Abstract
Primary amebic meningoencephalitis (PAM) is a rapidly fatal infection caused by the free-living ameba Naegleria fowleri. The drug of choice in treating PAM is the antifungal antibiotic amphotericin B, but its use is associated with severe adverse effects. Moreover, few patients treated with amphotericin B have survived PAM. Therefore, fast-acting and efficient drugs are urgently needed for the treatment of PAM. To facilitate drug screening for this pathogen, an automated, high-throughput screening methodology was developed and validated for the closely related species Naegleria gruberi. Five kinase inhibitors and an NF-kappaB inhibitor were hits identified in primary screens of three compound libraries. Most importantly for a preclinical drug discovery pipeline, we identified corifungin, a water-soluble polyene macrolide with a higher activity against Naegleria than that of amphotericin B. Transmission electron microscopy of N. fowleri trophozoites incubated with different concentrations of corifungin showed disruption of cytoplasmic and plasma membranes and alterations in mitochondria, followed by complete lysis of amebae. In vivo efficacy of corifungin in a mouse model of PAM was confirmed by an absence of detectable amebae in the brain and 100% survival of mice for 17 days postinfection for a single daily intraperitoneal dose of 9 mg/kg of body weight given for 10 days. The same dose of amphotericin B did not reduce ameba growth, and mouse survival was compromised. Based on these results, the U.S. FDA has approved orphan drug status for corifungin for the treatment of PAM.
INTRODUCTION
Primary amebic meningoencephalitis (PAM) is caused by the free-living ameba Naegleria fowleri and occurs most commonly in healthy children and young adults with recent recreational freshwater exposure. PAM due to N. fowleri has a worldwide distribution and occurs most frequently in tropical areas and during hot summer months; it is a rare disease in the United States (38). Infection results from water containing N. fowleri entering the nasal cavity, followed by migration of amebae to the brain via the olfactory nerve. Within the brain, N. fowleri causes extensive inflammation, hemorrhage, and necrosis, leading to death in 3 to 7 days (7, 18).
Optimum treatment for PAM has not been well defined. Amphotericin B remains a cornerstone of therapy for PAM, but this requires a high dosage, and its use is frequently associated with renal toxicity, manifested as azotemia and hypokalemia (34). Amphotericin B may also cause anemia, and many patients experience chills, fever, nausea, vomiting, and headache (23, 25). Moreover, only 10 persons with PAM have been treated successfully with amphotericin B, alone or in combination with other drugs (1, 2, 6, 17, 20, 21, 24, 28–31, 33–35). Therefore, fast-acting and efficient drugs are urgently needed for the treatment of PAM.
In this study, we developed, optimized, and employed a high-throughput screening (HTS) methodology to screen large diverse compound libraries for compounds with cytotoxicity to Naegleria. This assay measures ATP bioluminescence generated when luciferase catalyzes the transformation of luciferin into oxyluciferin, yielding PPi, AMP, and light in the presence of cellular ATP and oxygen (10). This assay is easily adapted to automated HTS procedures and represents a rapid, sensitive, and efficient assay to identify active compounds. In this study, the whole-organism screening was formatted to 96- and 384-well microtiter plates, thus providing increased throughput and improved interfacing with similarly formatted small-molecule libraries maintained in-house at the UCSF Small Molecule Discovery Center (SMDC; http://smdc.ucsf.edu/). The screening protocol is intended to streamline and accelerate the identification of hit compounds and scaffolds in vitro.
The screens were performed using libraries of commercially available compounds and a recently isolated compound, corifungin. Because of its efficacy in vitro, we then tested corifungin in a mouse model of PAM due to N. fowleri.
MATERIALS AND METHODS
Chemicals and reagents.
White, solid-bottom, tissue culture-treated 96-well and 384-well microplates were purchased from E&K Scientific (Santa Clara, CA). A CellTiter-Glo luminescence-based cell viability assay kit was purchased from Promega (Madison, WI); dimethyl sulfoxide (DMSO) and amphotericin B were purchased from Sigma-Aldrich (St. Louis, MO).
Maintenance of N. gruberi and N. fowleri.
The nonpathogenic strain Naegleria gruberi NEG-M (a gift of Zac Cande, University of California, Berkeley, CA) and the pathogenic strain N. fowleri ATCC 30808 were used for compound screening experiments. Trophozoites of N. gruberi were cultured axenically in M7 medium (ATCC, Manassas, VA) at 25°C (14), and trophozoites of N. fowleri were cultured axenically in 2% Bacto Casitone medium supplemented with 10% fetal bovine serum at 37°C (7). N. gruberi trophozoites were counted using a particle counter (Beckman Coulter, Fullerton, CA). All experiments were performed using trophozoites and cells harvested during the logarithmic phase of growth.
Compound collections.
A library of 1,083 known bioactive compounds was donated by Iconix Biosciences, Inc. (Foster City, CA), but only 910 compounds were soluble at 20 mM in undiluted DMSO, and these 910 compounds were used for screening. Kinase-targeted compound collections comprising 14,757 compounds were purchased from Asinex (Winston-Salem, NC) (1,639 compounds) and ChemDiv, Inc. (San Diego, CA) (13,118 compounds). The libraries were maintained as 1 mM and 5 mM stocks in 384-well plates at −40°C at the UCSF SMDC, which is juxtaposed to the UCSF Sandler Center. Corifungin was dissolved in water. The Iconix and Asinex compound libraries and corifungin were screened in a 96-well plate format, and the ChemDiv library was screened in a 384-well plate format.
Automated primary HTS using a cell viability assay.
Compounds were diluted by using a Biomek FXp laboratory automation workstation (Beckman Coulter) and a Matrix WellMate bulk dispenser (Thermo Fisher Scientific, Hudson, NH) to yield 125 μM compounds in 12.5% DMSO. Finally, the FXp station transferred 4 μl and 2 μl of diluted compounds to the 96- and 384-well screening plates, respectively, followed by addition of 96 μl (10,000 amebae) and 48 μl (2,500 amebae) of N. gruberi trophozoites in M7 medium to the respective plates by use of a WellMate dispenser, to achieve final concentrations of test compound and DMSO per well of 5 μM and 0.5%, respectively.
