Abstract
Antiretroviral-based microbicides applied topically to the vagina may play an important role in protecting women from HIV infection. Incorporation of the nucleoside reverse transcriptase inhibitor tenofovir (TFV) into intravaginal rings (IVRs) for sustained mucosal delivery may lead to increased microbicide product adherence and efficacy compared with those of conventional vaginal formulations. Formulations of a novel “pod IVR” platform spanning a range of IVR drug loadings and daily release rates of TFV were evaluated in a pig-tailed macaque model. The rings were safe and exhibited sustained release at controlled rates over 28 days. Vaginal secretion TFV levels were independent of IVR drug loading and were able to be varied over 1.5 log units by changing the ring configuration. Mean TFV levels in vaginal secretions were 72.4 ± 109 μg ml−1 (slow releasing) and 1.84 ± 1.97 mg ml−1 (fast releasing). The mean TFV vaginal tissue concentration from the slow-releasing IVRs was 76.4 ± 54.8 μg g−1 and remained at steady state 7 days after IVR removal, consistent with the long intracellular half-life of TFV. Intracellular tenofovir diphosphate (TFV-DP), the active moiety in defining efficacy, was measured in vaginal lymphocytes collected in the study using the fast-releasing IVR formulation. Mean intracellular TFV-DP levels of 446 ± 150 fmol/106 cells fall within a range that may be protective of simian-human immunodeficiency virus strain SF162p3 (SHIVSF162p3) infection in nonhuman primates. These data suggest that TFV-releasing IVRs based on the pod design have potential for the prevention of transmission of human immunodeficiency virus type 1 (HIV-1) and merit further clinical investigation.
INTRODUCTION
As the global human immunodeficiency virus (HIV)/AIDS pandemic enters its fourth decade, infection rates remain alarmingly high. The global incidence of HIV was estimated at 2.6 million in 2009, and 22 million more people are predicted to acquire HIV by 2031 (16, 42). Campaigns aimed at encouraging monogamy and condom use have had limited success in areas where marriage has been identified as the major risk factor for HIV acquisition in women (2, 7, 9, 14, 26). For decades, all attempts to develop a preexposure prophylaxis (PrEP) strategy for the prevention of HIV transmission have failed (5, 42). Recently, two large-scale clinical trials, iPrEx (12) and CAPRISA 004 (20), have shown that PrEP using antiretroviral-based microbicides can prevent infection in a significant proportion of individuals (16, 42).
In CAPRISA 004, participants who were instructed to use a pericoital vaginal gel containing 1% tenofovir (TFV) showed a 39% reduction in HIV transmission compared to those receiving placebo gel (20). In late November 2011, the Data and Safety Monitoring Board of the VOICE trial, a major HIV prevention study in women that included a TFV vaginal gel component (http://www.mtnstopshiv.org/node/3909), issued a statement on the decision to discontinue the use of the TFV gel due to lack of efficacy. While the reasons underlying these different outcomes remain to be elucidated, they highlight the pressing need for an improved understanding of the fundamental processes affecting efficacy.
Adherence remains a critical component in the development of effective PrEP for HIV prevention. High adherers in the CAPRISA 004 trial (gel adherence > 80%) showed a 54% lower HIV incidence than the control arm (20). Since intravaginal rings (IVRs) likely increase adherence, the development of IVR formulations of antiretroviral-based microbicides is an urgent global priority (11, 38, 40, 41, 43). Administration of pharmaceutical agents via IVRs represents a coitally independent approach for local or systemic delivery (46), thereby increasing efficacy and adherence to therapy while potentially decreasing toxic side effects compared to daily oral administration. IVRs have been shown to be well tolerated by women and are used extensively in contraception and in estrogen replacement therapy (49), with several products available commercially (27). Sustained vaginal delivery of antiviral agents from IVRs constitutes an attractive route to HIV PrEP in women, particularly in the developing world (32, 41).
Nonhuman primates are considered the most appropriate model for screening microbicide candidates (48). Many believe that rigorous safety and pharmacokinetic (PK) testing in nonhuman primates should be a key factor for advancing microbicide candidates to clinical trials (47, 48). The pig-tailed macaque model is particularly relevant because of its similarities with the human vaginal anatomy and physiology (47). Like humans, pig-tailed macaques generally have a 28-day menstrual cycle (36). Progesterone administration to synchronize their menstrual cycle during PK, safety, and efficacy studies is not required, providing a significant advantage, as this treatment has been shown to thin the vaginal epithelium, leading to an increase in permeability to chemical agents and viral particles (35). Culture-dependent (36) and independent (44, 50) studies on the pig-tailed macaque vaginal microbiota have shown that it is an excellent model for the human vaginal microbiome. Pig-tailed macaques have therefore been used extensively in the evaluation of vaginal products (36), including gel-based microbicide studies (37), prior to widespread use in women.
Female pig-tailed macaques receiving intravaginal application of 1% TFV gel 30 min prior to challenge with simian-human immunodeficiency virus strain SF162p3 (SHIVSF162p3) on a twice-weekly dose-and-challenge schedule were completely protected from infection, even after 20 exposures to virus (35). Topical delivery of TFV from IVRs in macaques has not been reported. A macaque model using a range of appropriately sized, unmedicated, silicone elastomer IVRs has been developed recently (38). We have developed IVRs that deliver TFV at controlled rates for up to 3 months (4, 28) and have evaluated these devices in the ovine model (29, 30). The IVRs were found to be safe, and they maintained steady-state TFV levels in vaginal secretions and tissue over the 28-day trial. The present study examines the PK and safety profiles of appropriately sized TFV-releasing IVRs in female pig-tailed macaques and is the first report demonstrating sustained vaginal TFV release from long-acting IVR devices in the macaque model.
MATERIALS AND METHODS
Study background.
The characteristics of the TFV IVR formulations used in the three studies, PK1 to PK3, are provided in Table 1. In the first study, PK1, IVRs were formulated to release TFV at twice the rate of human-sized rings from previous sheep studies (29, 30), simply by doubling the number of TFV pods. In PK2, the impact on release rate of increasing the drug load by 5-fold while maintaining the same IVR configuration (i.e., four PLA-coated pods, 0.79-mm2 delivery channels) (Table 1) was investigated. In the only terminal study, PK3, the IVR configuration was modified to increase the TFV release rate with the goal of attaining detectable intracellular tenofovir diphosphate (TFV-DP) levels in the pharmacological compartments of relevance for HIV PrEP efficacy (6). This was achieved by formulating the IVRs to release most of their TFV payload over the first 7 days of the study.
Table 1.
Physical characteristics of intravaginal rings used in pig-tailed macaque studiesa
| Physical characteristic | Values in each macaque study |
||
|---|---|---|---|
| PK1 | PK2 | PK3 | |
| No. of pods | 4 | 4 | 4 |
| TFV reservoir (mg) | 12 | 64 | 80 |
| Pod-coating polymer | PLA | PLA | PVA |
| Delivery channel SA (mm2) | 0.79 | 0.79 | 1.77b |
PLA, poly-d,l-lactide; PVA, polyvinyl alcohol; SA, surface area.
Three channels per pod.
Manufacture of silicone intravaginal rings.
