Abstract
Artemisinin-resistant malaria along the Thailand-Cambodian border is an important public health concern, yet mechanisms of drug action and their contributions to the development of resistance are poorly understood. The pharmacokinetics and pharmacodynamics of oral artesunate monotherapy were explored in a dose-ranging trial in an area of emerging artesunate resistance in western Cambodia. We enrolled 143 evaluable subjects with uncomplicated Plasmodium falciparum malaria in an open label study of directly observed artesunate monotherapy at 3 dose levels (2, 4, and 6 mg/kg of body weight/day) for 7 days at Tasanh Health Center, Tasanh, Cambodia. Clinical outcomes were similar among the 3 groups. Wide variability in artesunate and dihydroartemisinin concentrations in plasma was observed. No significant dose-effect or concentration-effect relationships between pharmacokinetic (PK) and parasite clearance parameters were observed, though baseline parasitemia was modestly correlated with increased parasite clearance times. The overall parasite clearance times were prolonged compared with the clearance times in a previous study at this site in 2006 to 2007, but this did not persist when the evaluation was limited to subjects with a comparable artesunate dose (4 mg/kg/day) and baseline parasitemia from the two studies. Reduced plasma drug levels with higher presentation parasitemias, previously hypothesized to result from partitioning into infected red blood cells, was not observed in this population with uncomplicated malaria. Neither in vitro parasite susceptibility nor plasma drug concentrations appeared to have a direct relationship with the pharmacodynamic (PD) effects of oral artesunate on malaria parasites. While direct concentration-effect relationships were not found, it remains possible that a population PK modeling approach that allows modeling of greater dose separation might discern more-subtle relationships.
INTRODUCTION
Oral artesunate (AS) is an important drug used in the control of multidrug-resistant Plasmodium falciparum malaria. However, the mechanism of action for this class of drugs, known as the artemisinins, and thus potential mechanisms for development of drug resistance remain poorly understood. Semimechanistic models of parasite dynamics have been proposed to explain the potential effects of drug on parasite sequestration in unmeasurable compartments (16, 18). Selective effects of drug on early parasite ring stages were found to explain differences in parasite clearance observed between patients treated in Thailand and Cambodia using intrahost mathematical modeling of pharmacokinetic (PK)-pharmacodynamic (PD) relationships (36). Autoinduction of metabolism has been suggested for drugs in this class (15, 17), representing a possible confounder to parasite-based mechanisms of resistance. The current models provide only limited explanations for artesunate resistance, creating an ongoing need for additional empirical data.
We recently reported evidence of artesunate resistance in malaria parasites from patients treated in a clinical trial conducted in western Cambodia in 2006 to 2007 (30, 31). This study showed delayed parasite clearance times and reduced artemisinin drug susceptibility in vitro in spite of what appeared to be therapeutic drug levels. However, pharmacologic collection times in that study were limited to 2 time points after dosing, prompting an effort to conduct more-detailed analysis. A dose-ranging clinical trial of artesunate monotherapy at 3 doses (2, 4, and 6 mg/kg of body weight/day once daily for 7 days) was conducted to better understand pharmacokinetic (PK)-pharmacodynamic (PD) relationships of oral artesunate monotherapy in 2008 to 2009 (7). The rates of adequate clinical and parasitological responses (ACPR) in all treatment arms to artesunate at 28 and 42 days were comparable to those observed in previous oral artesunate monotherapy studies in this geographic region (8, 9, 17, 26, 34). There were prolonged parasite clearance times observed compared to the clearance times in the study carried out in 2006, with more than 50% of patients remaining parasitemic 72 h after commencing artesunate treatment. The pharmacokinetics and pharmacodynamics of oral artesunate and dihydroartemisinin (DHA), its active metabolite, were explored to test whether alterations in pharmacokinetics could explain the apparent changes in parasite pharmacodynamics. We investigated dose-effect and concentration-effect relationships with key pharmacodynamic variables, including parasite clearance and in vitro parasite drug susceptibility to evaluate whether increasing the dose of oral artesunate might improve pharmacodynamic responses by reducing the rate of clinical failure or decreasing the parasite clearance time.
MATERIALS AND METHODS
Clinical study.
The U.S. Army Medical Component, Armed Forces Research Institute of Medical Sciences (AFRIMS), and the Center for Parasitology, Entomology and Malaria Control (CNM) in Cambodia conducted an open label study of oral artesunate (AS) monotherapy in Tasanh, Cambodia, from 2008 to 2009 (7) and compared the results to a study conducted at the same site from 2006 to 2007 (30). Both studies were approved by the Walter Reed Army Institute of Research institutional review board (IRB) and the National Ethical Committee for Health Research of Cambodia and complied with relevant federal guidelines and institutional policies. Adult patients with uncomplicated Plasmodium falciparum malaria from the Samlot district of Battambang Province in Cambodia were randomized into 3 groups of 2, 4, or 6 mg/kg of body weight/day directly observed oral artesunate monotherapy for 7 days with target treatment allocation of 150 subjects with a 2:1:2 ratio. The dose was rounded up to the nearest 1/4 tablet, with quality control analysis of study drug as described previously (7). However, the high-dose cohort (6 mg/kg) was halted due to dose-limiting neutropenia after 28 patients had been enrolled (6). Subjects were monitored for 42 days for clinical efficacy, safety, pharmacokinetic (PK) analysis, and parasite molecular and in vitro drug resistance phenotype characterization. Lean body mass (LBM) was estimated using the following formulas: LBM = (1.10 × weight) − 128 × [weight2/(100 × height)2] for men and LBM = (1.07 × weight) − 148 × [weight2/(100 × height)2] for women. For the purposes of the present analysis, subjects were categorized as late responders (LR) if they had persistent fever and parasitemia beyond 72 h of therapy and late treatment failures (LTF) if they had clinical and/or parasitological P. falciparum malaria recurrence within the follow-up period.
Pharmacokinetics.
PK blood samples were collected in heparinized tubes on the first day of AS dosing (day 0) at times 0 (predose), 15, 30 min, 1, 2, 4, 6, 8 h, and on the 7th day of AS dosing (day 6) at times 0 (predose) 2, 4, and 6 h. Samples were collected on ice and centrifuged immediately, and plasma was stored at −20°C for up to 2 weeks, followed by transfer to our reference lab in liquid nitrogen with storage at −80°C until analysis. AS and dihydroartemisinin (DHA) drug levels were determined by liquid chromatography-mass spectrometry (LC-MS) as previously described (38). The actual sample sizes for the pharmacokinetic analysis database were 75, 40, and 28 subjects who received 2, 4, and 6 mg/kg of AS, respectively. All late responders, late treatment failures, and neutropenia patients were included in the pharmacokinetic and pharmacodynamic analyses.
Pharmacodynamics.