Negative-control wells in the screening plates contained 0.5% DMSO, and positive-control wells contained 50 μM amphotericin B (Sigma-Aldrich). Assay plates were incubated for 48 h at 25°C. At the end of incubation, the assay plates were equilibrated to room temperature for 30 min, and 50 μl and 25 μl of CellTiter-Glo luminescent cell viability assay reagent (Promega) was added to each well of the 96-well and 384-well plates, respectively, using a WellMate dispenser. The plates were then placed on an orbital shaker at room temperature for 10 min to induce cell lysis. After lysis, the plates were again equilibrated at room temperature for 10 min to stabilize the luminescent signal. The resulting ATP bioluminescence of the trophozoites was measured at room temperature by use of an Analyst HT plate reader from Molecular Devices (Sunnyvale, CA).
Secondary screen for potency determination.
For confirmatory screens of trophozoites, hits from the primary screen were “cherry picked” from 5 mM stocks in 100% DMSO by use of the Biomek FXp workstation. For 8-point 50% effective concentration (EC50) determination experiments, 2.5-μl aliquots of stock compounds were diluted with 17.5 μl sterile water to yield a 625 μM working concentration for library compounds. Threefold serial dilutions were then performed, yielding a concentration range of 0.25 to 625 μM. From this dilution plate, 4 μl was transferred into the 96-well screening plate, followed by addition of 96 μl of trophozoites (10,000 amebae) to yield a final 8-point concentration range spanning 0.01 to 25 μM in 0.5% (final concentration) DMSO. The assays were performed in triplicate using the CellTiter-Glo luminescence-based cell viability assay.
HTS data analysis and statistics.
The raw Analyst data file from the Analyst HT plate reader was uploaded into SMDC's MySQL database by use of Pipeline Pilot 4.5.2 (SciTegic, Inc., San Diego, CA). The results of each screen were deposited into SMDC's database, where tables, heat maps, and scatter plots display concise representations of the screening results. Standard assay metrics such as Z′ were calculated for each plate. Percent inhibition relative to maximum and minimum reference signal controls was calculated using the following formula: % inhibition = [(mean for maximum reference signal control − experimental value)/(mean for maximum reference signal control − mean for minimum reference signal control)] × 100.
The cutoff was selected to identify active hits from the primary screen. A hit was defined as a compound showing at least 50% inhibition and a value 3 standard deviations above the mean for the population of compounds tested. Visualization and statistical analysis of secondary screening data were performed using GraphPad Prism 5.0 software.
In vitro studies of corifungin against N. fowleri.
To determine the effect of corifungin on the growth of N. fowleri, 3.7 × 106 amebae were incubated with 3.12 μM, 6.25 μM, 12.5 μM, 18.75 μM, or 25 μM corifungin for 72 h at 37°C. Control trophozoites were incubated with fresh medium only. Cell numbers were calculated by use of a hemocytometer at the end of incubation. The percentage of viable trophozoites due to treatment at different concentrations of corifungin was determined by the standard trypan blue exclusion method. Cells stained blue were considered nonviable. The 50% inhibitory concentration (IC50) was evaluated by the probit death test (12).
Transmission electron microscopy.
For ultrastructural analysis, N. fowleri trophozoites were incubated with different concentrations of corifungin for 72 h and then fixed with a solution of 2.5% glutaraldehyde in 0.1 M cacodylate buffer. Samples were postfixed with 1% (wt/vol) osmium tetroxide, dehydrated with ethanol and propylene oxide, and embedded in epoxy resin. Thin sections (60 to 90 nm) were contrast stained with uranyl acetate and lead citrate and observed under a Zeiss EM-910 transmission electron microscope (Carl Zeiss, Germany).
In vivo efficacy of corifungin.
Four-week-old male BALB/c mice were inoculated by intranasal instillation of 20 μl of fresh N. fowleri culture medium containing 2 × 104 trophozoites into a single naris. The strain of N. fowleri used in the study is highly virulent, and the time of death of mice is approximately 1 week with this inoculum. At 24 h postinfection, the mice were treated with either phosphate-buffered saline (PBS), amphotericin B at 9 mg/kg of body weight/day, or corifungin at 9 mg/kg/day (10 mice per group). The compound was administered intraperitoneally daily for 10 days. The mice were sacrificed at 17 days postinfection. Brains were cultured to see if live amebae were present. Brains were also fixed by vascular perfusion with a 4% solution of paraformaldehyde diluted in PBS. The brains were then carefully dissected, representative fragments of brain tissue were embedded in paraffin, and sections were stained with hematoxylin-eosin (7). The animal management protocols were approved by the CINVESTAV institutional committee (IACUC ID number 0244/05). All animals were euthanized by an overdose of sodium pentobarbital at the end of the experiments and were handled according to the guidelines of the 2000 AVMA Panel of Euthanasia.
RESULTS
HTS assay optimization.
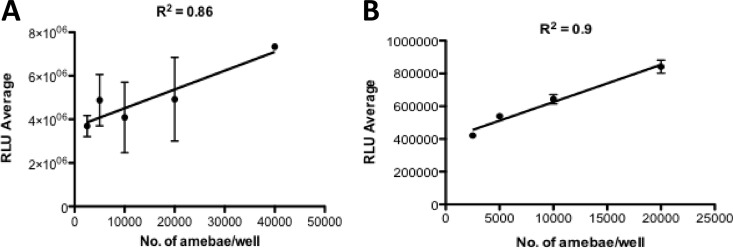
All screening studies in 96-well microtiter plates were performed with exponentially growing N. gruberi trophozoites (100,000 amebae/ml). This inoculum was chosen so that confluent but not excessive growth took place. Screening studies in 384-well plates were performed with an inoculum of 50,000 amebae/ml. For biosafety reasons, we performed the high-throughput screening with the nonpathogenic species N. gruberi instead of the pathogenic species N. fowleri. Because ATP is an essential cofactor for biogenesis in N. gruberi, we used a luciferase-based assay to validate the correlation between the number of viable trophozoites and their ATP level (10). The relationships between numbers of amebae seeded into 96- and 384-well plates and relative luminescence from the CellTiter-Glo assay of amebae showed strong linear correlations (R2 = 0.86 and 0.9) (Fig. 1). Trophozoites readily tolerated up to 0.5% DMSO, with no reduction of the growth rate. In our system, the EC50 for amphotericin B against N. gruberi, defined as the concentration of compound necessary to reduce the culture density to 50% of that in a DMSO-treated culture, was 0.3 μM in the 96-well format. This HTS assay was used to evaluate the activity of thousands of chemicals to identify potential drug leads and was performed at a single concentration of 5 μM.
