Abstract
The increasing prevalence of multidrug-resistant Gram-negative infections has led to the resurgence of systemic polymyxin B, but little is known about its pharmacokinetics. The objective of this study was to characterize the pharmacokinetics and renal disposition of polymyxin B. Eight female Sprague-Dawley rats (weight, 225 to 250 g) were administered a single intravenous polymyxin B dose (4 mg/kg of body weight). Serial serum samples were collected and assayed for major polymyxin B components using a validated ultraperformance liquid chromatography-tandem mass spectrometry method. The best-fit pharmacokinetic parameters of each component were derived and compared using one-way analysis of variance. Cumulative urine was also collected daily for 48 h and assayed for polymyxin B. Kidney drug concentrations were measured at 6 h (n = 3) and 48 h (n = 3) after the same dose. Additionally, three rats were administered 2 doses of intravenous polymyxin B (4 mg/kg) 7 days apart. Serial serum samples were collected pre- and post-renal insufficiency (induced by uranyl nitrate) and assayed for polymyxin B. The pharmacokinetic parameters of the major components did not appear to be significantly different (P > 0.05). Less than 1% of the dose was recovered unchanged in urine collected over 48 h following administration. Therapeutic drug concentrations persisted in kidney tissue at 48 h. The post-renal insufficiency to pre-renal insufficiency ratio of the area under the serum concentration-time curve from time zero to infinity was 1.33 ± 0.04. Polymyxin B components appear to have similar pharmacokinetics. Polymyxin B preferentially persists in kidneys, which suggests a selective uptake process in renal cells. A mechanism(s) other than renal excretion could be involved in polymyxin B elimination, and dosing adjustment in renal insufficiency may not be necessary.
INTRODUCTION
The increasing prevalence of multidrug resistance worldwide has led to the resurgence of polymyxin B (12, 18). However, despite being available for clinical use since the 1950s, little is known about the pharmacokinetics of this drug. Current dosing recommendations are not well supported by the pharmacokinetic and pharmacodynamic data, which may adversely affect the clinical outcome (3) as well as increase the risk of resistance development (7, 13). Since polymyxin B is currently the last line of defense against many strains of multidrug-resistant Gram-negative bacteria, optimal dosing of this agent is essential until new agents become available.
Polymyxin B is a peptide antibiotic commercially available as a mixture of several closely related cyclic amphiphilic molecules, of which polymyxin B1 (PB1), isoleucine-polymyxin B1 (Ile-PB1), polymyxin B2 (PB2), and polymyxin B3 (PB3) constitute most of the mixture (14). The relative abundance of these four components is fairly consistent among polymyxin B formulations produced by different manufacturers (5). However, whether the different components of polymyxin B exhibit similar pharmacokinetic properties has not been previously investigated.
The currently used dosing recommendations for polymyxin B are commonly based on the weight and the renal function of the patients (18). These recommendations were published in the 1970s, but whether polymyxin B is renally eliminated has not been comprehensively investigated. On the basis of the findings of earlier studies, approximately 60% of the dose was recovered in urine (6). However, in the majority of the earlier studies, polymyxin B was quantified using nonspecific microbiological assays. Since patients with multidrug-resistant bacterial infections are usually administered more than one antibiotic simultaneously, using a microbiological assay to quantify polymyxin B in clinical samples may be problematic. Recently, Zavascki et al. reported that only less than 1% of the dose of polymyxin B was recovered unchanged in the urine of a group of 8 critically ill patients (19). These findings suggest that nonrenal elimination might be the predominant clearance pathway for polymyxin B.
In this study, we examined the pharmacokinetics of major polymyxin B components in an animal model. We also examined the impact of renal insufficiency on the rate of elimination and total drug exposure. Identifying the extent of drug elimination via the renal route will justify dose adjustment in patients with impaired renal function.
MATERIALS AND METHODS
Antimicrobial agent.
Polymyxin B sulfate (USP) powder was purchased from Sigma-Aldrich (St. Louis, MO). Prior to each experiment, the drug was reconstituted and diluted to the desired concentrations with normal saline. The reconstituted drug solutions were stored at −70°C and used within 10 days. A prior stability study confirmed that the reconstituted drug was stable under these storage conditions (data not shown).
Animals.
Female Sprague-Dawley rats (weight, 225 to 249 g; Harlan, Indianapolis, IN) were used. In selected experiments, the animals were either single or double jugular vein cannulated, which had been performed by the vendor, to facilitate drug administration and blood collection. The rats received food and water ad libitum. All animals were cared for in accordance with the highest humane and ethical standards, as approved by the Institutional Animal Care and Use Committee of the University of Houston.