Silicone IVRs were prepared in a multistep process that has been described in detail elsewhere (4, 30). The torus-shaped devices were fabricated according to the dimensions (outer diameter [o.d.], 25 mm; inner diameter [i.d.], 15 mm; cross-sectional diameter, 5 mm) recommended by Promadej-Lanier et al. (38). The IVR device specifications are provided in Table 1. Ring blanks containing empty, evenly spaced cavities were fabricated by injection molding, and delivery channels were fashioned mechanically. Drug cores of ({[(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy}methyl)phosphonic acid (tenofovir [TFV]; Sinoway International, China) were polymer coated to afford so-called “pods.” Pods were placed in the cavities that subsequently were back-filled with room temperature cure silicone. An image of the medicated macaque IVR is shown in Fig. 1. Control rings contained silicone plugs of the same dimensions as the drug pods.
Fig 1.
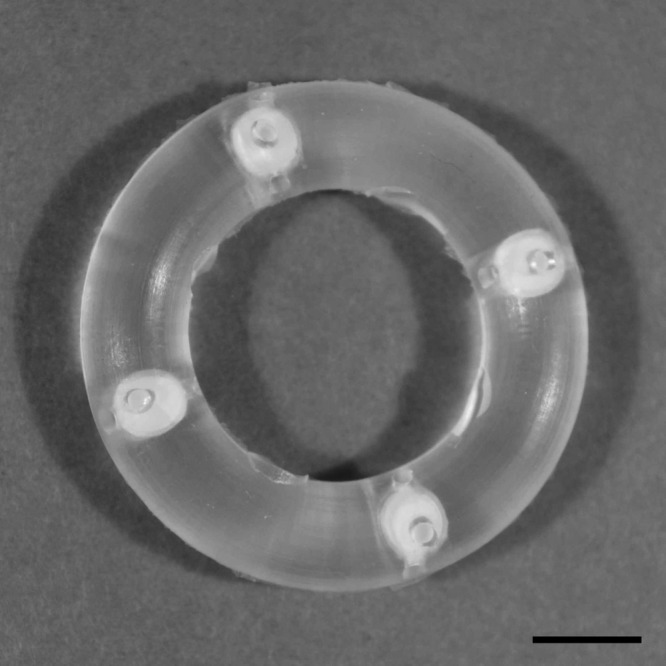
Photograph of a 4-pod macaque IVR. Scale bar, 5 mm.
In vitro studies.
The IVR formulations shown in Table 1 were evaluated in vitro as part of the quality control procedures prior to conducting in vivo studies and to establish an in vitro-in vivo correlation (IVIVC), as described below. All in vitro release studies were designed to mimic sink conditions, and detailed procedures are presented elsewhere (4). Briefly, the IVRs were placed in dissolution medium so that the delivery windows were facing up and not in contact with the jar surface. The medium consisted of a simplified vaginal fluid simulant (VFS) (34) consisting of 25 mM acetate buffer (pH 4.2) with NaCl added to achieve 220 mOs. The vessels were agitated in an orbital shaker at 25 ± 2°C and 60 rpm. Aliquots (200 μl) were removed at predetermined time points and were replaced with an equal volume of dissolution medium. This dilution of the in vitro release solution was taken into account in the concentration determinations for each absorption measurement. The concentration of TFV was measured as a function of time by UV-visible absorption spectroscopy. Concentrations were calculated from absorbance values at 260 nm using Beer's Law (18), and a linear concentration-absorbance dependence was observed over the measurement range.
Nonhuman primate studies.
Three pharmacokinetic (PK) studies were carried out at the Centers for Disease Control and Prevention (CDC) under approved CDC Institutional Animal Care and Use Committee protocols and standard guidelines according to the Guide for the Care and Use of Laboratory Animals (31). In the first study, PK1 (November 2009), six sexually mature female pig-tailed macaques (Macaca nemestrina) were used; four received medicated IVRs (Table 1), and two received blank control IVRs. The second study, PK2 (July 2010), used three sexually mature female pig-tailed macaques receiving medicated IVRs according to the characteristics shown in Table 1. The third study, PK3 (July 2011), consisted of a terminal PK study and used two SHIV-infected, sexually mature female pig-tailed macaques with medicated IVRs (Table 1). SHIV-infected macaques released from previous efficacy studies were used in PK3 since this study needs to be terminal in order to furnish sufficient vaginal tissue for the detection of intracellular TFV-DP levels in tissue monocytes. While it is acknowledged that the inclusion of only two animals impacts the statistical significance of the data, it is currently the only feasible experimental approach, as supported by other reports (6), because of the low availability of infected macaques. The IVRs were inserted on day 0 into the posterior vagina and retained for a period of 28 days for PK1 and PK2 or 14 days for PK3.
Vaginal colposcopy was used to confirm placement and retention of the vaginal rings. The animals were placed in ventral recumbency while under general anesthesia. A pediatric speculum was used to open the vaginal vault to visualize the IVR placement. A Rebel T1i/EOS 500D digital camera (15 megapixels; Canon, Melville, NY) was used along with a model number LR66238 colposcope (Carl Zeiss, Dublin, CA).
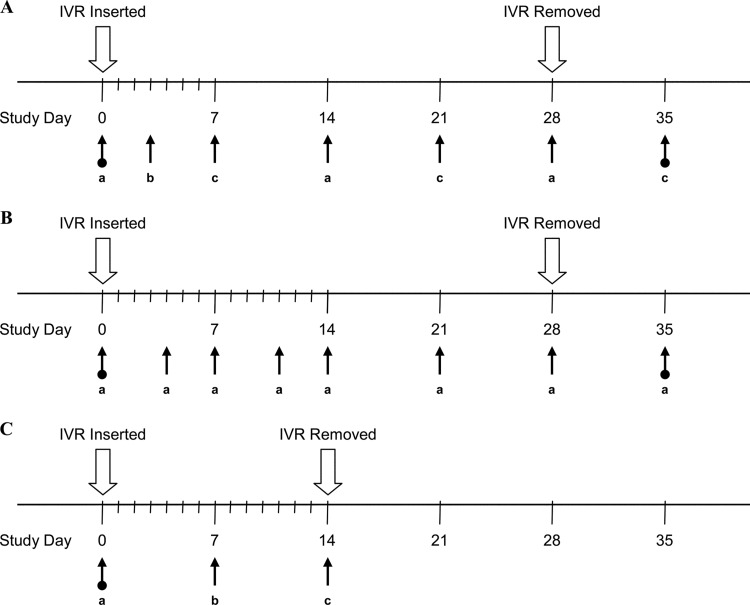
Study timelines and biological sample collection points are shown in Fig. 2 and include blood, vaginal secretion (Weck-Cel), cervicovaginal lavage (CVL) fluid, and tissue sample collection. Vaginal secretions were collected proximal (ectocervix) and distal (introitus) to the ring placement. For CVL fluid collection, phosphate-buffered saline solution (4 to 5 ml) was gently infused into the vaginal vault via a sterile 10-ml syringe attached to a sterile gastric feeding tube (size 5 or 8 French) of adjusted length and CVL fluid was drawn out with the same device. The CVL fluid was observed for the presence of blood or any other discoloration. In the nonterminal study (PK1), tissue collection consisted of vaginal biopsies. In the terminal study (PK3), the animals were necropsied on day 14 and the vaginal tissue obtained was sectioned into three areas, proximal, medial, and distal vaginal segments relative to the location of the IVR. Animals were sedated when procedures were performed, and after recovery from anesthesia, the animals were awake and mobile in their cages. Animal enrichment procedures are in place to promote the health and well-being of the animals.