Malaria whole-blood films for parasite density were collected by finger stick and/or venipuncture 8 times in the first 24 h (at 0, 2, 4, 6, 8, 12, 18, and 24 h) and then every 6 h until 2 negative smears were obtained. Thick and thin smears were prepared with counts per 200 white blood cells (WBC) on a thick film or per 5,000 red blood cells (RBC) on a thin film with a minimum of 200 oil immersion fields examined before a negative read was determined. Final outcome determination was adjusted for species using PCR genotyping using merozoite-specific protein 1 (MSP1), MSP2, and Glurp for all malaria recurrences using previously described methods (40). Fifty and 90% parasite clearance times (PCT50 and PCT90, respectively) were calculated using curve-fitting analysis with GraphPad Prism, version 5 (La Jolla, CA).
In vitro parasite drug susceptibility.
Enrolled subjects provided 8 ml of blood in sodium heparin tubes for a histidine-rich protein 2 (HRP-2) ex vivo drug susceptibility assay, and 50% inhibitory concentrations (IC50) for each parasite isolate were calculated. Susceptibility was measured against 6 drugs—artesunate (AS), dihydroartemisinin (DHA), lumefantrine (LUM), chloroquine (CQ), quinine (Q), and mefloquine (MQ). P. falciparum isolates were plated directly onto malaria drug-coated plates without prior culture adaptation, as previously described (7, 29).
Data analysis. (i) Pharmacokinetic analysis.
Pharmacokinetic parameters were calculated using noncompartmental analysis (NCA) with PK Solutions software, after determining that there was little difference between NCA and a one-compartment model calculated using Phoenix WinNonlin version 1.3 (Pharsight Corporation). The PK parameters determined were the elimination half-life (t1/2), maximum concentration in plasma by inspection (Cmax), time to reach Cmax (Tmax), the areas under the concentration-time curve (AUC) from 0 h to ∞ (AUC0–∞), 0 to 6 h (AUC0–6), 0 to 8 h (AUC0–8), and 2 to 6 h (AUC2–6), the mean residence time (MRT), the volume of distribution (V), and the clearance (CL). For extravascular models, the fraction of dose absorbed cannot be estimated. Therefore, V and CL for the models are actually V/F and CL/F where F is the estimated fraction absorbed.
(ii) Pharmacodynamic analysis.
The 100% parasite clearance time (PCT100) was defined as the time from the start of treatment until the first time the blood smear became negative for asexual parasites and remained negative for 2 consecutive measurements. PCT50 and PCT90 were calculated as the time to reduction of parasitemia to 50 and 90%, respectively, of the baseline parasitemia value (P0). PCT50 and PCT90 were calculated by interpolation using a curve-fitting model in GraphPad Prism, version 5 (La Jolla, CA). The PCT50 was calculated based on a dose-response relationship using a 4-parameter equation where Y = lower asymptote + (upper asymptote − lower asymptote)/{1 + 10[(log PCT50 − X) × Hill slope]} where Y is the number of parasites per microliter at time X where X is the log time in hours. PCT90 was calculated using the following equation: log PCT50 = log PCT90 − (1 − hill slope) × log [F/(100 − F)] where F is equal to 10. Parasite reduction ratios (percent parasite reduction ratio [%PRR]) were calculated as 100 minus the percentage reduction from P0 at 24, 48, and 72 h after treatment using the formula 1 − (Px/P0) where x is the number of hours from time zero. Linear relationships using nonparametric correlation tests were used to investigate the relationships between PK and PD parameters. Parasite clearance rates were also calculated using previously described methods (13), and compared with drug dose levels using one-way analysis of variance (ANOVA).
(iii) Statistical analysis.
Values of normally distributed data were expressed as means (95% confidence intervals [95% CI]) with standard deviations and nonnormally distributed data as medians with interquartile ranges (IQR). Means, medians, and/or geometric means were compared using ANOVA for normally distributed data, with nonparametric tests (Kruskal-Wallis) for nonnormally distributed data. For continuous variable PK-PD relationships, Spearman's correlation coefficients (ρ) were calculated using pairwise elimination for missing case data, and multiple linear and logistic regression analysis were performed using SPSS 16.0.
RESULTS
Pharmacokinetics.
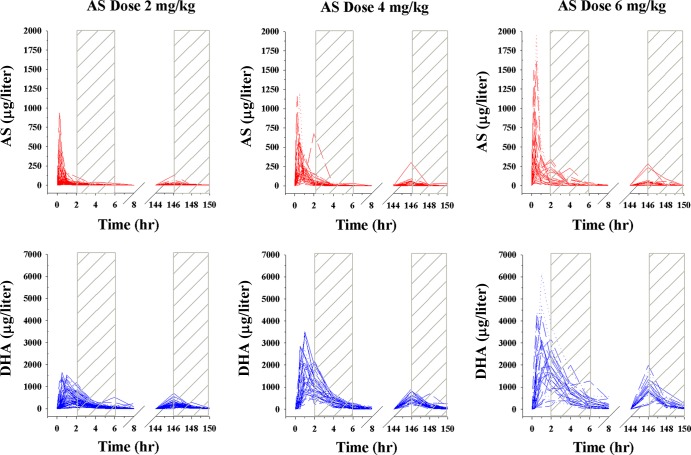
There was wide interindividual variability in plasma AS and DHA concentrations at day 0 and day 6 with a reduction in the AUC during the terminal elimination phase on day 6 (between 146 and 150 h), as seen in Fig. 1. The pharmacokinetic parameters for AS and DHA are shown in Table 1. Plasma AS levels were low on day 6, preventing accurate PK parameter calculations, so only day 0 values are shown. There was an approximate 2-fold reduction in the AUC of DHA between day 0 and day 6 regardless of dose, when comparable concentration-time points were compared (Table 2).
Fig 1.
Plasma drug concentration-time profiles of subjects included in the pharmacokinetic analysis. Artesunate (AS) concentrations are shown in red, while dihydroartemisinin (DHA) concentrations are shown in blue. A total of 75, 40, and 28 subjects were given 2, 4, and 6 mg/kg AS, respectively.
Table 1.
Summary of overall pharmacokinetic parameters for subjects taking oral artesunate for 7 days
| PK parameter | Geometric mean value for subjects given the following dose of AS: |
P valued | ||
|---|---|---|---|---|
| 2 mg/kg | 4 mg/kg | 6 mg/kg | ||
| AS on day 0a | ||||
| t1/2 (h) | 0.489 | 0.499 | 0.461 | 0.827 |
| Tmax (h) | 0.482 | 0.518 | 0.640 | 0.366 |
| Cmax (μg/liter) | 83.1 | 237 | 374 | <0.0001 |
| AUC0–8 (μg · h/liter) | 91.2 | 279 | 453 | <0.0001 |
| MRT (h) | 1.07 | 1.06 | 1.17 | 0.749 |
| V/F (liter/kg) | 14.2 | 10.1 | 8.56 | 0.051 |
| CL/F (liter/h/kg) | 20.1 | 13.9 | 12.9 | <0.0001 |
| DHA on day 0b | ||||
| t1/2 (h) | 0.838 | 0.913 | 1.13 | 0.170 |
| Tmax (h) | 1.26 | 1.15 | 1.44 | 0.310 |
| Cmax (μg/liter) | 554 | 1,515 | 2,129 | <0.0001 |
| AUC0–8 (μg · h/liter) | 1,331 | 3,854 | 5,989 | <0.0001 |
| MRT (h) | 2.06 | 2.21 | 2.84 | 0.008 |
| DHA on day 6c | ||||
| t1/2 (h) | 0.799 | 0.851 | 0.843 | 0.186 |
| Tmax (h) | 2.08 | 2.11 | 2.17 | 0.555 |
| Cmax (μg/liter) | 196 | 497 | 875 | <0.0001 |
| AUC0–8 (μg · h/liter) | 481 | 1267 | 2218 | <0.0001 |
| MRT (h) | 2.53 | 2.67 | 2.64 | 0.144 |
For the artesunate (AS) pharmacokinetic (PK) parameters for subjects given AS on day 0, 75, 40, and 28 subjects were given 2, 4, and 6 mg/kg of AS, respectively.