Fig 1.
Correlation between the number of viable N. gruberi trophozoites and ATP bioluminescence in 96-well (A) and 384-well (B) microtiter plates. Values plotted are means and standard deviations for triplicate wells. Each line represents the linear regression for plotted data.
Screening of the Iconix library.
To test the efficacy of the assay for use in large-scale automated screens and to identify new amebicidal agents, an Iconix library consisting of 910 FDA-approved and unapproved bioactive compounds was screened against N. gruberi. Fifteen compounds were primary hits, as defined by at least 50% inhibition of the single readout and a value 3 standard deviations above the mean for the entire population of compounds tested (Table 1). Amphotericin B, a compound with known activity against Naegleria, was present in the library and was also identified as a primary hit, confirming that our whole-cell HTS assay format could be used to identify compounds exhibiting activity against the pathogen. Bay-11-7085, a nuclear factor kappaB inhibitor, also showed strong inhibition but was not considered for further investigation because of its host cell toxicity (5, 16, 39).
Table 1.
Hits obtained after screening the Iconix library at 5 μM
| Compound | % Inhibition | EC50 (μM) |
|---|---|---|
| Amphotericin B | 99 | 0.3 |
| Bay-11-7085 | 99 | |
| Thimerosal | 98 | |
| Sporidesmin A | 93 | |
| Gentian violet | 78 | |
| Pyrithione zinc | 76 | |
| Brilliant green | 71 | |
| Emetine | 61 | |
| Clozapine | 56 | |
| Oxiconazole | 55 | |
| Tamoxifen citrate | 55 | |
| Metergoline | 53 | |
| Terbinafine | 53 | |
| 4,4′-Diethylaminoethoxyhexestrol | 52 | |
| Lomerizine | 51 |
Screening of the Asinex and ChemDiv libraries.
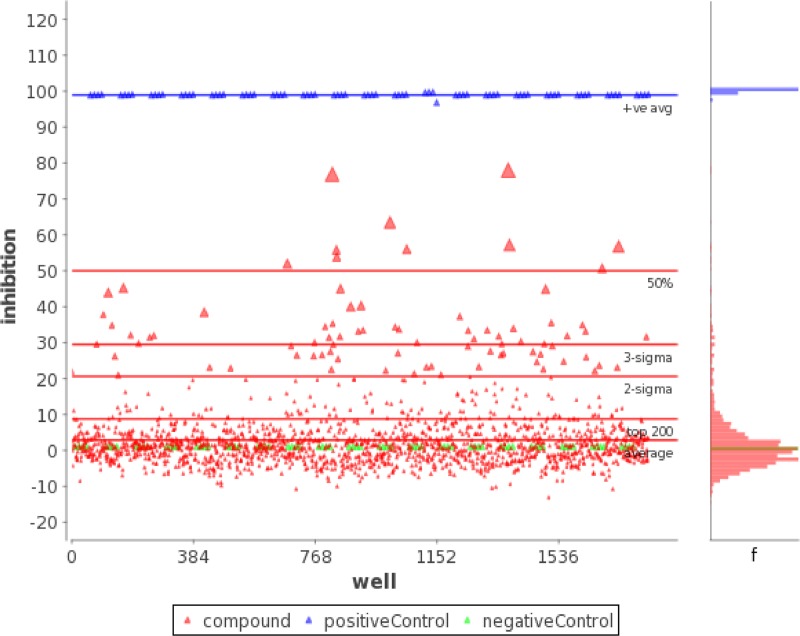
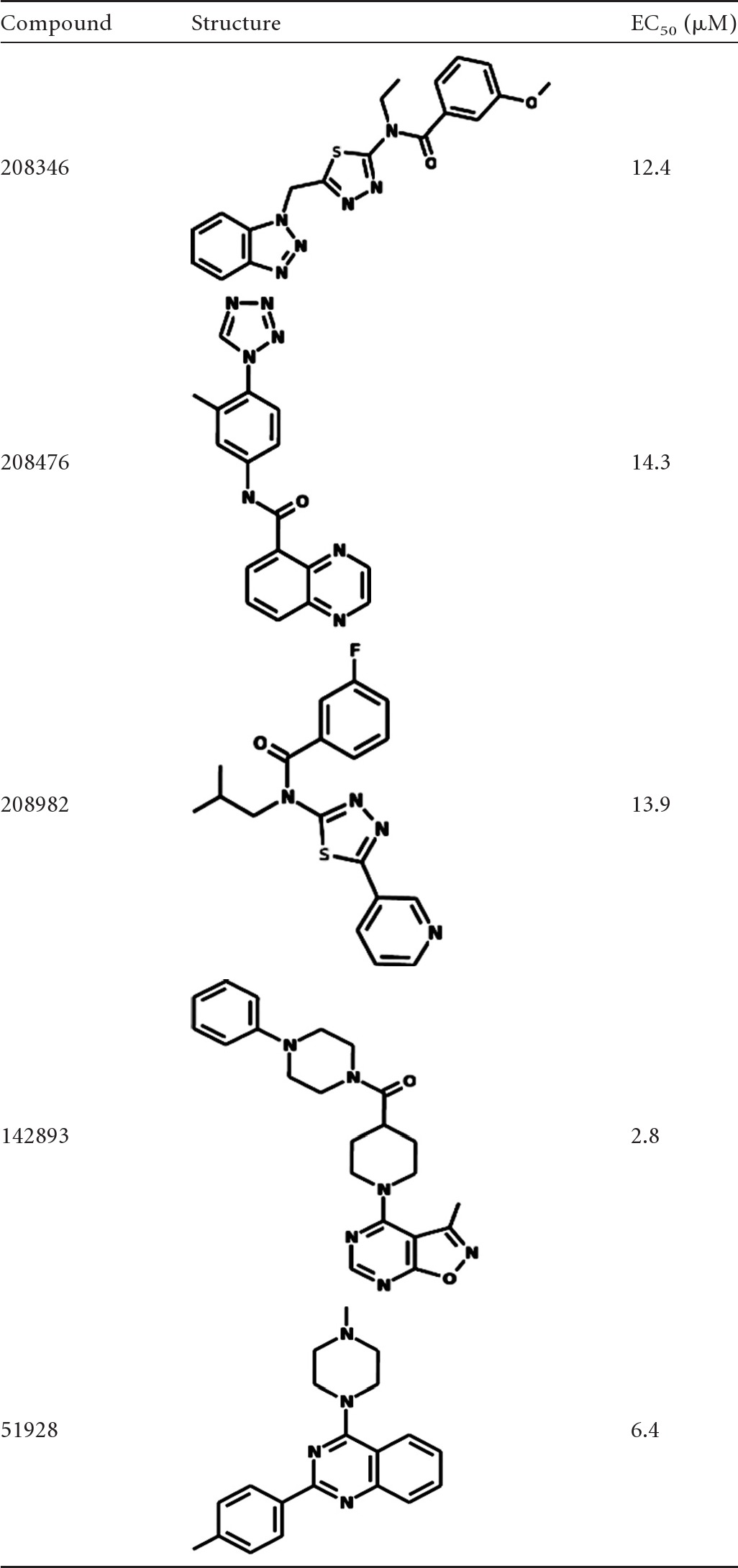
Of the 1,639 kinase-targeted compounds comprising the Asinex library, 10 compounds yielded at least 50% inhibition and a value 3 standard deviations above the mean in the primary screen (Fig. 2). Of these, only three compounds (208346, 208476, and 208982) were confirmed in the secondary screen, with much higher EC50s than that of amphotericin B (Table 2). After interrogating 13,118 compounds of the ChemDiv library at a concentration of 5 μM in 42 384-well plates, we identified two confirmed hits (142893 and 51982), both with higher EC50s than that of amphotericin B (Table 2). The assay showed excellent discrimination between active and inactive compounds, with a Z′ value (dimensionless calculation used to assess the quality of a high-throughput assay) of 0.95 ± 0.1.