Fig 2.
Macaque study timelines and biological sample collection points. (A) PK1 (4 medicated; 2 placebo). (a) Blood and CVL fluid. (b) CVL fluid collection. (c) Blood, CVL fluid, and tissue collection. Four predose sample collection points (days −21, −14, −5, and 0) and one postdose sample collection point (day 35). (B) PK2 (3 medicated). (a) Blood, CVL fluid, and vaginal secretion (Weck-Cel) collection. Two predose sample collection points (days −8 and 0) and one postdose sample collection point (day 35). (C) PK3 (2 medicated). (a) Blood collection. (b) Blood, CVL fluid, and vaginal secretion (Weck-Cel) collection. (c) Blood, CVL fluid, vaginal secretion (Weck-Cel), and tissue collection. Two predose sample collection points (days −14 and 0).
Blood and CVL fluid were obtained up to 3 weeks prior to the insertion of the ring in order to establish baseline cytokine profiles. Amicon ultra-4 10-kDa concentrators (Millipore, Billerica, MA) were used to concentrate the CVL fluids for cytokine analysis. Induction of mucosal inflammation was monitored by measuring vaginal and systemic cytokines as previously described using fluorescent multiplexed bead-based assays (Invitrogen, Carlsbad, CA, and Bio-Rad, Hercules, CA) in accordance with the manufacturer's instructions (38). Tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), macrophage inflammatory protein 1α (MIP-1α), regulated-upon-activation, normal T-cell-expressed, and secreted (RANTES), granulocyte colony-stimulating factor (G-CSF), macrophage inflammatory protein 1β (MIP-1β), interleukin-8 (IL-8), interleukin-1 receptor antagonist (IL-1Ra), eotaxin, interleukin-15 (IL-15), and interleukin-12 p40 (IL-12p40) were analyzed by the above-described methods. Although additional cytokines were assayed, those that were below the level of detection are not reported. Statistical analysis was performed using the Mann-Whitney U test.
Vaginal biopsy specimens were taken according to the timelines shown in Fig. 2 to assess the integrity of the vaginal tissue and the presence of mononuclear infiltrates in the mucosal tissue.
Sterile swabs were collected from all macaques to complete a microbiological characterization on days −14, 0, 7, 21, 28, and 35 in PK1. Each swab was placed individually in a Port-a-Cul (Becton, Dickinson) tube and transported to Magee Women's Research Institute within 24 h of collection for microbial analysis. The swabs were characterized for the presence of aerobic and anaerobic microorganisms. Details on the methods used to assess vaginal microflora have been provided elsewhere (13).
Used intravaginal implants were analyzed for residual drug content at Oak Crest Institute using the following methods. The silicone surrounding the drug pods was cut with a scalpel, and the excised material was dissolved in deionized water with sonication. The resulting solution was analyzed by high-performance liquid chromatography (HPLC), and these data were used to back-calculate the residual drug content according to published methods (29, 30).
A published method for plasma TFV (24) was adapted to analyze TFV in CVL fluid, vaginal secretions from Weck-Cel spears, plasma, and tissue samples. Biopsy samples were incubated with tenofovir-d6 as the internal standard in 100% methanol for 30 min and then sonicated for 30 min. The supernatant was transferred to a microtiter plate and evaporated to near dryness. A volume of 100 μl of buffer A (20 mM formic acid, 20 mM acetic acid; pH 3.2) was added to all samples and evaporated again to near dryness. Another 100 μl of buffer A was then added to all samples, and the plate was sealed and stored at −20°C until used. For CVL and biopsy samples, known negative samples were spiked to provide quality control samples. The method of detection was based on the procedures previously described (24), with the following changes. In this study, no column switching was performed, and a 150- by 2-mm Synergi Polar-RP column (Phenomenex) was used. Samples were analyzed using a 3200QTrap (Applied Biosystems) mass spectrometer equipped with a Prominence HPLC system (Shimadzu). The vaginal lymphocytes were processed from the tissue sections obtained in PK3, and the intracellular concentration of tenofovir diphosphate (TFV-DP) was determined as described previously (6). In addition, TFV-DP concentrations were measured in peripheral blood mononuclear cells (PBMCs) and mononuclear cells isolated from rectal tissue and the draining inguinal lymph nodes.
The TFV lower limits of quantitation (LLOQs) for CVL fluid and plasma were 5 and 7 ng ml−1, respectively, and the LLOQ was 15 ng g−1 in tissue samples. The intracellular TFV-DP LLOQ was 10 ng ml−1 (6, 25), which corresponds to 13 fmol/106 cells (25). All calibration curves had R2 values greater than 0.99.
RESULTS
In vitro studies.
In vitro cumulative release profiles for the three IVR formulations exhibited linear (R2 > 0.95) sustained release of TFV over the course of the studies, as is typical for pod IVRs (4, 29, 30). The cumulative release rates over these time periods are shown in Table 2.
Table 2.
In vitro and in vivo daily release rates and IVIVC of TFV IVRs
| Macaque study (no. of macaques) | Release rate (mg day−1)a |
IVIVCb | |
|---|---|---|---|
| In vitro | In vivo | ||
| PK1 (4) | 0.23 ± 0.033 | 0.37 ± 0.048c | 1.6c |
| PK2 (3) | 0.23 ± 0.040 | 1.08 ± 0.038 | 4.7 |
| PK3 (2) | 14.1 | NMd | NM |
Values are means and SDs.
Defined as the in vivo release rate divided by the in vitro release rate.
The in vivo release rate biased low because the complete 12-mg drug payload was delivered in less than 28 days according to residual drug measurements on the used IVRs.
Not measured, as the IVRs delivered most of their TFV payload within the first week of the 14-day study (Fig. 5) so that an accurate release rate, and hence the IVIVC, was not able to be determined by residual drug analysis.
Nonhuman primate studies: safety profiles.