For the dihydroartemisinin (DHA) PK values on day 0, 75, 40, and 27 subjects were given 2, 4, and 6 mg/kg of AS, respectively. One subject was excluded from the 6-mg/kg AS group because of an outlier value.
For the DHA PK values on day 0, 73, 38, and 25 patients were given 2, 4, and 6 mg/kg of AS, respectively. The sample size decreased for all three groups because there are no follow-up data for two subjects.
P value for Kruskal-Wallis one-way ANOVA.
Table 2.
Changes in mean area under the curve for DHA between days 0 and 6 for comparable time points
| AS dose (mg/kg) | No. of subjects | Day 0 |
Day 6 |
Day 0 compared to day 6 |
|||
|---|---|---|---|---|---|---|---|
| AUC0–6a | AUC2–6 | AUC0–6 | AUC2–6 | AUC0–6 | AUC2–6 | ||
| 2 | 73 | 1,067 | 652 | 554 | 330 | 1.93 | 1.97 |
| 4 | 38 | 2,853 | 1,801 | 1,263 | 775 | 2.26 | 2.33 |
| 6 | 25 | 4,850 | 3,221 | 1,394 | 1,472 | 2.03 | 2.19 |
AUC0–6 (area under the concentration-time curve from 0 to 6 h) as calculated from time points at 0, 2, 4 and 6 h on day 0 as they were collected on day 6 for purposes of comparison between day 0 and day 6 only.
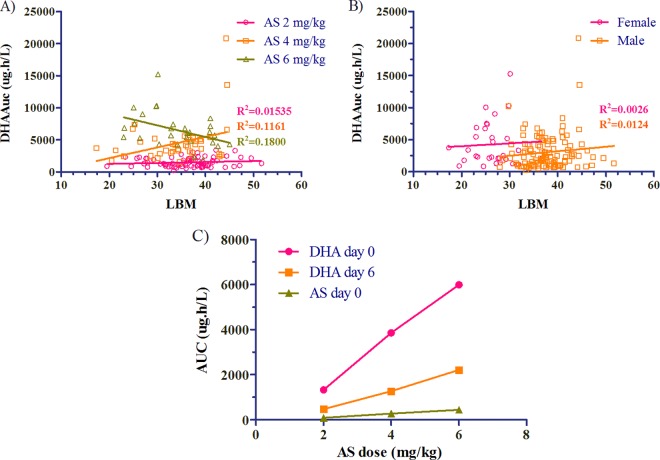
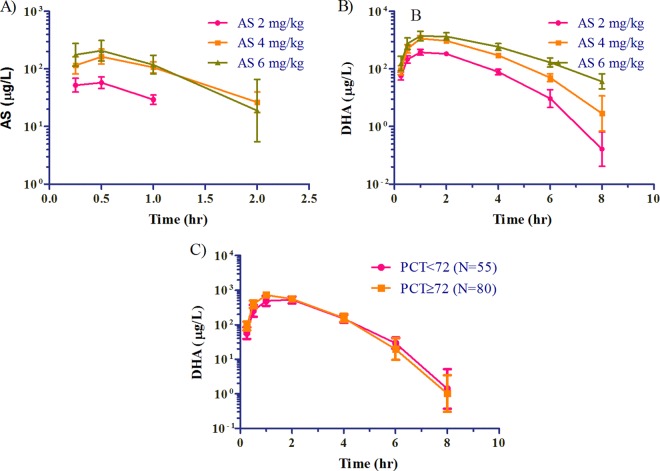
There were no significant differences in AS (not shown) or DHA exposure seen with lean body mass by either dose administered or sex (Fig. 2A and B). Linear dose proportionality of AUC and Cmax were observed with increasing doses of artesunate (Fig. 2C). While there were increases in day 0 AS and DHA concentrations by dose at 2, 4, and 6 mg/kg (Fig. 3A and B), there was no difference between mean plasma concentrations of DHA at any time point between those subjects who had persistent parasitemia at 72 h and those who did not (Fig. 3C). This lack of effect remained even after corrections for baseline parasitemia were made.
Fig 2.
(A and B) There was no relationship between lean body mass (LBM) and drug concentrations in blood based on AUC0–8 of DHA by dose (A) or sex (B) (31 females and 112 males). (C) Linear dose proportionality of geometric mean AUC and Cmax (not shown) was seen with increasing artesunate doses.
Fig 3.
(A and B) Geometric mean plasma AS and DHA concentration-time curves by dose. (C) Geometric mean concentration-time points are plotted against parasite clearance time (PCT) greater or less than 72 h.
Pharmacodynamics. (i) Dose-effect relationships.
