respir. J. 39:1275–1276 (Letter.) [DOI] [PubMed] [Google Scholar]
- 6. Bolhuis MS, et al. 2010. Clarithromycin significantly increases linezolid serum concentrations. Antimicrob. Agents Chemother. 54:5418–5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conte JE, Jr, Golden JA, Kipps J, Zurlinden E. 2002. Intrapulmonary pharmacokinetics of linezolid. Antimicrob. Agents Chemother. 46:1475–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cox H, Ford N. 2012. Linezolid for the treatment of complicated drug-resistant tuberculosis: a systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 16:447–454 [DOI] [PubMed] [Google Scholar]
- 9. Cynamon MH, Klemens SP, Sharpe CA, Chase S. 1999. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob. Agents Chemother. 43:1189–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dooley KE, et al. 17 July 2012, posting date World Health Organization group V drugs for the treatment of drug-resistant tuberculosis: unclear efficacy or untapped potential? J. Infect. Dis. doi:10.1093/infdis/jis460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dryden MS. 2011. Linezolid pharmacokinetics and pharmacodynamics in clinical treatment. J. Antimicrob. Chemother. 66(Suppl 4):iv7–iv15 [DOI] [PubMed] [Google Scholar]
- 12. Edelbroek PM, van der Heijden J, Stolk LM. 2009. Dried blood spot methods in therapeutic drug monitoring: methods, assays, and pitfalls. Ther. Drug Monit. 31:327–336 [DOI] [PubMed] [Google Scholar]
- 13. Emmons G, Rowland M. 2010. Pharmacokinetic considerations as to when to use dried blood spot sampling. Bioanalysis 2:1791–1796 [DOI] [PubMed] [Google Scholar]
- 14. Fortun J, et al. 2005. Linezolid for the treatment of multidrug-resistant tuberculosis. J. Antimicrob. Chemother. 56:180–185 [DOI] [PubMed] [Google Scholar]
- 15. Gebhart BC, Barker BC, Markewitz BA. 2007. Decreased serum linezolid levels in a critically ill patient receiving concomitant linezolid and rifampin. Pharmacotherapy 27:476–479 [DOI] [PubMed] [Google Scholar]
- 16. Harmelink IM, Alffenaar JW, Wessels AM, Greijdanus B, Uges DR. 2008. A rapid and simple liquid chromatography-tandem mass spectrometry method for the determination of linezolid in human serum. EJHP Science 14:3–7 [Google Scholar]
- 17. Koh WJ, et al. 30 May 2012. Daily 300 mg dose of linezolid for multidrug-resistant and extensively drug-resistant tuberculosis: updated analysis of 51 patients. J. Antimicrob. Chemother. 67:2056–2057 [Epub ahead of print.] doi:10.1093/jac/dks078 [DOI] [PubMed] [Google Scholar]
- 18. Li W, Tse FL. 2010. Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Biomed. Chromatogr. 24:49–65 [DOI] [PubMed] [Google Scholar]
- 19. McGee B, et al. 2009. Population pharmacokinetics of linezolid in adults with pulmonary tuberculosis. Antimicrob. Agents Chemother. 53:3981–3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pea F, et al. 2010. Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis. Antimicrob. Agents Chemother. 54:4605–4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prammananan T, Chaiprasert A, Leechawengwongs M. 2009. In vitro activity of linezolid against multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant (XDR)-TB isolates. Int. J. Antimicrob. Agents. 33:190–191 [DOI] [PubMed] [Google Scholar]
- 22. Sasaki T, et al. 2011. Population pharmacokinetic and pharmacodynamic analysis of linezolid and a hematologic side effect, thrombocytopenia, in Japanese patients. Antimicrob. Agents Chemother. 55:1867–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singla R, et al. 2012. Linezolid: an effective, safe and cheap drug for patients failing multidrug-resistant tuberculosis treatment in India. Eur. Respir. J. 39:956–962 [DOI] [PubMed] [Google Scholar]
- 24. Spooner N, Lad R, Barfield M. 2009. Dried blood spots as a sample collection technique for the determination of pharmacokinetics in clinical studies: considerations for the validation of a quantitative bioanalytical method. Anal. Chem. 81:1557–1563 [DOI] [PubMed] [Google Scholar]
- 25. Spooner N, Ramakrishnan Y, Barfield M, Dewit O, Miller S. 2010. Use of DBS sample collection to determine circulating drug concentrations in clinical trials: practicalities and considerations. Bioanalysis 2:1515–1522 [DOI] [PubMed] [Google Scholar]
- 26. Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. 2011. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J. Infect. Dis. 204:1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. U.S. Department of Health and Human Services, Food and Drug Administration 2001. Guidance for industry. Bioanalytical method validation. U.S. Department of Health and Human Services, Food and Drug Administration, Rockville, MD: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf [Google Scholar]
- 28. van Klingeren B, Dessens-Kroon M, van der Laan T, Kremer K, van Soolingen D. 2007. Drug susceptibility testing of Mycobacterium tuberculosis complex by use of a high-throughput, reproducible, absolute concentration method. J. Clin. Microbiol. 45:2662–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vinh DC, Rubinstein E. 2009. Linezolid: a review of safety and tolerability. J. Infect. 59(Suppl 1):S59–S74 [DOI] [PubMed] [Google Scholar]
- 30. Vu DH, Alffenaar JW, Edelbroek PM, Brouwers JR, Uges DR. 2011. Dried blood spots: a new tool for tuberculosis treatment optimization. Curr. Pharm. Des. 17:2931–2939 [DOI] [PubMed] [Google Scholar]
- 31. Vu DH, Koster RA, Alffenaar JW, Brouwers JR, Uges DR. 2011. Determination of moxifloxacin in dried blood spots using LC-MS/MS and the impact of the hematocrit and blood volume. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 879:1063–1070 [DOI] [PubMed] [Google Scholar]
- 32. Williams KN, et al. 2009. Promising antituberculosis activity of the oxazolidinone PNU-100480 relative to that of linezolid in a murine model. Antimicrob. Agents Chemother. 53:1314–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. World Health Organization 2010. Treatment of tuberculosis: guidelines, 4th ed World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf [Google Scholar]
- 34. Yew WW, Chau CH, Wen KH. 2008. Linezolid in the treatment of ‘difficult’ multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 12:345–346 [PubMed] [Google Scholar]