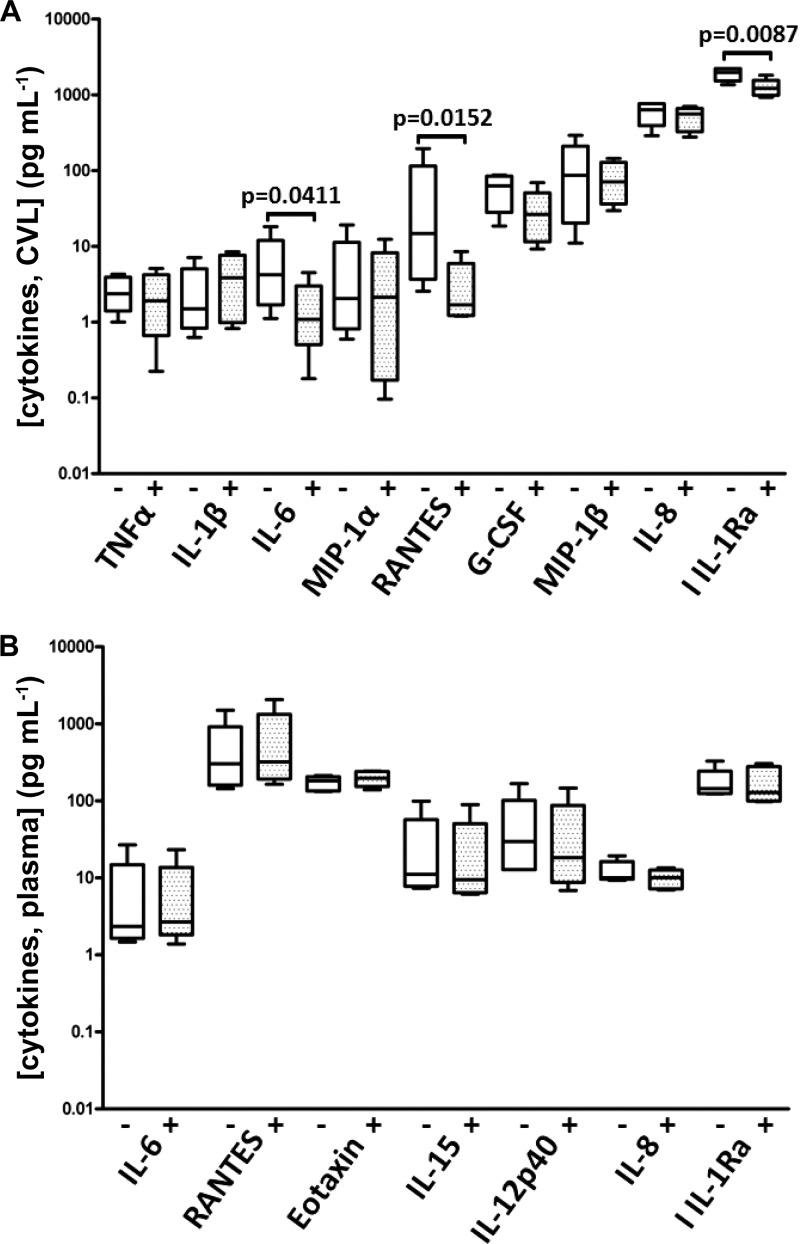
No ring expulsions or behavioral changes were noted throughout all studies, and adverse effects were not observed by colposcopy. Cytokine profiles, both locally and systemically, were stable when samples collected before and after ring removal were compared to those from when the ring was in place and releasing drug (Fig. 3). The proinflammatory cytokines TNF-α, IL-1β, MIP-1α, IL-8, and MIP-1β were all unchanged in the local mucosal environment, and no increases were observed systemically in the plasma.
Fig 3.
Cytokine levels (means and standard deviations) in vaginal lavage fluid (A) and plasma (B) from macaques (n = 6) in PK1. The + and − symbols denote time points when the samples were collected, with the IVR either absent (5 time points) or present (4 time points).
Details on the impact of the IVRs in PK1 on vaginal microflora have been presented elsewhere (13), and only a brief discussion is included below. The ring surfaces were covered with monolayers of epithelial cells. Two bacterial biofilm phenotypes were found to develop on these monolayers, and both had a broad diversity of bacterial cells closely associated with the extracellular material (13). These structures did not appear to impact the IVR release rates significantly.
IVIVC.
The in vitro-in vivo correlation (IVIVC) was analyzed to describe a framework that minimizes in vivo studies required in future research. In vitro experiments therefore may guide parameter selection in the development of sustained-release formulations. The calculated IVIVC for PK1 and PK2 is given in Table 2 and indicates that release rates in vivo are ca. 5 times as fast as those measured in vitro. Note that the in vivo release rate for PK1 is biased low because the complete 12-mg drug payload was delivered in less than 28 days according to residual drug measurements on the used IVRs. No IVIVC is presented for PK3, as this study was designed to have most of the drug delivered in the first 7 days of the study (see Discussion).
Nonhuman primate studies: pharmacokinetics.
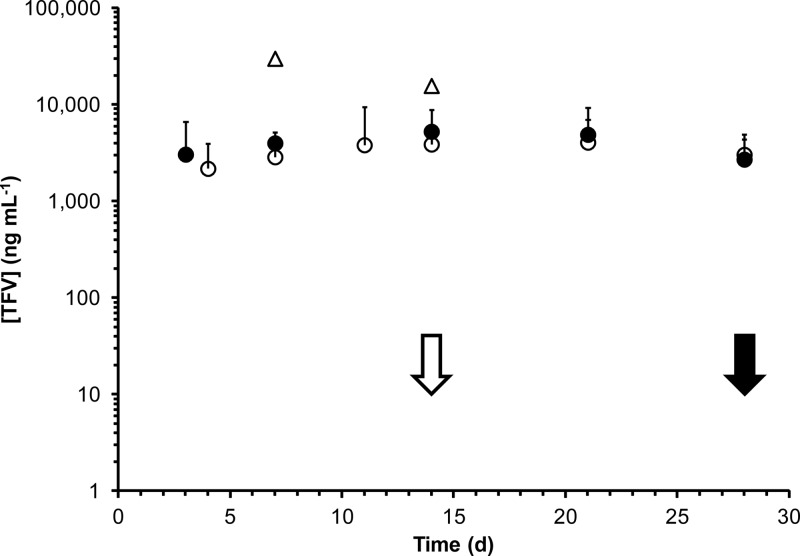
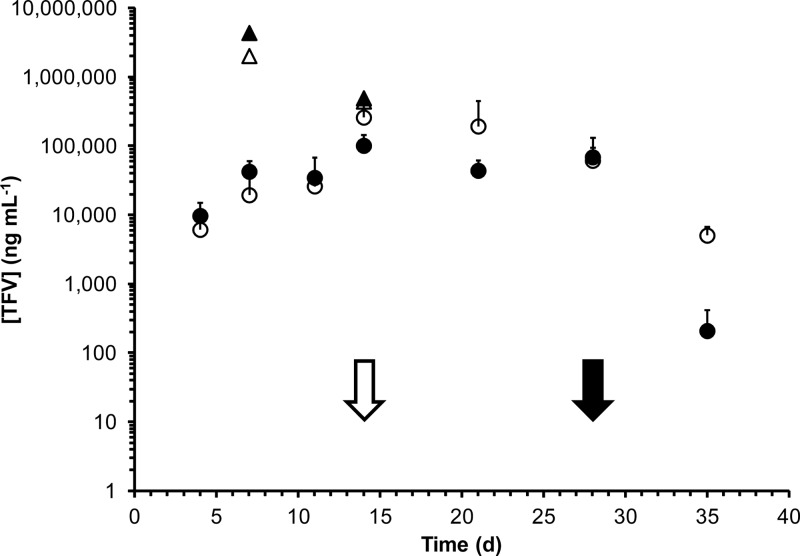
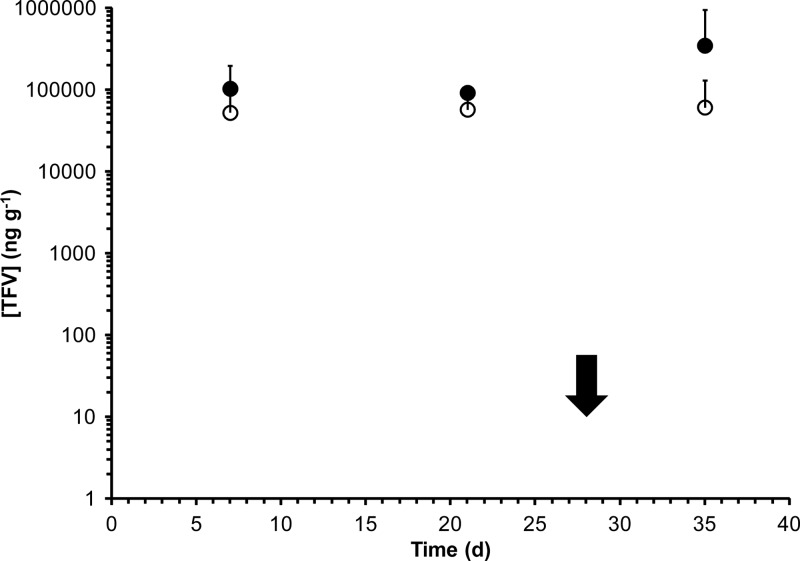
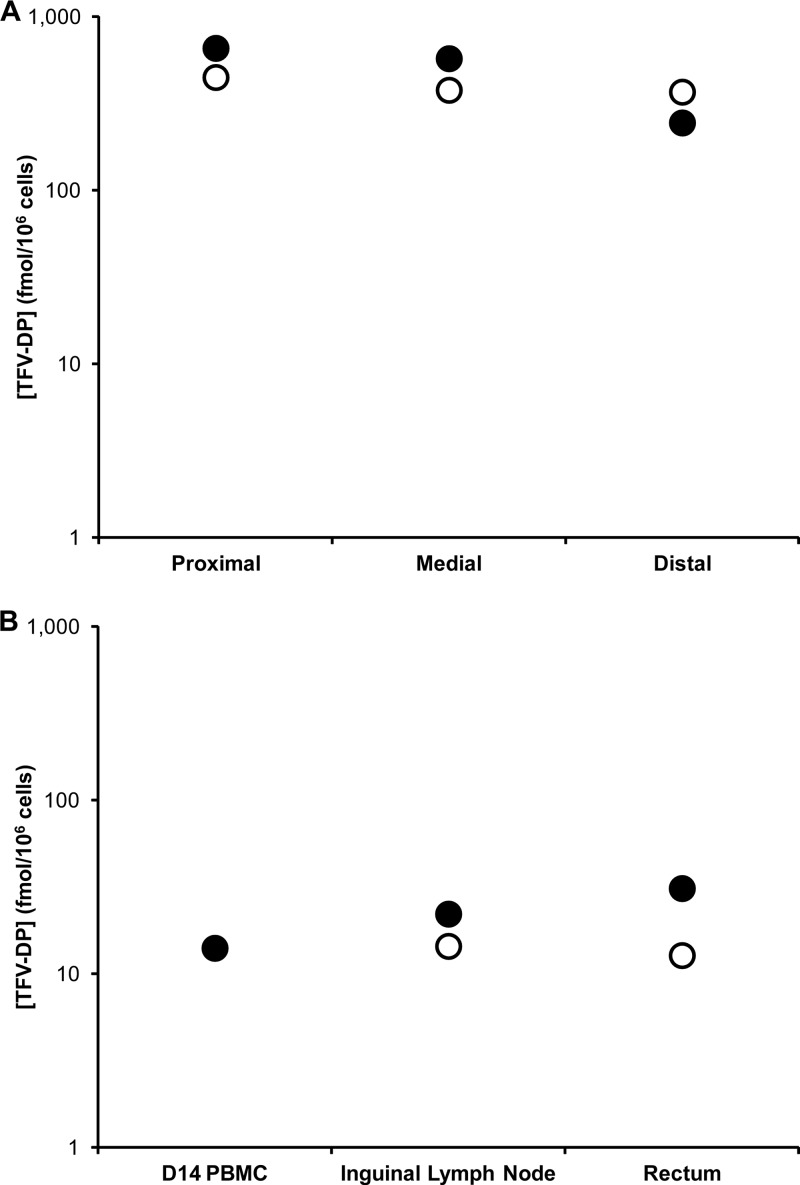