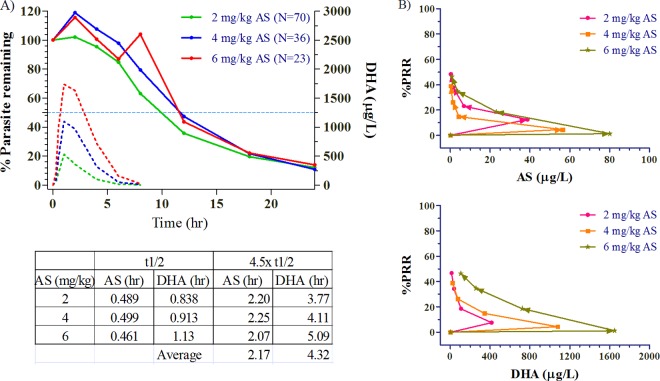
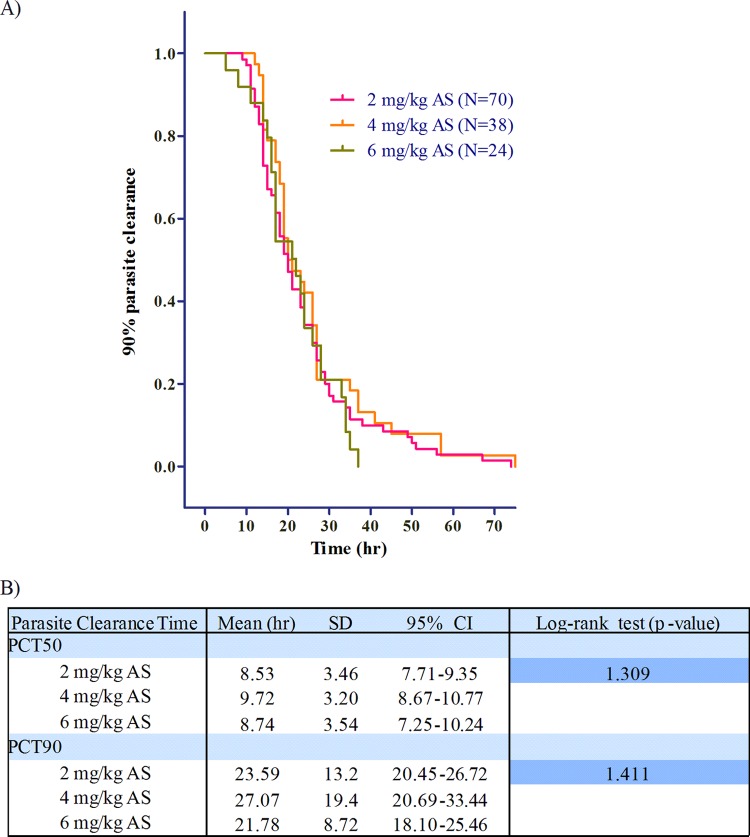
There was continuous reduction in parasitemia over the first 24 h following the first dose of each drug, persisting well beyond the effective clearance (4.5× half-lives) of AS at 2.94 h and DHA at 4.67 h (Fig. 4A). Figure 4B plots median concentration versus time-specific parasite reduction ratios (percent parasite reduction ratio [%PRR]) at each time point for both AS and DHA at the 3 dose levels. Regardless of dose, 50% PRR was reached on the first day at approximately 8 to 12 h. There was no apparent dose-effect or concentration-effect relationship of the drug on either clinical outcome or parasite clearance. This was true not only for parasite clearance times but also parasite clearance rates (not shown). As previously reported, there were no clinically or statistically significant differences in 28- or 42-day treatment outcomes between dosing groups with 2 subjects each having late treatment failure (LTF) in the 2- and 6-mg/kg groups and 1 subject with LTF in the 4-mg/kg group (7). The mean times to 50% (not shown) and 90% parasite clearance (PCT90) were not significantly different by dosing group compared by Kaplan-Meier survival analysis (Fig. 5A). Mean values were used for this analysis due to normal distribution of parasite clearance time data.
Fig 4.
(A) Parasite clearance as the mean percentage of baseline values over the first 24 h for the 3 dose groups. The broken lines show the median concentration-time profiles of AS and DHA. The geometric mean half-life (t1/2) and 4.5× half-life values appear in the accompanying table below the graph. (B) Concentration-effect relationships on percent parasite reduction (percent parasite reduction ratio [%PRR]) for AS and DHA at the 3 dose levels.
Fig 5.
(A) Kaplan-Meier survival analysis of time to 90% parasite clearance. The x axis represents the time to reach 90% parasite clearance. (B) Mean times to 50% or 90% parasite clearance (PCT50 or PCT90) by the log rank test at the 3 doses of artesunate. The mean, standard deviation (SD), and 95% confidence interval (95% CI) values are shown.
(ii) Concentration-effect relationships.
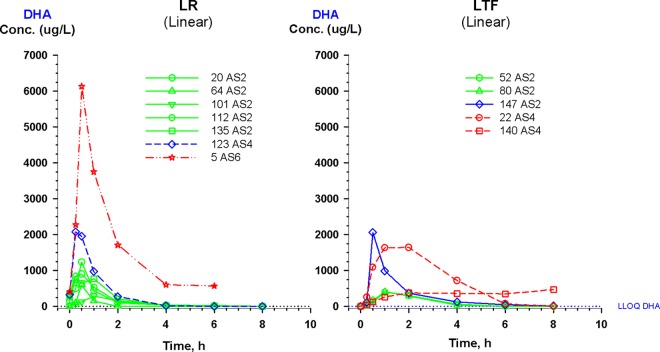
There was variability in the concentration-time curves for patients classified as late responders and late treatment failures (LR and LTF, respectively), particularly with LR (Fig. 6). Notably, none of the LR went on to become an LTF. Only 1 of the 5 LTF subjects had AS and DHA drug concentrations that were more than 1 standard deviation below the mean for their respective dosing group. There were no statistically significant correlations between pharmacokinetic variables, including AUC, Cmax, or clearance of AS or DHA with pharmacodynamic variables, including PCT50, PCT90, or PCT100 or parasite reduction ratio (PRR) at 12, 24, 48, or 72 h using nonparametric correlation analysis (Spearman's ρ [Table 3]). We compared the ratios of AUC and Cmax for DHA (in nanograms per milliliter) to the IC50 for DHA (in ng/ml) on day 0 to see whether the failures had lower values. However, no correlations were found for these ratios, either for the study population as a whole or for LTF or LR patients.
Fig 6.
Detailed plasma concentration-time curve plots for 7 subjects classified as late responders (LR) and 5 subjects classified as late treatment failures (LTF). Notably, no LR subjects went on to become LTF. Subject identification (ID) numbers are followed by artesunate dosing groups (AS2, AS4, and AS6 for artesunate doses of 2, 4, and 6 mg/kg, respectively). The lower limit of quantitation (LLOQ) by LC-MS for DHA is shown as a dotted line.
Table 3.