Fig 2.
Scatterplot of percent inhibition of each well from 19 96-well plates for the Asinex library. Ten compounds yielded both 50% inhibition and a value 3 standard deviations above the mean for the population of compounds tested in the primary screen (at 5 μM). Positive controls included amphotericin B-treated N. gruberi, and negative controls included 0.5% DMSO-treated N. gruberi.
Table 2.
Structures and EC50s of the Asinex and ChemDiv library hits
Effects of corifungin against N. gruberi and N. fowleri in vitro.
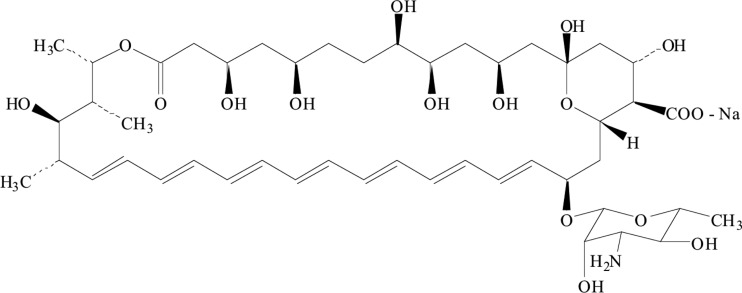
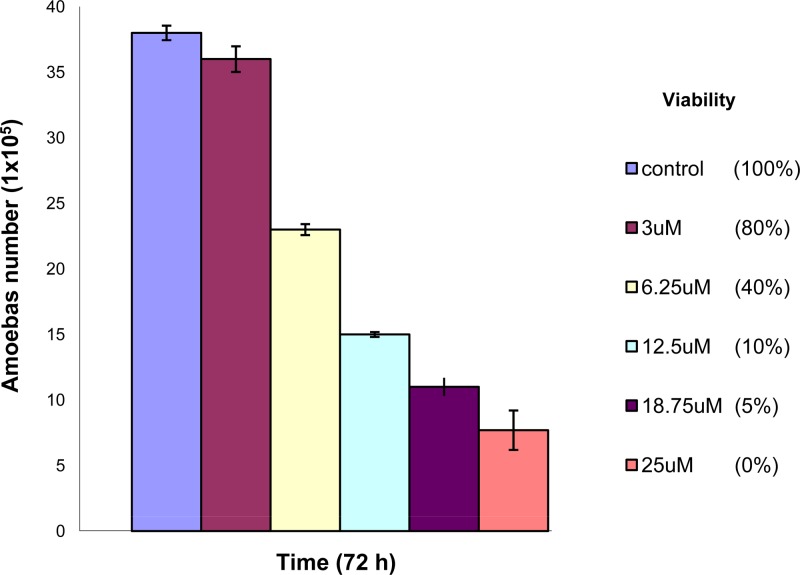
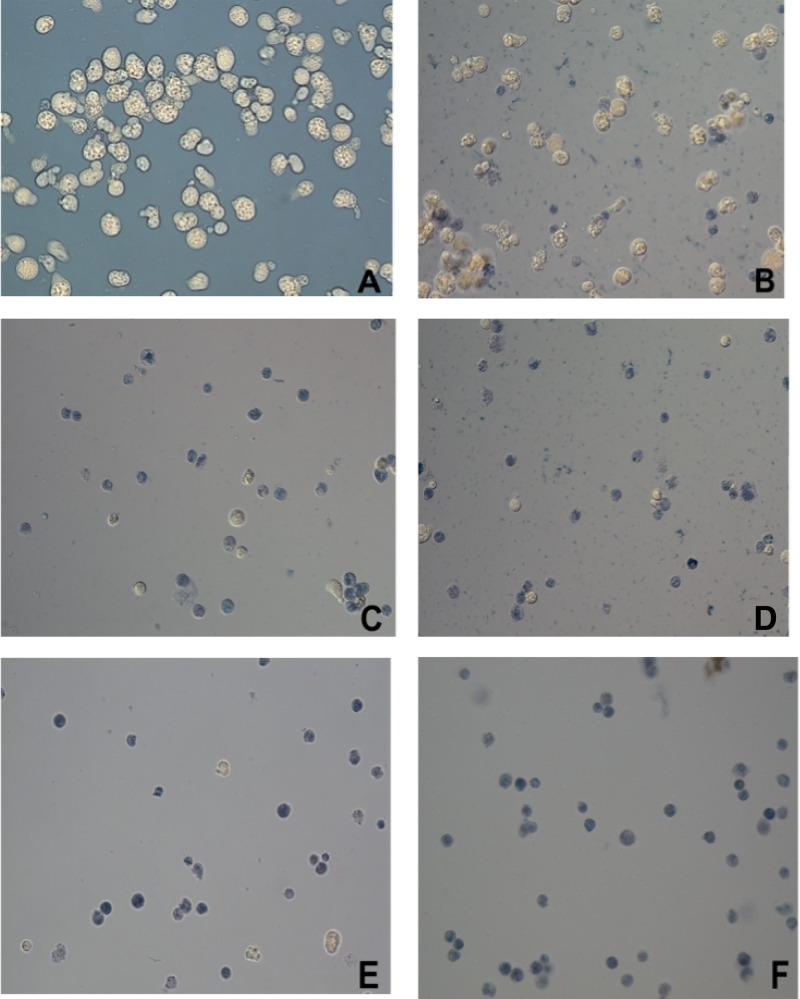
Since corifungin (molecular weight, 947.4; molecular formula, C47H73NO17Na) belongs to the same class of antifungals as amphotericin B (Fig. 3), its activity was tested against nonpathogenic N. gruberi and pathogenic N. fowleri. Corifungin inhibited N. gruberi growth at 8 μM (100% growth inhibition). Its EC50 against N. gruberi was 0.2 μM, which is lower than the EC50 of amphotericin B. N. fowleri trophozoites were incubated with different concentrations of corifungin (3.12 μM, 6.25 μM, 12.5 μM, 18.75 μM, and 25 μM) for 72 h, and the percent viability of N. fowleri was determined by the standard trypan blue exclusion method. The percentages of ameba cell death at 3.12 μM, 6.25 μM, 12.5 μM, 18.75 μM, and 25 μM were 20, 60, 90, 95, and 100%, respectively (Fig. 4 and 5).