Drug distributions and concentrations across key pharmacokinetic compartments are summarized in Table 3. Cervicovaginal lavage (CVL) fluid TFV levels for all three studies are shown in Fig. 4, and vaginal secretion TFV concentrations are shown in Fig. 5. While TFV levels in CVL fluid and vaginal secretion samples were poorly correlated (R2 values of 0.079 and 0.007 for secretions collected proximally and distally to the IVRs, respectively), the mean values in PK2 suggest that, on average, a 22-fold dilution of the vaginal fluid results from the lavage procedure: mean secretion levels over the vaginal tract in PK2, 72.4 ± 109 μg ml−1; mean CVL fluid levels in PK2, 3.31 ± 2.59 μg ml−1 (Table 3). Due to the poor correlation between CVL fluid and vaginal fluid analyses, all TFV measurements in CVL fluid are uncorrected. Macaque plasma levels were below the LLOQ (7 ng ml−1) for all samples. TFV tissue levels in vaginal biopsy specimens (PK1) are shown in Fig. 6. Tissue TFV-DP levels were measured in all four compartments (PBMCs, mononuclear cells isolated from vaginal and rectal tissue, and draining inguinal lymph nodes) in PK3, as shown in Fig. 7.
Table 3.
Drug distributions and concentrations across key pharmacokinetic compartments in macaques following administration from TFV IVRsa
| Study (no. of macaques) | TFV concn |
Intracellular TFV-DP concn (fmol/106 cells) |
|||||
|---|---|---|---|---|---|---|---|
| CVL fluid (μg ml−1) | Secretions (μg ml−1) | Tissue (μg g−1) | D14 PBMCs | Vagina | Inguinal lymph node | Rectum | |
| PK1 (4) | 3.98 ± 3.02 | NM | 97.8 ± 61.3,b 55.0 ± 39.7,c 76.4 ± 54.8 | NM | NM | NM | NM |
| PK2 (3) | 3.31 ± 2.59 | 50.3 ± 42.3,b 94.6 ± 146,c 72.4 ± 109 | NM | NM | NM | NM | NM |
| PK3 (2) | 22.8 ± 17.9d | 2.44 × 103 ± 2.37 × 103,b,d 1.24 × 103 ± 1.59 × 103,c,d 1.84 × 103 ± 1.97 × 103 | NM | 7 | 446 ± 150 | 18 | 22 |
Values are means and SDs, where applicable. Values in bold font are means and SDs of samples collected proximally and distally to the IVR. NM, not measured. Plasma TFV levels were below the 7 ng ml−1 lower limit of quantitation (LLOQ).
Ectocervix (proximal to the IVR).
Introitus (distal to the IVR).
Only day 7 data were used in these calculations, as the IVRs delivered most of their TFV payload within the first week of the 14-day study (Fig. 5).
Fig 4.
Tenofovir levels (means and standard deviations) in vaginal lavage fluid from macaque in vivo studies. Solid circles, PK1 (n = 4); open circles, PK2 (n = 3); open triangles, PK3 (n = 2); solid arrow, IVRs removed in PK1 and PK2; open arrow, IVRs removed in PK3.
Fig 5.