Spearman's correlation coefficients (ρ) of plasma drug pharmacokinetic and parasitological variables compared to pharmacodynamic outcomes
| Pharmacokinetic or parasitological parameter | Spearman's correlation coefficienta |
||||||
|---|---|---|---|---|---|---|---|
| Parasite clearance time |
Parasite reduction ratio |
P0 | |||||
| 50% | 90% | 100% | 24 h | 48 h | 72 h | ||
| Parasite density at time zero (P0) | 0.204 | 0.025 | 0.377 | 0.002 | 0.028 | −1.410 | 1.000 |
| PK parameters | |||||||
| AS on day 0 | |||||||
| AUC0–8 | 0.125 | 0.092 | 0.067 | −0.054 | −0.020 | −0.049 | 0.052 |
| Cmax | 0.076 | 0.072 | 0.058 | −0.450 | 0.004 | 0.045 | 0.051 |
| CL/F | −0.058 | 0.007 | 0.045 | −0.032 | −0.119 | −0.155 | −0.064 |
| V/F | 0.050 | −0.027 | −0.006 | 0.007 | −0.015 | −0.067 | −0.035 |
| DHA on day 0 | |||||||
| AUC0–8 | 0.125 | 0.095 | 0.091 | −0.068 | −0.068 | −0.029 | 0.073 |
| Cmax | 0.079 | 0.077 | 0.088 | −0.034 | −0.064 | −0.014 | 0.064 |
| CL | −1.010 | −0.080 | −0.090 | 0.071 | 0.054 | 0.048 | −0.086 |
| V/F | −0.088 | −0.026 | −0.060 | −0.015 | −0.052 | −0.012 | −0.191 |
| DHA on day 6 | |||||||
| AUC | 0.027 | 0.068 | 0.122 | −0.068 | −0.087 | −0.035 | 0.014 |
| Cmax | 0.010 | 0.057 | 0.120 | −0.063 | −0.077 | −0.039 | −0.013 |
| CL | 0.102 | 0.054 | −0.044 | −0.017 | −0.018 | −0.037 | −0.042 |
| V/F | 0.034 | 0.040 | −0.022 | −0.008 | −0.053 | −0.013 | −0.087 |
| In vitro drug susceptibility (IC50b) | |||||||
| DHA | 0.200 | 0.117 | 0.223 | −0.104 | −0.098 | −0.217 | 0.262 |
| AS | 0.242 | 0.157 | 0.223 | −0.118 | −0.073 | −0.170 | 0.294 |
| LUM | −0.130 | −0.015 | −0.012 | −0.009 | −0.023 | 0.020 | −0.090 |
| CQ | 0.057 | −0.060 | 0.041 | 0.092 | 0.047 | −0.042 | 0.181 |
| Q | −0.178 | −0.149 | −0.114 | 0.123 | 0.093 | 0.108 | −0.118 |
| MQ | −0.122 | −0.040 | −0.050 | 0.006 | 0.029 | 0.032 | −0.013 |
Spearman's correlation coefficients (ρ) of the indicated pharmacokinetic or parasitological parameter compared to the pharmacodynamic outcomes expressed as 50, 90, and 100% parasite clearance times and parasite reduction ratios at 24, 48, and 72 h. Statistically significant correlations are highlighted in boldface type (P < 0.05).
IC50s are 50% inhibitory drug concentrations in vitro.
(iii) Pharmacodynamics and in vitro drug susceptibility.
There were modest but statistically significant correlations between the pharmacodynamic parasite clearance variables and in vitro drug resistance of fresh parasite isolates as measured by IC50 collected at time zero using an HRP-2 assay against 6 drugs (AS, DHA, CQ, LUM, Q, and MQ). PCT50 and PCT100 were modestly correlated with increases in the IC50s of both AS (ρ = 0.242 and 0.223, respectively) and DHA (ρ = 0.200 and 0.222, respectively), and there was an inverse correlation between IC50 and PRR72 (ρ = −0.217 for DHA and −0.170 for AS). Conversely, higher baseline parasitemia (P0) correlated with modest but significantly increased IC50s for AS (ρ = 0.294), DHA (ρ = 0.262), and CQ (ρ = 0.181), and there was a modest inverse correlation with the IC50 of Q (ρ = −0.118) (Table 3). We compared the ratios of AUC and Cmax for DHA (in ng/ml) to the IC50 value for DHA (in ng/ml) on day 0 to see whether failures had lower values. However, no correlations between these ratios were found, either for the study population as a whole or LTF or LR patients.
(iv) Effects of baseline parasitemia.
There was a modest correlation between PCT50 and PCT100 and parasitemia at baseline (ρ = 0.204 and 0.377, respectively, and P < 0.001 [Table 3]). The geometric mean P0 was 2-fold higher in subjects who had persistent parasitemia at 72 h (20,914 parasites/μl) than those who had cleared parasites at 72 h (8,991 parasites/μl) (P = 0.002). There was no measurable effect of P0 on PK parameters of AS or DHA with the exception of a small (though statistically significant) inverse effect on V/F.
Key results from studies conducted at the Tasanh site in Cambodia in 2006 (30) and 2009 (7) are compared in Table 4 for all subjects and for only those subjects with comparable dose (4 mg/kg) and enrollment parasitemia (between 1,000 and 100,000 parasites/μl). The 2006-2007 and 2008-2009 studies had different enrollment criteria for baseline P. falciparum parasitemia—100 to 100,000 parasites/μl versus 1,000 to 200,000 parasites/μl, respectively. The median parasite density on enrollment was significantly higher in 2009 (P value < 0.01). Though parasite clearance times (PCT) and the proportion of patients still parasitemic at 72 h were significantly different between the two overall study populations, when comparing only patients taking 4 mg/kg AS with between 1,000 and 100,000 parasites/μl at enrollment, the differences in parasite clearance times were no longer statistically significant. This was also true for the log rank test for this comparable patient subset when Kaplan-Meier survival analysis (not shown) was applied to the patient population that was still parasitemic over the first 72 h (Table 4).
Table 4.
Baseline characteristics and clinical and parasitological responses in two studies conducted at the Tasanh site in Cambodiaa
| Baseline characteristic or clinical or parasitological response | Median (interquartile range) for all patients enrolled and randomized to 4 mg/kg AS for 7 days |
P value | Median (interquartile range) for only patients randomized to 4 mg/kg with a parasite density of 1,000 to 100,000/μl |
P value | ||
|---|---|---|---|---|---|---|
| Tasanh site in 2006 (n = 60) | Tasanh site in 2009 (n = 38) | Tasanh site in 2006 (n = 45) | Tasanh site in 2009 (n = 35) | |||
| Baseline characteristics | ||||||
| Age (yr) | 29.0 (20.0–44.0) | 22.0 (20.0–36.0) | 0.10 | 27.0 (20.0–41.0) | 22.0 (20.0–38.0) | 0.31 |
| Temp (°C) | 38.0 (37.0–38.9) | 38.1 (37.7–39.1) | 0.12 | 38.0 (37.2–39.2) | 38.1 (37.6–39.2) | 0.51 |
| Parasite density (no. of cells/μl) | 4,325 (982–18,643) | 24,791 (6,714–51,498) | <0.01 | 6,562 (3,892–33,452) | 23,531 (6,389–39,393) | 0.03 |
| IC50 vs dihydroartemisinin concn (μg/liter) | 1.16 (0.79–1.54) | 2.59 (1.52–3.99) | <0.01 | 1.19 (0.82–1.53) | 2.59 (1.49–4.04) | <0.01 |
| Clinical and parasitological responses | ||||||
| Cmax of dihydroartemisinin (μg/liter) | 1,226 (744–1,845) | 1,467 (889–2,126) | 0.25 | 1,345 (942–1,928) | 1,468 (894–2,184) | 0.69 |
| Patients with adequate clinical and parasitological responses (%) | 93.3 | 94.7 | 0.78 | 91.1 | 94.4 | 0.57 |
| Fever clearance time (h) | 23.8 (10.1–30.1) | 17.6 (9.65–28.8) | 0.46 | 22.9 (10.0–29.4) | 17.6 (10.0–29.2) | 0.73 |
| Parasite clearance time (h) | 62.0 (38.0–86.6) | 78.0 (64.4–96.1) | <0.01 | 66.4 (43.0–91.0) | 78.0 (61.5–96.1) | 0.18 |
| Patients with persistent parasitemia after 72 h of treatment (%) | 31.7 | 57.9 | 0.01 | 42.2 | 57.1 | 0.18 |
| P value for log rank test of Kaplan-Meier survival analysis | P = 0.02 | P = 0.41 | ||||
Two studies were conducted at the Tasanh site in Cambodia in 2006 (30) and 2009 (7). Here we compare the values for baseline characteristics and clinical and parasitological responses controlling for dose and enrollment parasitemia. Statistically significant differences are highlighted in boldface type with P values calculated by the Mann-Whitney U test or by the chi-squared and Fisher's exact tests where proportions were compared between treatment groups.