Single-dose pharmacokinetics.
Eight rats were used to derive the pharmacokinetic parameters of the four major components of polymyxin B (polymyxin B1, isoleucine-polymyxin B1, polymyxin B2, and polymyxin B3) following intravenous administration. The rats were given a single slow intravenous bolus dose of 4 mg of polymyxin B/kg of body weight over 1 to 2 min via one cannula. Selection of the dose was guided by prior dose escalation studies to determine the maximum tolerated intravenous dose. Serial blood samples were collected at 0.5, 2, 4, and 6 h postdose via the other cannula. The blood samples were allowed to clot on ice, and serum was obtained by centrifugation at 6,000 × g for 10 min. Serum was stored at −70°C until analysis. The serum samples were assayed for major components of polymyxin B using the ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method (11) and purified standards (17) described previously. The ADAPT II program was used to fit various models (e.g., one- and two-compartment open models) to the observed serum polymyxin B concentrations over time (2). The Akaike information criterion (AIC) was used to select the best-fit model, and the principle of parsimony was applied. The best-fit pharmacokinetic parameters of each of the major polymyxin B components were derived and compared using one-way analysis of variance. P values of <0.05 were considered significant.
Renal tissue concentration and urinary excretion.
Six rats were administered a single intravenous dose of 4 mg/kg of polymyxin B; three rats were euthanized at 6 h, and the other three rats were euthanized at 48 h. One kidney from each rat was collected to determine the polymyxin B concentration in renal tissues. The kidneys were wrapped in Parafilm and stored at −70°C until analysis. On the day of the analysis, the kidneys were thawed at room temperature and homogenized in deionized water. Eight rats were housed individually in metabolic cages and were administered a single intravenous dose of 4 mg/kg of polymyxin B. The cumulative urine of each rat was collected daily for up to 48 h following administration of polymyxin B to estimate the fraction of drug excreted unchanged in the urine. Urine samples were stored at −70°C until analysis. The urine and the kidney homogenates were assayed for the major components of polymyxin B as described above.
Pharmacokinetics in renal insufficiency.
The pharmacokinetics and urinary excretion of polymyxin B1 (the most abundant component in the USP mixture) were further studied in renal insufficiency using a self-controlled design. Three rats were administered a single intravenous dose of 4 mg/kg of polymyxin B. Serial blood samples were collected at 0.5, 2, 4, and 6 h postdose, and the cumulative urine (over 48 h) of each rat was collected following administration of polymyxin B. After a 5-day washout period, the rats were administered a single intravenous dose of 5 mg/kg of uranyl nitrate (NOAH Technologies Corporation, San Antonio, TX) to induce transient renal dysfunction (4). Serum creatinine was measured for each rat at baseline and daily following uranyl nitrate treatment using a clinical chemistry analyzer (Piccolo Xpress; Abaxis, Inc., Union City, CA). Once a considerable elevation in serum creatinine was observed (≥5 times the baseline level), the rats were administered a second intravenous dose of 4 mg/kg of polymyxin B. Serial blood sampling and cumulative urine collection were repeated. The blood and urine samples were processed as outlined above. The pharmacokinetic parameters and the amount of polymyxin B1 recovered unchanged in the urine were compared before and after renal insufficiency using a paired t test. P values of <0.05 were considered significant.
Urinary excretion upon repeated administration.
In order to examine the effect of repeated administration on the urinary excretion of polymyxin B, three rats were administered 5 mg/kg of polymyxin B subcutaneously every 24 h for 10 days. Selection of the dose was guided by previous studies, which demonstrated that the dose was well tolerated and did not result in a significant change in renal function for up to 10 days. Furthermore, prior pharmacokinetic studies revealed a drug exposure, as estimated by the area under the serum concentration-time curve (AUC) from time zero to infinity (AUC0–∞), comparable to that achieved with an intravenous dose of 4 mg/kg (data not shown). The cumulative urine (over 24 h) of each rat was collected on days 1, 5, and 10. The urine samples were processed and assayed for polymyxin B1 as described above to estimate the fraction of drug excreted unchanged in urine.
RESULTS
Single-dose pharmacokinetics.