Tenofovir levels (means and standard deviations) in vaginal secretions from macaque in vivo studies. Solid circles, ectocervix (proximal to IVR) in PK2 (n = 3); open circles, introitus (distal to IVR) in PK2 (n = 3); solid triangles, ectocervix (proximal to IVR) in PK3 (n = 2); open triangles, introitus (distal to IVR) in PK3 (n = 2); solid arrow, IVRs removed in PK2; open arrow, IVRs removed in PK3.
Fig 6.
Tissue TFV levels (means and standard deviations) in vaginal biopsy specimens from macaque in vivo study PK1 (n = 4). Solid circles, ectocervix (proximal to IVR); open circles, introitus (distal to IVR); solid arrow, IVRs removed.
Fig 7.
Intracellular TFV-DP concentrations in macaque in vivo study PK3 (n = 2) in mononuclear cells obtained from the proximal, medial, and distal vaginal compartments relative to the location of the IVR (A) and from rectal tissue, inguinal lymph node, and blood (B). The TFV pod IVR was inserted on day 0 and left in place for 14 days. Solid circles, animal identification (ID) PVC2; open circles, animal ID PIF2.
DISCUSSION
Three TFV pod IVR formulations were evaluated for safety and PK in female pig-tailed macaques (Table 1). TFV is a nucleotide analog inhibitor of reverse transcriptase, an essential enzyme that transcribes the viral single-stranded RNA genome into double-stranded viral DNA prior to incorporation into the host cell DNA, and has been used in many recent clinical PrEP trials. The pod IVR design consists of polymer-coated solid TFV cores, referred to as pods, positioned in an unmedicated ring, with delivery channels in the impermeable IVR structure exposing the TFV pod to vaginal fluids (4).
Practical benefits of the pod IVR design.
Conventional IVR designs consist of devices that contain the solid drug homogeneously dispersed throughout the polymeric matrix, so-called “matrix IVRs,” and IVRs with a drug-loaded polymer layer below the surface of the ring, positioned between nonmedicated polymer layers, so-called “sandwich” or “reservoir rings” (27). The pod IVR design described here is novel (27) because it consists of polymer-coated drug cores, referred to as pods, that are embedded in an unmedicated ring. This approach leads to a number of important benefits, which have been discussed in detail elsewhere (4). The drug release rates from the pod IVRs are determined by the pod's biocompatible polymer membrane(s) and by the characteristics (e.g., number, geometry, and cross-sectional area) of the delivery channels in the impermeable IVR structure (4, 28) and not by the drug loading, as with conventional IVR designs. This was demonstrated in PK1 and PK2, in which a 5-fold increase in drug loading, but an otherwise identical IVR configuration, resulted in statistically equivalent drug levels in CVL fluid over the 28-day studies. Constant daily release rates (i.e., no initial burst) were observed with pod IVRs, as opposed to those observed with matrix IVRs (27). The release rate from each pod can be titrated to the requirements of the application over a wide dynamic range by changing the IVR configuration (4), as demonstrated here for TFV, for which in vitro release rates spanning a factor of 60 were achieved (Table 2). Finally, controlled and sustained release is independent of the ring material, which acts as a scaffold to hold the pods, offering flexibility in drug and polymer choice that may be important for future large-scale production.
The development of IVR formulations to deliver antiretroviral active pharmaceutical ingredients (APIs) for HIV prevention will have to follow the guiding principles of microbicide design in being effective, well tolerated, user-friendly, and affordable (17, 23). In a recent review on IVR technology for topical HIV prophylaxis, Kiser and colleagues stated that “the academic drug-delivery community often overlooks practical issues such as API chemical and physical stability, cost and reproducibility, and manufacturability, all of which should be seriously considered when designing drug-delivery devices for use in the developing world” (22). The modular design of the pod IVR is key to enabling cost-effective manufacturability, which is accomplished in three major steps: (i) injection molding of an empty ring using established and inexpensive injection molding processes with no API present, unlike conventional technologies in which the API is dispersed in the ring polymer (27); (ii) core tableting and polymer coating, identical to the production method for tablet-based oral dosage forms, a mature technology; (iii) IVR assembly, which can be accomplished manually at first and using automation at higher production volumes. The only API-dependent manufacturing step in this three-stage process is the initial tableting to form the API cores. Once cores are fabricated, the procedures for coating to produce pods and assembly of the final IVR are identical, regardless of the API.
Local safety of the IVRs.
Local inflammation is a concern when devices are inserted in a body compartment for extended periods of time. Mucosal levels of the proinflammatory cytokines TNF-α, IL-1β, MIP-1α, IL-8, and MIP-1β remained stable throughout the study period in all animals, with no significant increases observed as a result of the IVRs (Fig. 3). The only statistically significant changes observed were slight decreases in IL-6, RANTES, and IL-1Ra. In addition, there were no changes observed in systemic cytokine patterns. This is typical and has been observed previously in pig-tailed macaques with IVRs (38). The vaginal microflora was undisturbed as a result of the IVRs during the course of the study (13). One of the control animals developed bacterial vaginosis (BV)-like flora following insertion of the IVR, evidenced by a drop in H2O2-producing bacterial populations and a concomitant infection by Gardnerella vaginalis which subsided after the IVR was removed. The microbial biofilm communities that developed on the IVR surfaces did not lead to any observable, negative local toxicity outcomes (13).
Drug distribution and concentration.
Figures 4 and 5 demonstrate that sustained release of TFV, exemplified by steady-state drug levels, was achieved over the course of both studies. The CVL fluid TFV levels from PK1 (mean and standard deviation [SD], 3.98 ± 3.02 μg ml−1) and PK2 (mean and SD, 3.31 ± 2.59 μg ml−1) were statistically similar based upon a naïve analysis comparing means and standard deviations on the means despite the fact that the second set of IVRs contained five times as much drug but were designed to have the same release profiles. Steady-state TFV concentrations in vaginal secretions (Table 3), means of measurements from both sampling locations, were 25 times higher in the fast-releasing formulation used in PK3 (1,840 ± 1,970 μg ml−1) than in the slower-releasing IVRs used in PK2 (72.4 ± 109 μg ml−1), reflective of the 30-fold difference in release rate observed in vitro (Table 2). Mean TFV levels in vaginal secretions and tissue were not correlated with the sampling location, i.e., proximal and distal to the IVR (Table 3). These observations differ from previous reports in which drug levels in the sampled compartments at the ectocervix (proximal to the IVR) were approximately an order of magnitude higher than those at the introitus (distal to the IVR) (19, 40). The higher water solubility of TFV compared to that of the more-insoluble drugs used in previous studies (19, 40) is a possible explanation for the difference. The absence of a TFV concentration gradient in the vaginal tract, along with the steady-state drug levels observed in biological fluids from day 3 onwards, is consistent with results from two-dimensional compartmental modeling of IVR drug delivery indicating that vaginal fluid flow and production are key determinants of drug distribution (11).