DISCUSSION
In a study of 143 patients in western Cambodia receiving 7 days of directly observed oral artesunate monotherapy, we found that plasma drug levels of artesunate and DHA and corresponding rates of parasite clearance were highly variable in this population. Where we had expected to see an increase in parasite clearance times and/or relative reductions in parasite burdens with increasing drug concentrations, we did not find clear relationships between plasma drug concentration or PK parameters on pharmacodynamic efficacy variables, including clinical outcomes, parasite clearance times, or relative reductions in parasitemia. Despite this variability, the 28- and 42-day treatment efficacies of all 3 dose levels were equal to or higher in some cases than those previously reported for various 5- to 7-day regimens of artesunate monotherapy, which have ranged between 85 and 100% in Southeast Asia and Africa (1, 8, 9, 17, 26, 34). Parasite clearance times were longer in our study than those reported in previous monotherapy studies in the region (PCT range, 40 to 55 h), including previous data from the Tasanh site in Cambodia in 2006 (30), and similar to those reported from a concurrent study in Pailin, Cambodia (12).
Distinguishing the host, pharmacokinetic, and intrinsic parasite factors responsible for resistance remains a daunting challenge. Semimechanistic models of parasite dynamics have been developed to explain the potential effects of drug on parasite sequestration in unmeasurable compartments (18). Development of similar models for our data set may shed further light on the lack of an observed relationship on the plasma drug levels and parasite clearance based on light microscopy. A recent PK-PD model demonstrating selective effects of drug on early parasite ring stages in Southeast Asia helped to identify a source for focus of further investigation of resistance mechanisms (36). It is possible that developing similar modeling approaches would reveal subtle relationships in our data set that were not apparent with the limited approach taken here.
While dose-response effects with AS monotherapy have been observed previously (12), our findings indicate a lack of dose response between 2, 4, and 6 mg/kg/day of oral artesunate. Despite the lack of dose response, we found evidence for a postantibiotic effect with persistent reduction in parasitemia over 24 h after both AS and DHA had been effectively cleared from the body. This suggests that artemisinin action against blood stage parasites may be dependent on concentration rather than time. This is supported by hysteresis seen in our data set with a lag following the peak concentration to the onset of maximal drug effect. This would indicate that pharmacodynamic effects could be increased by increasing the dose to achieve a higher Cmax. However, this approach was not supported by our data which showed that increasing the artesunate dose to 6 mg/kg did not influence clinical or parasitological outcomes, despite achieving higher Cmax. Alternative explanations for this postantibiotic effect would include the persistence of drug in unmeasured or unmeasurable compartments or persistent activity of drug at concentrations below the limit of quantification. The former hypothesis would suggest that AS and DHA at the doses used were in excess of the amount needed to bind parasitized erythrocytes at the parasitemias observed in this population with uncomplicated P. falciparum malaria. This finding is supported by a prior prediction that increases in parasite clearance responses are unlikely at oral doses above 2 mg/kg, based on single-dose maximum effect (Emax) modeling (2). Parasite dormancy has also been suggested in animal studies as a possible explanation for parasite recrudescence despite apparently adequate drug levels (21).
Parasitemia at baseline had an impact on the pharmacodynamics of parasite clearance, with a positive correlation between P0 and parasite clearance times, as well as a loss of statistical differences in the parasite clearance times found in our 2006 and 2009 Tasanh studies when subjects with comparable drug doses and baseline parasitemia were compared. While the loss of significance in our data set could be attributable to sampling effect (the number of subjects taking the 4-mg/kg dose was reduced from 98 to 80 volunteers in our analysis), this finding has been reported previously (20) and suggests that baseline parasitemia is an important methodological consideration that should be controlled for when interpreting parasite clearance data in clinical trials of artemisinins.
There was a modest influence of intrinsic parasite artemisinin susceptibility ex vivo (IC50) on pharmacodynamic outcomes, but an equal influence of baseline parasitemia (P0) on the in vitro results by nonparametric correlation analysis. Using a ratio of pharmacokinetic parameters and IC50 did not reveal a relationship with parasite reduction or clearance. Given the effects of P0 on in vitro drug susceptibility test results with modest but significant correlations seen between increasing P0 and increasing IC50, this relationship should be taken into account when interpreting in vitro field isolate data.
Despite a lack of dose response for antimalarial activity, artesunate did produce a dose-limiting neutropenia in 5 of 28 subjects, as reported previously (6). It is unclear at this time if the effect on the neutrophil could be reduced by using higher doses over shorter periods, but given the lack of additional efficacy at higher doses, it does not appear that this strategy is likely to be effective.
Pharmacokinetic data were comparable to the previously observed results following oral dosing with artesunate in patients with uncomplicated P. falciparum malaria (4, 10, 32, 33), with somewhat higher values for PK parameters, including AUC and Cmax than those seen in studies of healthy volunteers (5, 33, 39). This could be attributable in part to a lack of standardization in PK analysis methods among laboratories. Oral data presented here contrast with recently reported intravenous (i.v.) data for good manufacturing process (GMP) intravenous artesunate, which found not only rapid hydrolysis of AS to DHA but also much higher Cmax values for AS than for DHA (22). The comparison suggests high first pass metabolism of AS when given by the oral route.
Our pharmacokinetic data revealed a reduction in drug exposure over 7 days of oral therapy, similar to previous reports. Autoinduction of metabolism has been suggested for drugs in this class (16, 17), representing a possible confounder to parasite-based mechanisms of resistance. Though some reports have attributed the observed decline in levels to the autoinduction of metabolism by artesunate (17), previous data from Southeast Asia suggest that this phenomenon may in fact be attributable to a transition from the infected to uninfected state over the course of therapy. Studies of healthy volunteers revealed reduced conversion of AS to DHA, reduced plasma drug levels, and little change in drug exposure over the course of 5 to 7 days of monotherapy compared to malaria patients where increased initial drug levels and a pronounced decrease in exposure over the course of therapy was seen (11, 37, 39). This suggests that host pharmacokinetics may be influenced by the presence of the parasite itself, though the mechanisms for this remain unproven. It is unclear whether this is a result of changes in drug metabolism, protein binding, drug distribution, and/or interactions with infected red blood cells that occur in malaria patients. Both reduced conversion of AS to DHA and reductions in penetration into the uninfected red blood cell could be possible explanations. Further, it remains unclear whether the observed reductions in drug concentration over the course of AS monotherapy are enough to account for treatment failures.