Fig 3.
Chemical structure of corifungin.
Fig 4.
Effect of corifungin on N. fowleri growth. Trophozoites were incubated with different concentrations of corifungin for 72 h, and cell numbers were calculated at the end of incubation. Values plotted are means and standard deviations for three independent experiments. The cell viability was evaluated using trypan blue stain.
Fig 5.
N. fowleri viability determination by the trypan blue exclusion method. Trophozoites were incubated with different concentrations of corifungin for 72 h. (A) Amebae with fresh medium only. (B) Amebae with 3.12 μM corifungin. (C) Amebae with 6.25 μM corifungin. (D) Amebae with 12.5 μM corifungin. (E) Amebae with 18.75 μM corifungin. (F) Amebae with 25 μM corifungin. Cells stained blue were considered nonviable. Magnification, ×40.
Transmission electron microscopy.
We also performed transmission electron microscopy to assess ultrastructural changes in N. fowleri induced by different concentrations of corifungin at 72 h (Fig. 6; see Fig. S1 in the supplemental material). We observed damage in the mitochondria and to surface and internal ameba membranes at 3.12 μM corifungin. Cell debris also appeared inside cytoplasmic vacuoles at 3.12 μM corifungin (see Fig. S1A and B in the supplemental material). There was mitochondrial swelling and a total disruption of the cytoplasmic membrane at 6.25 μM and 12.5 μM corifungin (Fig. 6C; see Fig. S1C and D in the supplemental material). Total lysis of the amebae took place at 18.75 μM and 25 μM corifungin (Fig. 6D to F; see Fig. S1E and F in the supplemental material).
Fig 6.
Transmission electron microscopy of N. fowleri trophozoites incubated with different concentrations of corifungin for 72 h. (A) Trophozoite treated with fresh medium only. (B) Higher-magnification view of panel A. (C) Trophozoite treated with 12.5 μM corifungin. The alteration of the plasma membrane is evident (arrows), and also the mitochondria are dilated and cristae are lost (arrowheads). (D) Trophozoite treated with 18.75 μM corifungin. The plasma membrane of the ameba is fragmented (arrows). Arrowheads indicate mitochondria. (E and F) Trophozoites treated with 25 μM corifungin. The amebae show complete lysis. Bars, 2 μm.
In vivo efficacy of corifungin against N. fowleri.
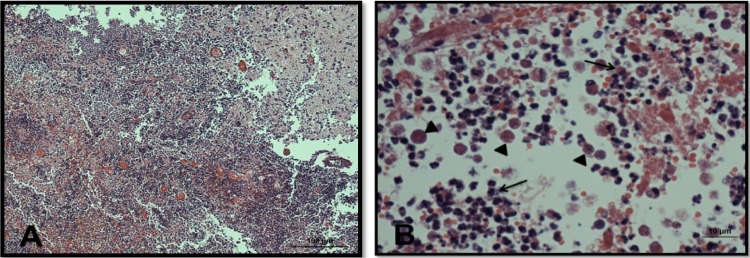
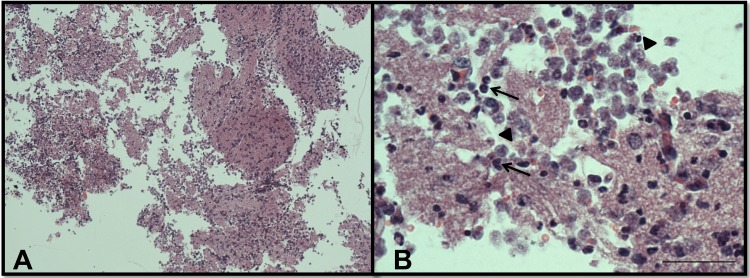
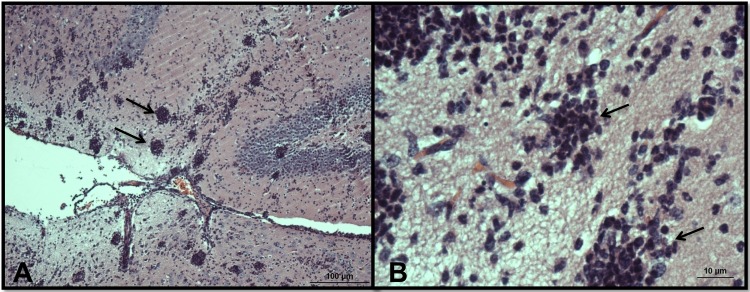
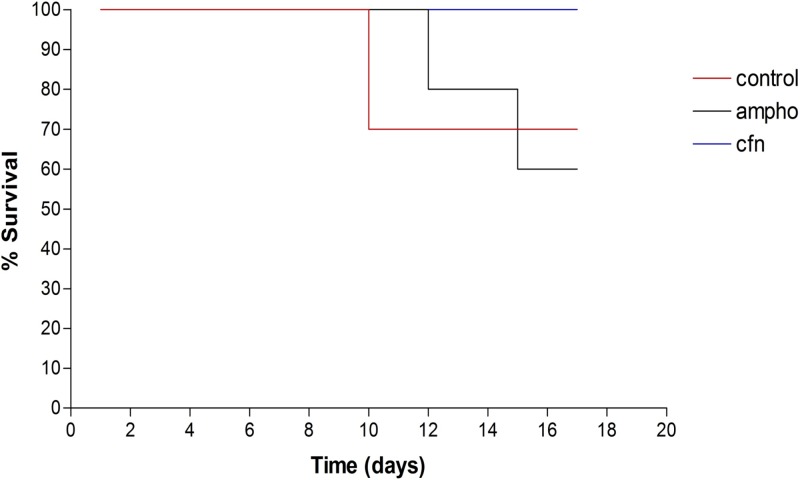
In PBS-only control mice, we observed numerous N. fowleri trophozoites and associated inflammatory cells, mainly neutrophils, in brain tissue (Fig. 7; see Fig. S2 in the supplemental material). There was extensive brain tissue necrosis. Amphotericin B at 9 mg/kg/day for a total of 10 days did not eliminate amebae and inflammatory cells from the olfactory bulbs (Fig. 8; see Fig. S3 in the supplemental material). No detectable amebae were found with an intraperitoneal dose of corifungin of 9 mg/kg/day for a total of 10 days. The olfactory bulbs showed multiple discrete, spherical inflammatory foci without trophozoites. Some areas of the brain showed a focal tissue vacuolization near the inflammation sites (Fig. 9; see Fig. S4 in the supplemental material). Corifungin at 9 mg/kg/day resulted in 100% survival of mice throughout the observation period, whereas similar survival was not observed with amphotericin B at 9 mg/kg/day (Fig. 10 and Table 3). The difference in survival between control and amphotericin B-treated mice was not statistically significant (P > 0.05), whereas the difference in survival between corifungin-treated and control mice was statistically significant (P < 0.001). The difference in survival between corifungin-treated and amphotericin B-treated mice was also statistically significant (P < 0.05).
Fig 7.
Light microscopy of olfactory bulbs in mice infected with N. fowleri and treated with PBS. (A) Olfactory bulbs show a mixed-cell inflammatory reaction with many neutrophils. Necrotic brain tissue is evident. Magnification, ×10. (B) Higher-magnification view of the same area, showing trophozoites (arrowheads) and polymorphonuclear cells (arrows). Magnification, ×60. The tissues were stained with hematoxylin and eosin.
Fig 8.