Mean TFV levels in vaginal secretions and tissue were equal (Table 3, PK1 and PK2), based on the assumption that vaginal tissue has a density of 1 g ml−1 (33). The results differ significantly from measurements in vaginal compartments of sheep receiving TFV from IVRs (30), for which drug levels in vaginal secretions were two orders of magnitude higher than those in tissue biopsy specimens. This observation can be attributed to different ratios of fluid volume to epithelial cell surface area in the vaginal tract of both animal models along with differences in TFV release rates from both formulations. Mean, steady-state TFV levels from the slow-releasing IVR formulation in vaginal tissue at the ectocervix and introitus were 97.8 ± 61.3 μg g−1 and 55.0 ± 39.7 μg g−1, respectively, five times as high as TFV concentrations measured in cervical (19.3 μg g−1) and vaginal (18.3 μg g−1) tissue 24 h after intravaginal administration of 1% TFV gel in rhesus macaques (33). Intravaginal administration of TFV from IVRs resulted in distribution of the drug to rectal tissue lymphocytes, with a 20-fold decrease in intracellular TFV-DP levels relative to that in vaginal tissue lymphocytes (Table 3). Similar results were obtained by Nuttall et al. using TFV gel formulations in rhesus macaques, although these investigators were unable to detect the intracellular phosphorylated moiety (33). These data suggest that a TFV-based microbicide applied vaginally may hold potential for protecting against rectal exposure to HIV-1. Systemic TFV levels were below the 7-ng ml−1 LLOQ in all studies, a benefit of the topical, sustained-release delivery system as it avoids the large peaks and troughs characteristic of oral- and gel-based formulations.
While TFV concentrations in vaginal secretions decreased sharply after the removal of the IVR (Fig. 5), tissue levels remained at steady state (Fig. 6), consistent with the long intracellular plasma TFV half-life of 0.6 days in humans (3) and macaques (8, 10). It is widely believed (1, 17) that vaginal tissue is the key pharmacological compartment in determining the efficacy of HIV prevention by TFV (see additional discussion below). Our observation that high TFV levels in whole vaginal tissues were sustained in spite of decreasing lumenal concentrations following IVR removal therefore suggests that the IVR may potentially be removed for extended periods of time without loss of efficacy.
Implications for pharmacodynamic outcomes.
The pivotal role of multicompartmental pharmacokinetic-pharmacodynamic (PK-PD) modeling in microbicide development has been described by Hendrix et al. (17). In general terms, such a model is fundamentally important in understanding the relationships between active drug concentrations in the relevant compartments (PK) and preventive effects (PD), such as the efficacy in preventing infection following vaginal exposure to SHIVSF162p3. Our results feed back into the model and identify concentration targets for in vitro and animal model studies that should be revised based on human biomarker outcomes. Clinically, biomarkers predict seroconversion outcomes as the PD efficacy endpoint, and determined concentrations in active-site targets combined with multicompartment PK modeling allow rational selection of dose and frequency, informing future clinical study design. A recent PK-PD analysis of data from the CAPRISA 004 trial suggests that women with TFV cervicovaginal fluid concentrations greater than 1,000 ng ml−1 were significantly protected from HIV infection (21). Mean TFV vaginal secretion concentrations in pig-tailed macaques were 100- and 2,000-fold higher than this protective level from slow- and fast-releasing IVRs, respectively (Table 3), suggesting a potential for favorable PD and efficacy outcomes with these formulations.
To demonstrate antiviral activity against HIV, TFV must undergo in vivo phosphorylation by intracellular kinases to produce the active moiety, TFV-DP (1, 39, 45). Measurement of TFV-DP within susceptible CD4+ cells is critical in relating drug exposure to drug efficacy. Because TFV-DP is ionized and trapped intracellularly, it persists with a longer half-life than the parent drug in plasma (1). In HIV-infected patients receiving TFV, Hawkins et al. reported intracellular TFV-DP kinetics in peripheral blood mononuclear cells (PBMCs) to be highly variable, with intracellular half-lives ranging from 60 to more than 175 h (15). Intracellular TFV-DP concentrations resulting from the fast-releasing TFV in the terminal pig-tailed macaque study (PK3) are presented in Fig. 7 and Table 3 and are compared with those measured in PBMCs. These cells have traditionally been the reference tissue for nucleoside reverse transcriptase inhibitor (NRTI) studies in vivo (45) because they are accessible and present in high number, in addition to being clinically relevant because they contain CD4+ T lymphocytes, the main HIV target (1). The findings presented here agree with previous studies (1), suggesting that TFV-DP accumulates in tissues relevant for HIV prevention—i.e., the vaginal tract in the case of topical delivery from IVRs—and that concentrations according to cell or tissue type may be different from those in the reference PBMC compartment. The correlation of intracellular TFV-DP levels in vaginal tissue lymphocytes at the time of vaginal exposure to SHIVSF162p3 and the associated efficacy in protecting pig-tailed macaques was described recently (6). Vaginal application of a 1% TFV gel 30 min prior to each virus exposure was 100% efficacious, whereas the efficacy dropped to 74% when animals were dosed with the same gel once weekly on day 0 and exposed to virus twice weekly on days 0 and 3 when the intracellular TFV-DP levels were substantially lower (35).
Conclusion.
The administration of TFV from pod IVRs to pig-tailed macaques demonstrated preliminary safety and exhibited sustained and controllable drug release over 28 days. Relating the PK data to PD outcomes from studies in humans and macaques suggests that TFV-releasing IVRs based on the pod design have potential in the prevention of transmission of HIV-1 and merit further investigation in a clinical setting.
ACKNOWLEDGMENTS
We thank the National Institutes of Health (grant numbers 5R21AI079791 and 5R21AI076136) for funding support. This project has been funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under interagency agreement no. Y1-AI-0681-01.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The authors have no commercial or other associations that might pose a conflict of interest. The use of trade names is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, the Public Health Service, or the Centers for Disease Control and Prevention.
We thank Debra Hanson at the Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA, for statistical consultation.
