Abstract
Cultures of 3T6 cells were plated in serum-free medium and grown in the presence of insulin (1 microgram/ml) and epidermal growth factor (0.5 ng/ml). External ATP (250 microM) applied to such cultures caused a rapid efflux of acid-soluble pools labeled with [3H]uridine, 2-deoxy[3H]glucose, or 86Rb+ and allowed the entry of p-nitrophenylphosphate. This increase in passive membrane permeability depended on ATP concentration, pH, and time of ATP contact with the cells, and it was not produced by GTP, UTP, or Pi. In the presence of compounds that decrease intracellular ATP, low concentrations of external ATP (40 microM) caused a massive synergistic stimulation of efflux. The efflux of acid-soluble pools was stopped (sealing) by bringing the cultures of 3T6 cells to neutral pH in the presence of Ca2+ and Mg2+. Exposure of 3T6 cells grown in serum-free medium to [gamma-32P]ATP under the conditions of permeabilization led to the selective labeling of a membrane protein with a molecular weight of 44,000 as revealed by NaDodSO4 polyacrylamide gel electrophoresis and autoradiography. The results show that the control of membrane permeability by ATP is completely independent of serum-deprived proteins. Furthermore, the protein band (Mr, 44 x 10(3)) that shows selective labeling by [32P]ATP during permeabilization is not an adsorbed serum component.
Full text
PDF
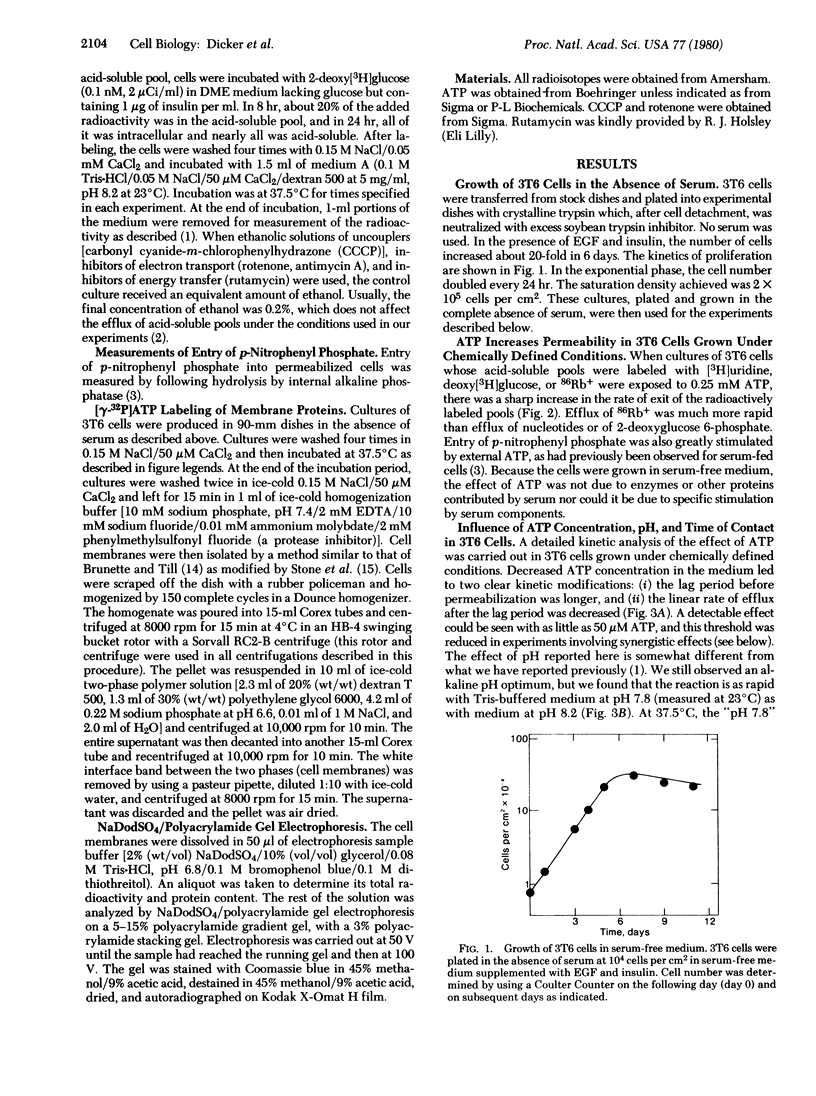
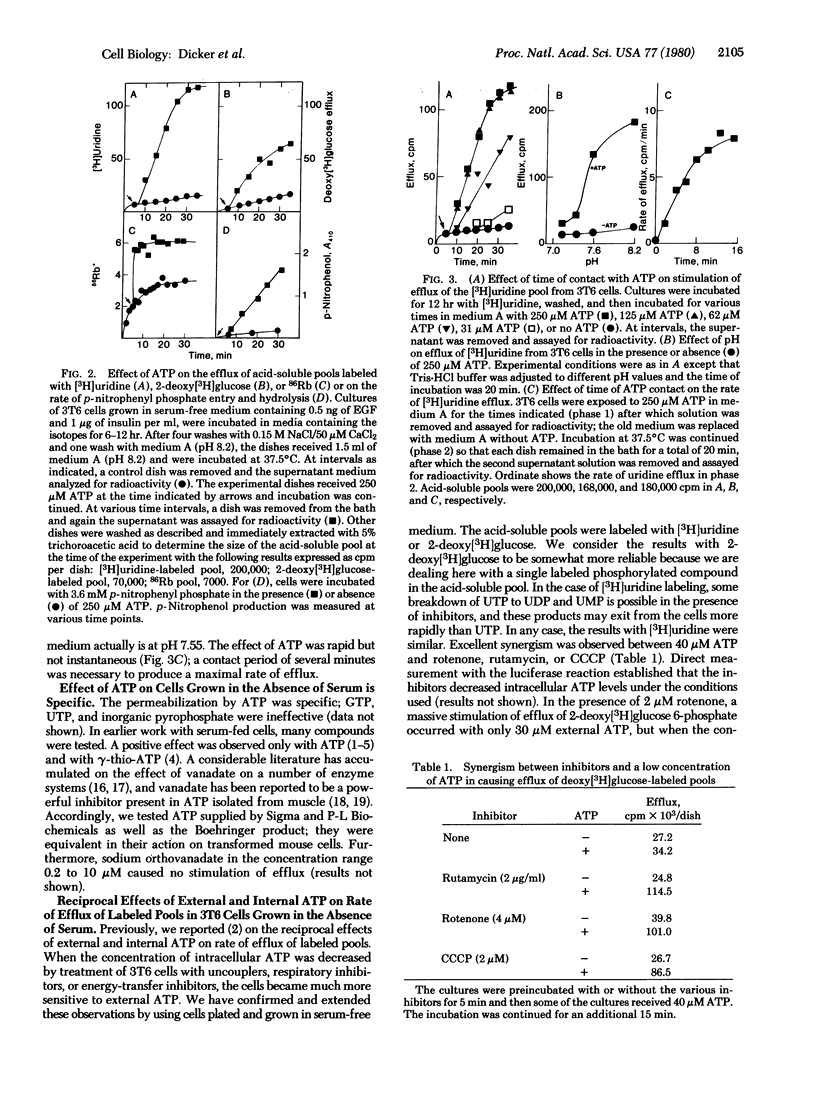
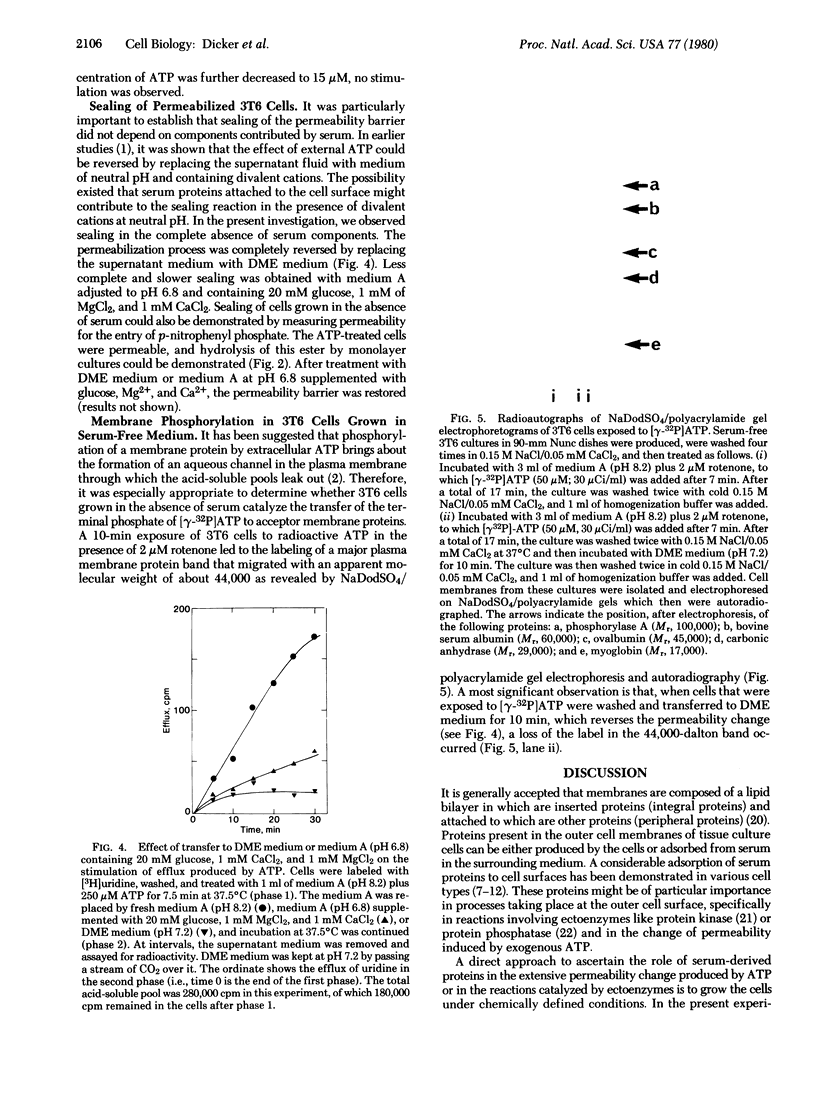

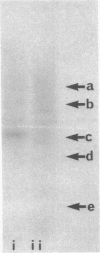
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaugé L. A., Glynn I. M. Commercial ATP containing traces of vanadate alters the response of (Na+ + K+) ATPase to external potassium. Nature. 1978 Apr 6;272(5653):551–552. doi: 10.1038/272551a0. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Cantley L. G., Josephson L. A characterization of vanadate interactions with the (Na,K)-ATPase. Mechanistic and regulatory implications. J Biol Chem. 1978 Oct 25;253(20):7361–7368. [PubMed] [Google Scholar]