Light microscopy of olfactory bulbs in mice infected with N. fowleri and treated intraperitoneally with 9 mg/kg/day amphotericin B for a total of 10 days. (A) Olfactory bulbs show several amebae (arrowheads) and a diffuse mixed-cell inflammatory infiltrate associated with widespread tissue necrosis (arrows). Magnification, ×10. (B) Higher-magnification view of the same area. Magnification, ×60. The tissues were stained with hematoxylin and eosin.
Fig 9.
Light microscopy of olfactory bulbs in mice infected with N. fowleri and treated intraperitoneally with 9 mg/kg/day corifungin for a total of 10 days. (A) Olfactory bulbs show multiple spherical inflammatory foci (arrows) without trophozoites. Some areas of the brain have a discrete vacuolization adjacent to the inflammation, but the parenchyma is well preserved, with no evidence of tissue necrosis. Magnification, ×10. (B) Higher-magnification view of the same zone. Magnification, ×60. The tissues were stained with hematoxylin and eosin.
Fig 10.
Survival of N. fowleri-infected mice treated with amphotericin B (ampho) or corifungin (cfn). At 24 h postinoculation, mice (n = 10) were treated once daily with 9 mg/kg of amphotericin B or corifungin for 10 days. Control mice received PBS.
Table 3.
Survival and mean times to death of mice inoculated with N. fowleri and treated for 10 days with amphotericin B or corifungin
| Treatment group (treatment dose [mg/kg/day])a | No. of surviving mice/total no. of mice (%)b | Mean time to death (days)c |
|---|---|---|
| Control | 7/10 (70) | 12 |
| Amphotericin B (9) | 6/10 (60) | 11 |
| Corifungin (9) | 10/10 (100) | None died |
The control group received PBS.
Mice were held for 17 days after inoculation, and the cumulative percentage was recorded on a daily basis.
The mean time to death was calculated based only on dead mice.
DISCUSSION
Traditional methods for the identification of amebicidal compounds are unsuitable for the discovery of anti-Naegleria compounds in a cost-efficient and timely manner. In the present study, we developed an automated, HTS-compatible assay of Naegleria viability that is suitable for identifying amebicidal compounds. This luciferase-based assay is modified from high-throughput screens previously used to measure proliferation, metabolic activity, and cytotoxicity with a number of cell types, including trypanosomes (9, 22, 32, 37). Because of the high virulence of N. fowleri, we first developed the assay for use with the nonpathogenic species N. gruberi and then rescreened active compounds against pathogenic N. fowleri. Though there are differences between N. fowleri and N. gruberi, N. gruberi has been validated as a comparative model for the closely related pathogenic species N. fowleri (26). Analysis of the N. gruberi genome revealed features indicative of a common eukaryotic ancestor (13).
A wide range of antiparasitic, antimicrobial, and other pharmacologic agents have been evaluated against N. fowleri, but these agents have shown limited activity against the pathogen (15, 27, 31). The current drug of choice in treating PAM is the antifungal antibiotic amphotericin B. However, amphotericin B has significant toxicity and is a hydrophobic molecule with negligible solubility in aqueous solutions (11). In interrogating compound libraries for activity against N. gruberi, we purposely selected two different kinds of libraries: one comprising both FDA-approved and known bioactive compounds and the other being a kinase-targeted collection. “Repurposing” of FDA-approved drugs offers shortened development timelines and decreased risk, with compounds having already passed regulatory clinical trials with full toxicological and pharmacokinetic profiles (3). The underlying rationale for selecting kinase-targeted collections was based on the knowledge that kinases are druggable (36) and that the genome of N. gruberi encodes at least 256 predicted protein kinases (13). In the initial HTS, we identified several hits against N. gruberi, but none of them showed a lower EC50 than that of amphotericin B in vitro. Nevertheless, they may represent a viable starting point for future medicinal chemistry optimization. Bay-11-7085, a known bioactive compound, showed strong inhibition (Table 1) but was not considered because it is not FDA approved and has host cell toxicity (5, 16, 39). Five confirmed kinase-targeted hits also had higher EC50s than that of amphotericin B (Table 2).