The four major components of polymyxin B were satisfactorily detected in rat serum for up to 6 h after intravenous administration. However, the polymyxin B2 and polymyxin B3 peaks in the chromatograms were not completely resolved, and the concentrations of polymyxin B3 were negligible in the majority of the samples. Therefore, the two components were combined into a single entity. The observations were satisfactorily described by a one-compartment linear model. Good model fits were achieved for all the different components (r2 ≥ 0.9). The derived pharmacokinetic parameters of the components are shown in Table 1. The polymyxin B1 AUC0–∞ was found to be 6.62 ± 2.13 μg · h/ml. On the basis of the best-fit parameter estimates (11), the projected human equivalent AUC following a standard dose is expected to be 73 ± 27 μg · h/ml. The pharmacokinetic parameters of the major components did not appear to be significantly different (P > 0.05).
Table 1.
Pharmacokinetics of major polymyxin B componentsa
| Component | kel (h−1) | t1/2 (h) | V (ml) | CL (ml/min) |
|---|---|---|---|---|
| PB1 | 0.51 ± 0.15 | 1.46 ± 0.39 | 198.1 ± 44.12 | 1.65 ± 0.62 |
| Ile-PB1 | 0.51 ± 0.15 | 1.47 ± 0.39 | 184.8 ± 49.21 | 1.52 ± 0.46 |
| PB2 + PB3 | 0.54 ± 0.12 | 1.33 ± 0.27 | 194.6 ± 64.84 | 1.69 ± 0.52 |
| P | 0.86 | 0.69 | 0.88 | 0.80 |
Data are means ± standard deviations. kel, elimination rate constant; t1/2, elimination half-life; V, volume of distribution; CL, total body clearance.
Renal tissue concentration and urinary excretion.
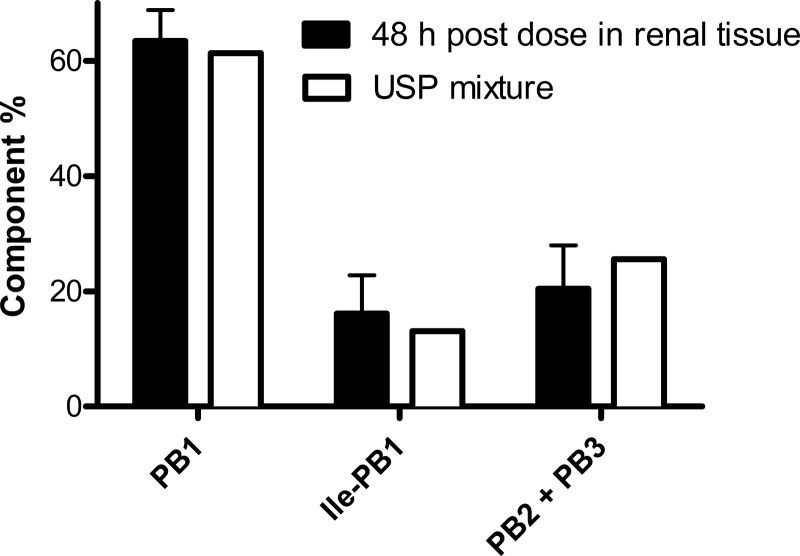
The concentrations of polymyxin B1, isoleucine polymyxin B1, and polymyxin B2 plus polymyxin B3 were 13.04 ± 3.09, 3.01 ± 1.87, and 2.78 ± 0.46 μg/g, respectively, in the kidneys at 6 h postdose. In comparison, the polymyxin B1 serum concentration was only 440 ± 0.02 ng/ml at 6 h. Long after the serum concentration fell below the limit of detection (at 48 h), therapeutic concentrations of polymyxin B persisted in kidney tissue. The concentrations of polymyxin B1, isoleucine polymyxin B1, and polymyxin B2 plus polymyxin B3 were 0.80 ± 0.21, 0.20 ± 0.03, and 0.26 ± 0.09 μg/g, respectively, in the kidneys. In addition, the relative proportions of the components in the kidney tissue at 48 h were found to be comparable to those in the USP mixture (Fig. 1), indicating no preferential accumulation of any of the components. Polymyxin B1 was detected in the urine, but only less than 1% of the dose was recovered unchanged during the first 48 h after administration. This implied that a mechanism(s) other than renal excretion could be involved in the elimination of polymyxin B.
Fig 1.
Comparison of percentages of major polymyxin B components. Data are means ± 95% confidence intervals.
Pharmacokinetics in renal insufficiency.