Our data add further evidence to the hypothesis that the site of drug action and appropriate place to measure pharmacokinetics may be within the erythrocyte. The formation of artemisinin adducts both with malaria parasite proteins and the membranes of disrupted but not intact red blood cells have been described previously (3, 19, 28). Further, selective uptake and concentration of artemisinins within malaria parasite-infected red blood cells is well documented, with many-fold-higher partition coefficients for infected red blood cells compared to uninfected cells, where partitioning of AS and DHA is only roughly 70% that of plasma (24, 25). This may be due to increases in directional transportation or intracellular binding (e.g., interactions with the malaria parasite) across infected red blood cell membranes or the effects of intracellular pH on drug ionization (14, 15, 27). Given that AS and DHA concentrate in infected red blood cells at many-fold-higher concentrations compared to uninfected cells, it might be expected that higher parasitemias would correlate with lower plasma drug levels due to partitioning into a currently unmeasurable storage pool within the red blood cells. Unfortunately, a validated nonradioactive method for measuring red blood cell drug levels remains elusive. Increased baseline parasitemias did not correlate with reduced plasma drug levels in our study, though it has been postulated that a clinically significant effect on PK parameters would be likely to occur only at very high levels of parasitemia (5% or greater) not generally seen in uncomplicated malaria (23). A prior review of mass balance studies would also argue against this possibility (35).
A limitation of our PK analysis was omission of an interim collection point between the 0- and 2-h time points on day 6, so AUC over the period of peak drug concentration could not be compared accurately. While some have fitted a one-compartment model for DHA, we found little difference in individual PK parameters between a one-compartment analysis (not shown) and a noncompartmental analysis. Our analysis would be strengthened by a modeling approach, particularly to address the possibility that the dose levels selected for the study were not sufficiently different to distinguish concentration-dependent pharmacodynamic effects on the malaria parasite. These limitations may have impacted our ability to detect underlying relationships where one truly existed.
The apparent lack of relationship between plasma pharmacokinetic and pharmacodynamic outcomes raises a question regarding the utility of routinely collecting these data in clinical trials. However, given the paucity of overall understanding of the mechanisms of action or resistance to artemisinins, further clinical data are clearly needed to add potential iterative validity to existing models. Improved study design methods, including the use of adaptive protocols, and a Bayesian approach to trial design using pretrial simulations based on existing data may help to improve understanding of the pharmacology of this important class of drugs. The relationship between intraerythrocytic drug levels and pharmacodynamic outcomes is of particular interest and likely to yield more insight into how this class of drugs works.
ACKNOWLEDGMENTS
We thank the staff of CNM—Sok Peou, Ses Sarim, Thay Kheang Heng, Va Soch, You Yom, Kong Nareth, Tim Sothi, San Savoen, Nou Samon, and Soklyda Chan. We thank Koy Lenin (Medical Monitor) of the Battambang Referral Hospital. We thank the following individuals at AFRIMS, Bangkok, Thailand—Maneerat Rasameesaroj, Roongnapha Apinan, Kuntida Tangthongchaiwiriya, Panita Gosi, Sathit Pichyangkul, Nichapat Uthaimongkol, Nilawan Buanthong, Mali Ittiverakul, Doungngoen Khamnithakul, Suriya Teopipthaporn, Chaiyawat Mathavarat, Worachet Kuntawunginn, Sittidech Surasri, Montri Arsanok, Siratchana Sundrakes, Piyaporn Siangam, Panjaporn Chaichana, Kristanai Yingyuen, and the staff of AFRIMS Research Support.
This study was supported by the U.S. Army Research and Materiel Command. This study was funded by the Bill & Melinda Gates Foundation through the World Health Organization (grant 48821) and the Global Emerging Infections Surveillance and Response System (a Division of the Armed Forces Health Surveillance Center). P.R. is a staff member of the World Health Organization.
The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions, policy, or views of the World Health Organization. The opinions or assertions contained herein are the views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Footnotes
Published ahead of print 6 August 2012
REFERENCES
- 1. Alin MH, et al. 1995. Efficacy of oral and intravenous artesunate in male Tanzanian adults with Plasmodium falciparum malaria and in vitro susceptibility to artemisinin, chloroquine, and mefloquine. Am. J. Trop. Med. Hyg. 53:639–645 [DOI] [PubMed] [Google Scholar]
- 2. Angus BJ, Thaiaporn I, Chanthapadith K, Suputtamongkol Y, White NJ. 2002. Oral artesunate dose-response relationship in acute falciparum malaria. Antimicrob. Agents Chemother. 46:778–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asawamahasakda W, Benakis A, Meshnick SR. 1994. The interaction of artemisinin with red cell membranes. J. Lab. Clin. Med. 123:757–762 [PubMed] [Google Scholar]
- 4. Batty KT, et al. 1998. A pharmacokinetic and pharmacodynamic study of intravenous vs oral artesunate in uncomplicated falciparum malaria. Br. J. Clin. Pharmacol. 45:123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benakis A, Paris M, Loutan L, Plessas CT, Plessas ST. 1997. Pharmacokinetics of artemisinin and artesunate after oral administration in healthy volunteers. Am. J. Trop. Med. Hyg. 56:17–23 [DOI] [PubMed] [Google Scholar]
- 6. Bethell D, et al. 2010. Dose-dependent risk of neutropenia after 7-day courses of artesunate monotherapy in Cambodian patients with acute Plasmodium falciparum malaria. Clin. Infect. Dis. 51:e105–e114 doi:10.1086/657402 [DOI] [PubMed] [Google Scholar]
- 7. Bethell D, et al. 2011. Artesunate dose escalation for the treatment of uncomplicated malaria in a region of reported artemisinin resistance: a randomized clinical trial. PLoS One 6:e19283 doi:10.1371/journal.pone.0019283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bunnag D, Viravan C, Looareesuwan S, Karbwang J, Harinasuta T. 1991. Clinical trial of artesunate and artemether on multidrug resistant falciparum malaria in Thailand. A preliminary report. Southeast Asian J. Trop. Med. Public Health 22:380–385 [PubMed] [Google Scholar]
- 9. Bunnag D, Viravan C, Looareesuwan S, Karbwang J, Harinasuta T. 1991. Double blind randomised clinical trial of oral artesunate at once or twice daily dose in falciparum malaria. Southeast Asian J. Trop. Med. Public Health. 22:539–543 [PubMed] [Google Scholar]