- Cantley L. C., Jr, Josephson L., Warner R., Yanagisawa M., Lechene C., Guidotti G. Vanadate is a potent (Na,K)-ATPase inhibitor found in ATP derived from muscle. J Biol Chem. 1977 Nov 10;252(21):7421–7423. [PubMed] [Google Scholar]
- Cherington P. V., Smith B. L., Pardee A. B. Loss of epidermal growth factor requirement and malignant transformation. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3937–3941. doi: 10.1073/pnas.76.8.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J. B., Racker E. Reversible inhibition of (Na+, K+) ATPase by Mg2+, adenosine triphosphate, and K+. Biochemistry. 1977 Jan 11;16(1):152–158. doi: 10.1021/bi00620a026. [DOI] [PubMed] [Google Scholar]
- Forni G., Green I. Heterologous sera: a target for in vitro cell-mediated cytotoxicity. J Immunol. 1976 Jun;116(6):1561–1565. [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Hayashi I., Sato G. H. Replacement of serum by hormones permits growth of cells in a defined medium. Nature. 1976 Jan 15;259(5539):132–134. doi: 10.1038/259132a0. [DOI] [PubMed] [Google Scholar]
- Hogg N. M. A comparison of membrane proteins of normal and transformed cells by lactoperoxidase labeling. Proc Natl Acad Sci U S A. 1974 Feb;71(2):489–492. doi: 10.1073/pnas.71.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makan N. R., Heppel L. A. Control of glycolysis and the pentose phosphate shunt in transformed 3T3 cultures rendered permeable by ATP. J Cell Physiol. 1978 Jul;96(1):87–94. doi: 10.1002/jcp.1040960111. [DOI] [PubMed] [Google Scholar]
- Makan N. R. Induction of permeability change and restoration of membrane permeability barrier in transformed cell cultures. Exp Cell Res. 1978 Jul;114(2):417–427. doi: 10.1016/0014-4827(78)90501-3. [DOI] [PubMed] [Google Scholar]
- Makan N. R. Role of cytoplasmic ATP in the restoration and maintenance of a membrane permeability barrier in transformed mammalian cells. J Cell Physiol. 1979 Dec;101(3):481–492. doi: 10.1002/jcp.1041010314. [DOI] [PubMed] [Google Scholar]