Footnotes
Published ahead of print 10 September 2012
REFERENCES
- 1. Anderson PL, et al. 2011. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J. Antimicrob. Chemother. 66:240–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anglewicz PA, Bignami-Van Assche S, Clark S, Mkandawire J. 2010. HIV risk among currently married couples in rural Malawi: what do spouses know about each other? AIDS Behav. 14:103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barditch-Crovo P, et al. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baum MM, et al. 2012. An intravaginal ring for the simultaneous delivery of multiple drugs. J. Pharm. Sci. 101:2833–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. D'Cruz OJ, Uckun FM. 2004. Clinical development of microbicides for the prevention of HIV infection. Curr. Pharm. Des. 10:315–336 [DOI] [PubMed] [Google Scholar]
- 6. Dobard C, et al. 2012. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J. Virol. 86:718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dunkle KL, et al. 2008. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet 371:2183–2191 [DOI] [PubMed] [Google Scholar]
- 8. Durand-Gasselin L, et al. 2009. Nucleotide analogue prodrug tenofovir disoproxil enhances lymphoid cell loading following oral administration in monkeys. Mol. Pharm. 6:1145–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eyawo O, et al. 2010. HIV status in discordant couples in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect. Dis. 10:770–777 [DOI] [PubMed] [Google Scholar]
- 10. Garcia-Lerma JG, et al. 2010. Intermittent prophylaxis with oral truvada protects macaques from rectal SHIV infection. Sci. Transl. Med. 2:14ra4 doi:10.1126/scitranslmed.3000391 [DOI] [PubMed] [Google Scholar]
- 11. Geonnotti AR, Katz DF. 2010. Compartmental transport model of microbicide delivery by an intravaginal ring. J. Pharm. Sci. 99:3514–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grant RM, et al. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gunawardana M, et al. 2011. Microbial biofilms on the surface of intravaginal rings worn in non-human primates. J. Med. Microbiol. 60:828–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guthrie BL, et al. 2009. Sexually transmitted infections among HIV-1-discordant couples. PLoS One 4:e8276 doi:10.1371/journal.pone.0008276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hawkins T, et al. 2005. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J. Acquir. Immune Defic. Syndr. 39:406–411 [DOI] [PubMed] [Google Scholar]
- 16. Hecht R, et al. 2010. Financing of HIV/AIDS programme scale-up in low-income and middle-income countries, 2009-31. Lancet 376:1254–1260 [DOI] [PubMed] [Google Scholar]
- 17. Hendrix CW, Cao YJ, Fuchs EJ. 2009. Topical microbicides to prevent HIV: clinical drug development challenges. Annu. Rev. Pharmacol. Toxicol. 49:349–375 [DOI] [PubMed] [Google Scholar]
- 18. Ingle JD, Crouch SR. 1988. Spectrochemical analysis. Prentice Hall, Old Tappan, NJ [Google Scholar]
- 19. Johnson TJ, et al. 2012. Safe and sustained vaginal delivery of pyrimidinedione HIV-1 inhibitors from polyurethane intravaginal rings. Antimicrob. Agents Chemother. 56:1291–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karim QA, et al. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karim SSA, Kashuba ADM, Werner L, Karim QA. 2011. Drug concentrations After topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet 378:279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kiser PF, Johnson TJ, Clark JT. 2012. State of the art in intravaginal ring technology for topical prophylaxis of HIV infection. AIDS Rev. 14:62–77 [PubMed] [Google Scholar]
- 23. Klasse PJ, Shattock RJ, Moore JP. 2006. Which topical microbicides for blocking HIV-1 transmission will work in the real world? PLoS Med. 3:e351 doi:10.1371/journal.pmed.0030351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuklenyik Z, et al. 2009. Effect of mobile phase pH and organic content on LC-MS analysis of nucleoside and nucleotide HIV reverse transcriptase inhibitors. J. Chromatogr. Sci. 47:365–372 [DOI] [PubMed] [Google Scholar]
- 25. Kuklenyik Z, et al. 2009. On-line coupling of anion exchange and ion-pair chromatography for measurement of intracellular triphosphate metabolites of reverse transcriptase inhibitors. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877:3659–3666 [DOI] [PubMed] [Google Scholar]
- 26. London S. 2008. Most heterosexual HIV transmission in urban Rwanda and Zambia occurs in married or cohabiting couples. Int. Fam. Plan. Perspect. 34:146 [Google Scholar]
- 27. Malcolm RK, Edwards KL, Kiser P, Romano J, Smith TJ. 2010. Advances in microbicide vaginal rings. Antiviral Res. 88:S30–S39 [DOI] [PubMed] [Google Scholar]
- 28. Moss JA, et al. 2010. In vitro release of tenofovir and acyclovir from a silicone vaginal ring platform, abstr 259. Abstr. Microbicides 2010, Pittsburgh, PA, 22 to 26 May 2010 [Google Scholar]
- 29. Moss JA, et al. 2012. Tenofovir and tenofovir disoproxil pharmacokinetics from intravaginal rings. AIDS 26:707–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moss JA, et al. 2012. Simultaneous delivery of tenofovir and acyclovir via an intravaginal ring. Antimicrob. Agents Chemother. 56:875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. National Research Council 2011. Guide for the care and use of laboratory animals. Department of Health, Education, and Welfare (DHEW) publication no. NIH 86-23. The National Academies Press, Washington, DC [Google Scholar]
- 32. Nel A, et al. 2009. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J. Acquir. Immune Defic. Syndr. 51:416–423 [DOI] [PubMed] [Google Scholar]
- 33. Nuttall J, et al. 2012. The pharmacokinetics of tenofovir following intravaginal and intrarectal administration of tenofovir gel to rhesus macaques. Antimicrob. Agents Chemother. 56:103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Owen DH, Katz DF. 1999. A vaginal fluid simulant. Contraception 59:91–95 [DOI] [PubMed] [Google Scholar]
- 35. Parikh UM, et al. 2009. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel Containing tenofovir alone or with emtricitabine. J. Virol. 83:10358–10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patton DL, Sweeney YC, Rabe LK, Hillier SL. 1996. The vaginal microflora of pig-tailed macaques and the effects of chlorhexidine and benzalkonium on this ecosystem. Sex. Transm. Dis. 23:489–493 [DOI] [PubMed] [Google Scholar]
- 37. Patton DL, Sweeney YTC, Paul KJ. 2008. A summary of preclinical topical microbicide vaginal safety and chlamydial efficacy evaluations in a pigtailed macaque model. Sex. Transm. Dis. 35:889–897 [DOI] [PubMed] [Google Scholar]
- 38. Promadej-Lanier N, et al. 2009. Development and evaluation of a vaginal ring device for sustained delivery of HIV microbicides to non-human primates. J. Med. Primatol. 38:263–271 [DOI] [PubMed] [Google Scholar]
- 39. Pruvost A, et al. 2005. Measurement of intracellular didanosine and tenofovir phosphorylated metabolites and possible interaction of the two drugs in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 49:1907–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Romano J, et al. 2009. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res. Hum. Retrovir. 25:483–488 [DOI] [PubMed] [Google Scholar]
- 41. Saxena BB, et al. 2009. Sustained release of microbicides by newly engineered vaginal rings. AIDS 23:917–922 [DOI] [PubMed] [Google Scholar]
- 42. Shattock RJ, Warren M, McCormack S, Hankins CA. 2011. Turning the tide against HIV. Science 333:42–43 [DOI] [PubMed] [Google Scholar]
- 43. Smith DJ, et al. 2008. An evaluation of intravaginal rings as a potential HIV prevention device in urban Kenya: behaviors and attitudes that might influence uptake within a high-risk population. J. Womens Health (Larchmt.) 17:1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spear GT, et al. 2010. Identification of rhesus macaque genital microbiota by 16S pyrosequencing shows similarities to human bacterial vaginosis: implications for use as an animal model for HIV vaginal infection. AIDS Res. Hum. Retroviruses 26:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stein DS, Moore KHP. 2001. Phosphorylation of nucleoside analog antiretrovirals: a review for clinicians. Pharmacotherapy 21:11–34 [DOI] [PubMed] [Google Scholar]
- 46. Valenta C. 2005. The use of mucoadhesive polymers in vaginal delivery. Adv. Drug Deliv. Rev. 57:1692–1712 [DOI] [PubMed] [Google Scholar]
- 47. Van Rompay KKA. 2010. Evaluation of antiretrovirals in animal models of HIV infection. Antiviral Res. 85:159–175 [DOI] [PubMed] [Google Scholar]
- 48. Veazey RS. 2008. Microbicide safety/efficacy studies in animals—macaques and small animal models. Curr. Opin. HIV AIDS 3:567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yoo JW, Lee CH. 2006. Drug delivery systems for hormone therapy. J. Control. Release 112:1–14 [DOI] [PubMed] [Google Scholar]
- 50. Yu RR, et al. 2009. A Chinese rhesus macaque (Macaca mulatta) model for vaginal lactobacillus colonization and live microbicide development. J. Med. Primatol. 38:125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]