- 10. de Vries PJ, Dien TK. 1996. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs 52:818–836 [DOI] [PubMed] [Google Scholar]
- 11. Diem Thuy LT, Ngoc Hung L, Danh PT, Na-Bangchang K. 2008. Absence of time-dependent artesunate pharmacokinetics in healthy subjects during 5-day oral administration. Eur. J. Clin. Pharmacol. 64:993–998 [DOI] [PubMed] [Google Scholar]
- 12. Dondorp AM, et al. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flegg JA, Guerin PJ, White NJ, Stepniewska K. 2011. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar. J. 10:339 doi:10.1186/1475-2875-10-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fujioka H, Aikawa M. 1993. Morphological changes of clefts in Plasmodium-infected erythrocytes under adverse conditions. Exp. Parasitol. 76:302–307 [DOI] [PubMed] [Google Scholar]
- 15. Ginsburg H, Kutner S, Zangwil M, Cabantchik ZI. 1986. Selectivity properties of pores induced in host erythrocyte membrane by Plasmodium falciparum. Effect of parasite maturation. Biochim. Biophys. Acta 861:194–196 [DOI] [PubMed] [Google Scholar]
- 16. Gordi T, et al. 2005. A semiphysiological pharmacokinetic model for artemisinin in healthy subjects incorporating autoinduction of metabolism and saturable first-pass hepatic extraction. Br. J. Clin. Pharmacol. 59:189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hassan Alin M, Ashton M, Kihamia CM, Mtey GJ, Bjorkman A. 1996. Multiple dose pharmacokinetics of oral artemisinin and comparison of its efficacy with that of oral artesunate in falciparum malaria patients. Trans. R. Soc. Trop. Med. Hyg. 90:61–65 [DOI] [PubMed] [Google Scholar]
- 18. Hietala SF, et al. 2010. Population pharmacokinetics and pharmacodynamics of artemether and lumefantrine during combination treatment in children with uncomplicated falciparum malaria in Tanzania. Antimicrob. Agents Chemother. 54:4780–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hong YL, Yang YZ, Meshnick SR. 1994. The interaction of artemisinin with malarial hemozoin. Mol. Biochem. Parasitol. 63:121–128 [DOI] [PubMed] [Google Scholar]
- 20. Ittarat W, et al. 2003. Recrudescence in artesunate-treated patients with falciparum malaria is dependent on parasite burden not on parasite factors. Am. J. Trop. Med. Hyg. 68:147–152 [PubMed] [Google Scholar]
- 21. LaCrue AN, Scheel M, Kennedy K, Kumar N, Kyle DE. 2011. Effects of artesunate on parasite recrudescence and dormancy in the rodent malaria model Plasmodium vinckei. PLoS One 6:e26689 doi:10.1371/journal.pone.0026689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Q, et al. 2009. Pharmacokinetic profiles of artesunate after single intravenous doses at 0.5, 1, 2, 4, and 8 mg/kg in healthy volunteers: a phase I study. Am. J. Trop. Med. Hyg. 81:615–621 [DOI] [PubMed] [Google Scholar]
- 23. Li Q, Milhous WK, Weina PJ. 2007. Artemisinins in malaria therapy, p 91–92 Nova Science, New York, NY [Google Scholar]
- 24. Li Q, Xie LH, Haeberle A, Zhang J, Weina P. 2006. The evaluation of radiolabeled artesunate on tissue distribution in rats and protein binding in humans. Am. J. Trop. Med. Hyg. 75:817–826 [PubMed] [Google Scholar]
- 25. Li QG, et al. 1998. Pharmacology and toxicology of artelinic acid: preclinical investigations on pharmacokinetics, metabolism, protein and red blood cell binding, and acute and anorectic toxicities. Trans. R. Soc. Trop. Med. Hyg. 92:332–340 [DOI] [PubMed] [Google Scholar]
- 26. Looareesuwan S, et al. 1997. Monotherapy with sodium artesunate for uncomplicated falciparum malaria in Thailand: a comparison of 5- and 7-day regimens. Acta Trop. 67:197–205 [DOI] [PubMed] [Google Scholar]
- 27. Maguire PA, Sherman IW. 1990. Phospholipid composition, cholesterol content and cholesterol exchange in Plasmodium falciparum-infected red cells. Mol. Biochem. Parasitol. 38:105–112 [DOI] [PubMed] [Google Scholar]
- 28. Meshnick SR, Thomas A, Ranz A, Xu CM, Pan HZ. 1991. Artemisinin (qinghaosu): the role of intracellular hemin in its mechanism of antimalarial action. Mol. Biochem. Parasitol. 49:181–189 [DOI] [PubMed] [Google Scholar]
- 29. Noedl H, Attlmayr B, Wernsdorfer WH, Kollaritsch H, Miller RS. 2004. A histidine-rich protein 2-based malaria drug sensitivity assay for field use. Am. J. Trop. Med. Hyg. 71:711–714 [PubMed] [Google Scholar]
- 30. Noedl H, et al. 2010. Artemisinin resistance in Cambodia: a clinical trial designed to address an emerging problem in Southeast Asia. Clin. Infect. Dis. 51:e82–e89 doi:10.1086/657120 [DOI] [PubMed] [Google Scholar]
- 31. Noedl H, Schaecher YSK, Smith BL, Socheat D, Fukuda MM. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium. N. Engl. J. Med. 359:2619–2620 [DOI] [PubMed] [Google Scholar]
- 32. Onyamboko MA, et al. 2011. Pharmacokinetics and pharmacodynamics of artesunate and dihydroartemisinin following oral treatment in pregnant women with asymptomatic Plasmodium falciparum infections in Kinshasa DRC. Malar. J. 10:49 doi:10.1186/1475-2875-10-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Orrell C, et al. 2008. Pharmacokinetics and tolerability of artesunate and amodiaquine alone and in combination in healthy volunteers. Eur. J. Clin. Pharmacol. 64:683–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Price R, et al. 1998. Artesunate versus artemether for the treatment of recrudescent multidrug-resistant falciparum malaria. Am. J. Trop. Med. Hyg. 59:883–888 [DOI] [PubMed] [Google Scholar]
- 35. Roffey SJ, Obach RS, Gedge JI, Smith DA. 2007. What is the objective of the mass balance study? A retrospective analysis of data in animal and human excretion studies employing radiolabeled drugs. Drug Metab. Rev. 39:17–43 [DOI] [PubMed] [Google Scholar]
- 36. Saralamba S, et al. 2011. Intrahost modeling of artemisinin resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 108:397–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tan B, et al. 2009. Population pharmacokinetics of artesunate and dihydroartemisinin following single- and multiple-dosing of oral artesunate in healthy subjects. Malar. J. 8:304 doi:10.1186/1475-2875-8-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Teja-Isavadharm P, et al. 2010. A simplified liquid chromatography-mass spectrometry assay for artesunate and dihydroartemisinin, its metabolite, in human plasma. Molecules 15:8747–8768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Teja-Isavadharm P, et al. 2001. Comparative pharmacokinetics and effect kinetics of orally administered artesunate in healthy volunteers and patients with uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 65:717–721 [DOI] [PubMed] [Google Scholar]
- 40. Viriyakosol S, et al. 1995. Genotyping of Plasmodium falciparum isolates by the polymerase chain reaction and potential uses in epidemiological studies. Bull. World Health Organ. 73:85–95 [PMC free article] [PubMed] [Google Scholar]