We focused on corifungin because it had a lower EC50 than that of amphotericin B against N. gruberi. Moreover, as a sodium salt of amphotericin B, it is water soluble (>100 mg/ml). Given the rapid mortality of PAM patients infected by N. fowleri, an efficient, fast-acting, water-soluble, nontoxic drug remains a highly desirable alternative to the conventional treatment.
We have shown in vitro that corifungin effectively kills both nonpathogenic N. gruberi and pathogenic N. fowleri. Ultrastructural analysis showed that low concentrations of corifungin damaged both internal and surface membranes of N. fowleri (Fig. 6C to F; see Fig. S1A to F in the supplemental material). It is generally believed that amphotericin B is a membrane-active drug that forms channel-like structures (pores) spanning the lipid bilayer (4, 8, 28). Based on our transmission electron microscopy study and the structural similarities of amphotericin B and corifungin, we believe that the mechanism of action of corifungin against N. fowleri is similar to that of amphotericin B. We also observed that corifungin targeted mitochondria of N. fowleri, and corifungin was shown to target mitochondria much more effectively than amphotericin B in Aspergillus terreus as well (J. B. Tunac, unpublished observations).
Corifungin is well tolerated in animals, with minimal toxicity. In rats, it is safe up to a level of 250 mg/kg/day when administered by oral gavage for 28 days (pre-investigational new drug document submitted to the U.S. FDA). In the in vivo efficacy testing of corifungin against N. fowleri, we administered a single dose of 9 mg/kg daily for 10 days. This dose was selected based on a previous report that 10-mg/kg amphotericin B treatment on days 3, 7, and 11 resulted in 40% survival during 1 month of observation (19). In our study, 9-mg/kg corifungin treatment resulted in 100% survival among N. fowleri-infected mice throughout the 17-day postinfection observation period, whereas the same dose of amphotericin B produced only 60% survival, similar to that of PBS controls (Table 3). The difference in survival between control and amphotericin B-treated mice was not statistically significant. The survival of mice due to corifungin treatment was maintained longer as well (Fig. 10).
Histopathological studies of the mouse brain at day 17 postinfection showed the presence of amebae and significant tissue lysis in the olfactory bulbs of amphotericin B-treated mice (Fig. 8; see Fig. S3 in the supplemental material), whereas corifungin treatment led to complete elimination of microscopically detectable amebae and to preservation of brain parenchyma (Fig. 9; see Fig. S4 in the supplemental material). Ameba elimination with corifungin was accompanied by a focal mononuclear cell infiltrate likely representing an immune response to dead or dying organisms (Fig. 9; see Fig. S4 in the supplemental material). This contrasted with the diffuse neutrophil-rich inflammatory infiltrate seen in untreated or amphotericin B-treated mice (Fig. 7 and 8; see Fig. S2 and S3 in the supplemental material). Because of its solubility, corifungin may penetrate the blood-brain barrier more effectively than amphotericin B, although this has not been tested. Based on the results presented here, the U.S. FDA has approved an orphan drug designation for corifungin for the treatment of primary amebic meningoencephalitis.
In summary, we have developed an HTS assay for Naegleria and have shown that robust and reproducible results can be generated from this HTS. We identified corifungin as a water-soluble drug with a higher activity than that of amphotericin B. Considering its in vitro and in vivo efficacy and recent orphan drug designation, corifungin is a novel and promising therapeutic option for treatment of PAM.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Sandler Foundation and by NIAID grant 5U01AI077822.
Compound libraries were kindly provided by Michelle R. Arkin and Steven Chen of the UCSF Small Molecule Discovery Center. We are grateful to Zac Cande of the University of California, Berkeley, for providing the N. gruberi NEG-M strain and for many helpful discussions.
Footnotes
Published ahead of print 6 August 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Anderson K, Jamieson A. 1972. Primary amoebic meningoencephalitis. Lancet i:902–903 [DOI] [PubMed] [Google Scholar]
- 2. Apley J, et al. 1970. Primary amoebic meningoencephalitis in Britain. Br. Med. J. 1:596–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ashburn TT, Thor KB. 2004. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 3:673–683 [DOI] [PubMed] [Google Scholar]
- 4. Baginski M, Sternal K, Czub J, Borowski E. 2005. Molecular modelling of membrane activity of amphotericin B, a polyene macrolide antifungal antibiotic. Acta Biochim. Pol. 52:655–658 [PubMed] [Google Scholar]
- 5. Berger N, Ben Bassat H, Klein BY, Laskov R. 2007. Cytotoxicity of NF-kappaB inhibitors Bay 11-7085 and caffeic acid phenethyl ester to Ramos and other human B-lymphoma cell lines. Exp. Hematol. 35:1495–1509 [DOI] [PubMed] [Google Scholar]
- 6. Brown RL. 1991. Successful treatment of primary amebic meningoencephalitis. Arch. Intern. Med. 151:1201–1202 [PubMed] [Google Scholar]
- 7. Cervantes-Sandoval I, Serrano-Luna Jde J, Garcia-Latorre E, Tsutsumi V, Shibayama M. 2008. Characterization of brain inflammation during primary amoebic meningoencephalitis. Parasitol. Int. 57:307–313 [DOI] [PubMed] [Google Scholar]
- 8. Chattopadhyay A, Jafurulla M. 2011. A novel mechanism for an old drug: amphotericin B in the treatment of visceral leishmaniasis. Biochem. Biophys. Res. Commun. 416:7–12 [DOI] [PubMed] [Google Scholar]