- Mastro A. M., Rozengurt E. Endgoenous protein kinase in outer plasma membrane of cultured 3T3 cells. Nature of the membrane-bound substrate and effect of cell density, serum addition, and oncogenic transformation. J Biol Chem. 1976 Dec 25;251(24):7899–7906. [PubMed] [Google Scholar]
- Mierzejewski K., Rozengurt E. Stimulation of DNA synthesis and cell division in a chemically defined medium: effect of epidermal growth factor, insulin and vitamin B12 on resting cultures of 3T6 cells. Biochem Biophys Res Commun. 1976 Nov 22;73(2):271–278. doi: 10.1016/0006-291x(76)90703-8. [DOI] [PubMed] [Google Scholar]
- Patterson M. K., Jr Alterations in membrane structure and function associated with neoplastic transformation in vitro. J Natl Cancer Inst. 1974 Nov;53(5):1493–1498. doi: 10.1093/jnci/53.5.1493. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. A Specific effect of external ATP on the permeability of transformed 3T3 cells. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1581–1588. doi: 10.1016/0006-291x(75)90207-7. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A., Friedberg I. Effect of exogenous ATP on the permeability properties of transformed cultures of mouse cell lines. J Biol Chem. 1977 Jul 10;252(13):4584–4590. [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Reciprocal control of membrane permeability of transformed cultures of mouse cell lines by external and internal ATP. J Biol Chem. 1979 Feb 10;254(3):708–714. [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Stone K. R., Smith R. E., Joklik W. K. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974 Mar;58(1):86–100. doi: 10.1016/0042-6822(74)90143-3. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarone G., Comoglio P. M. Binding of serum polypeptides to the plasma membrane outer surface. FEBS Lett. 1976 Sep 1;67(3):364–367. doi: 10.1016/0014-5793(76)80565-0. [DOI] [PubMed] [Google Scholar]