- 9. Cooksey RC, Crawford JT, Jacobs WR, Jr, Shinnick TM. 1993. A rapid method for screening antimicrobial agents for activities against a strain of Mycobacterium tuberculosis expressing firefly luciferase. Antimicrob. Agents Chemother. 37:1348–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Debnath A, et al. 2012. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat. Med. 18:956–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Falk R, Domb AJ, Polacheck I. 1999. A novel injectable water-soluble amphotericin B-arabinogalactan conjugate. Antimicrob. Agents Chemother. 43:1975–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finney DJ. 1971. Probit analysis, 3rd ed University Press, Cambridge, England [Google Scholar]
- 13. Fritz-Laylin LK, et al. 2010. The genome of Naegleria gruberi illuminates early eukaryotic versatility. Cell 140:631–642 [DOI] [PubMed] [Google Scholar]
- 14. Fulton C, Webster C, Wu JS. 1984. Chemically defined media for cultivation of Naegleria gruberi. Proc. Natl. Acad. Sci. U. S. A. 81:2406–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goswick SM, Brenner GM. 2003. Activities of azithromycin and amphotericin B against Naegleria fowleri in vitro and in a mouse model of primary amebic meningoencephalitis. Antimicrob. Agents Chemother. 47:524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernandez-Gutierrez S, et al. 2006. NF-kappaB signaling blockade by Bay 11-7085 during early cardiac morphogenesis induces alterations of the outflow tract in chicken heart. Apoptosis 11:1101–1109 [DOI] [PubMed] [Google Scholar]
- 17. Jain R, Prabhakar S, Modi M, Bhatia R, Sehgal R. 2002. Naegleria meningitis: a rare survival. Neurol. India 50:470–472 [PubMed] [Google Scholar]
- 18. Jarolim KL, McCosh JK, Howard MJ, John DT. 2000. A light microscopy study of the migration of Naegleria fowleri from the nasal submucosa to the central nervous system during the early stage of primary amebic meningoencephalitis in mice. J. Parasitol. 86:50–55 [DOI] [PubMed] [Google Scholar]
- 19. Kim JH, et al. 2008. Effect of therapeutic chemical agents in vitro and on experimental meningoencephalitis due to Naegleria fowleri. Antimicrob. Agents Chemother. 52:4010–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lawande RV, John I, Dobbs RH, Egler LJ. 1979. A case of primary amebic meningoencephalitis in Zaria, Nigeria. Am. J. Clin. Pathol. 71:591–594 [DOI] [PubMed] [Google Scholar]
- 21. Loschiavo F, Ventura-Spagnolo T, Sessa E, Bramanti P. 1993. Acute primary meningoencephalitis from entamoeba Naegleria fowleri. Report of a clinical case with a favourable outcome. Acta Neurol. 15:333–340 [PubMed] [Google Scholar]
- 22. Mackey ZB, et al. 2006. Discovery of trypanocidal compounds by whole cell HTS of Trypanosoma brucei. Chem. Biol. Drug Des. 67:355–363 [DOI] [PubMed] [Google Scholar]
- 23. McCurdy DK, Frederic M, Elkinton JR. 1968. Renal tubular acidosis due to amphotericin B. N. Engl. J. Med. 278:124–130 [DOI] [PubMed] [Google Scholar]
- 24. Poungvarin N, Jariya P. 1991. The fifth nonlethal case of primary amoebic meningoencephalitis. J. Med. Assoc. Thai. 74:112–115 [PubMed] [Google Scholar]
- 25. Proffitt RT, Satorius A, Chiang SM, Sullivan L, Adler-Moore JP. 1991. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J. Antimicrob. Chemother. 28(Suppl B):49–61 [DOI] [PubMed] [Google Scholar]
- 26. Sanchez-Flores A. 2011. A new piece of the eukaryotic puzzle. Nat. Rev. Microbiol. 9:769. [DOI] [PubMed] [Google Scholar]
- 27. Schuster FL, Guglielmo BJ, Visvesvara GS. 2006. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J. Eukaryot. Microbiol. 53:121–126 [DOI] [PubMed] [Google Scholar]
- 28. Schuster FL, Visvesvara GS. 2004. Opportunistic amoebae: challenges in prophylaxis and treatment. Drug Resist. Updat. 7:41–51 [DOI] [PubMed] [Google Scholar]
- 29. Seidel JS, et al. 1982. Successful treatment of primary amebic meningoencephalitis. N. Engl. J. Med. 306:346–348 [DOI] [PubMed] [Google Scholar]
- 30. Singh SN, Patwari AK, Dutta R, Taneja N, Anand VK. 1998. Naegleria meningitis. Indian Pediatr. 35:1012–1015 [PubMed] [Google Scholar]
- 31. Soltow SM, Brenner GM. 2007. Synergistic activities of azithromycin and amphotericin B against Naegleria fowleri in vitro and in a mouse model of primary amebic meningoencephalitis. Antimicrob. Agents Chemother. 51:23–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sykes ML, Avery VM. 2009. A luciferase based viability assay for ATP detection in 384-well format for high throughput whole cell screening of Trypanosoma brucei brucei bloodstream form strain 427. Parasit. Vectors 2:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vargas-Zepeda J, et al. 2005. Successful treatment of Naegleria fowleri meningoencephalitis by using intravenous amphotericin B, fluconazole and rifampicin. Arch. Med. Res. 36:83–86 [DOI] [PubMed] [Google Scholar]
- 34. Visvesvara GS. 2010. Amebic meningoencephalitides and keratitis: challenges in diagnosis and treatment. Curr. Opin. Infect. Dis. 23:590–594 [DOI] [PubMed] [Google Scholar]
- 35. Wang A, Kay R, Poon WS, Ng HK. 1993. Successful treatment of amoebic meningoencephalitis in a Chinese living in Hong Kong. Clin. Neurol. Neurosurg. 95:249–252 [DOI] [PubMed] [Google Scholar]
- 36. Weigelt J, McBroom-Cerajewski LD, Schapira M, Zhao Y, Arrowsmith CH. 2008. Structural genomics and drug discovery: all in the family. Curr. Opin. Chem. Biol. 12:32–39 [DOI] [PubMed] [Google Scholar]
- 37. Xia M, et al. 2008. Compound cytotoxicity profiling using quantitative high-throughput screening. Environ. Health Perspect. 116:284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoder JS, Eddy BA, Visvesvara GS, Capewell L, Beach MJ. 2010. The epidemiology of primary amoebic meningoencephalitis in the U. S. A., 1962–2008. Epidemiol. Infect. 138:968–975 [DOI] [PubMed] [Google Scholar]
- 39. Zahradka P, et al. 2002. NF-kappaB activation is essential for angiotensin II-dependent proliferation and migration of vascular smooth muscle cells. J. Mol. Cell. Cardiol. 34:1609–1621 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.