A considerable elevation in serum creatinine (6.5 to 9.0 times the baseline level) was observed at approximately 48 h after uranyl nitrate treatment. The pharmacokinetic parameters of polymyxin B1 before and after the onset of renal insufficiency are shown in Table 2. Renal function had no significant impact on the elimination half-life (P > 0.05). However, the total body clearance appeared to be slightly reduced. Nevertheless, the total drug exposure (AUC0–∞) was not considerably altered. The mean post- to pre-renal insufficiency AUC0–∞ ratio was 1.33. Renal function also appeared to have no impact on the extent of polymyxin B urinary excretion; less than 1% of the dose was recovered unchanged in urine at 48 h following administration, both before and after induction of renal insufficiency. These results corroborated our previous findings that a mechanism(s) other than renal excretion could be involved in the elimination of polymyxin B.
Table 2.
Pharmacokinetics of polymyxin B1 pre- and post-renal insufficiencya
| Time of evaluation | t1/2 (h) | V (ml) | CL (ml/min) | AUC0–∞ (μg · h/ml) |
|---|---|---|---|---|
| Pre-renal insufficiency | 1.56 ± 0.16 | 209.10 ± 8.38 | 1.56 ± 0.21 | 6.42 ± 0.91 |
| Post-renal insufficiency | 1.81 ± 0.40 | 184.00 ± 44.44 | 1.17 ± 0.13 | 8.50 ± 1.07 |
| P | 0.47 | 0.45 | 0.02 | 0.004 |
Data are means ± standard deviations. t1/2, elimination half-life; V, volume of distribution; CL, total body clearance; AUC0–∞, area under the serum concentration-time curve from time zero to infinity.
Urinary excretion upon repeated administration.
Less than 1% of the dose was excreted unchanged in urine on days 1, 5, and 10. Using the dose investigated, the extent of polymyxin B urinary excretion was not affected by repeated administration.
DISCUSSION
Recently, several attempts have been made to characterize the pharmacokinetics and pharmacodynamics of polymyxin B (11, 17, 19). Knowledge of the pharmacokinetics/pharmacodynamics as well as the toxicodynamics of the drug could lead to the design of dosing regimens that yield maximum effectiveness and minimal toxicity. A major barrier is the use of the USP mixture, which is composed of multiple components (14). Our group has previously examined the pharmacokinetics of polymyxin B1 following intravenous administration to a group of patients with multidrug-resistant Gram-negative bacterial infections (11). However, since purified standards of other components were not available at that time, we were able to characterize the pharmacokinetics only of polymyxin B1. Zavascki et al. examined the pharmacokinetics of intravenous polymyxin B in a group of 8 critically ill patients (19). Since their chromatographic separation also lacked the resolution necessary to separate the different components of the same molecular weight, they used a combined approach (sum of major chromatographic peak areas) to quantify polymyxin B in their samples. This approach assumed similar pharmacokinetics for all components, which was not well established.
In this study, we examined if the four major components of polymyxin B have similar pharmacokinetic properties using a rat model. The serum samples were assayed for the different components using a validated multiplexed UPLC-MS/MS method and purified standards of the components. The pharmacokinetics of the different components appeared to be similar. Recently, our group has also reported that the in vitro potencies of the different components were comparable (17). Collectively, these findings suggest that the different polymyxin B components exhibit similar pharmacological properties. This would justify the use of polymyxin B1 (the most abundant component) as a representative to describe the pharmacokinetics and renal disposition of polymyxin B in subsequent studies.
We next examined the extent of accumulation of polymyxin B in renal tissue following a single intravenous dose. Even though the serum concentration of the drug at 6 h was barely detectable, a very high concentration of polymyxin B was detected in renal tissue (more than 30-fold the serum concentration). At 48 h, therapeutic concentrations of polymyxin B persisted in kidney tissue. This implies that polymyxin B has a preferential affinity to renal tissues, which resulted in its accumulation and prolonged residence in the kidneys. These findings were somewhat consistent with those of earlier studies (8, 9). Persistent accumulation of polymyxin B in kidneys may have clinical implications on its nephrotoxicity (1, 15).
We also examined the extent of renal elimination of polymyxin B in a rat model. In contrast to an earlier report (6), only less than 1% of the dose was recovered unchanged in the urine collected over 48 h following a single intravenous dose. Therefore, the specific renal clearance was negligible. Furthermore, the extent of renal excretion did not increase upon repeated administration. Consistent with our results, Zavascki et al. also reported very low recovery (<1%) of polymyxin B in urine following intravenous administration to a group of 8 critically ill patients (19). In earlier studies, nonselective microbiological assays were used to quantify polymyxin B. The presence of microbiologically active metabolites in the urine is a possible explanation for such a discrepancy in the magnitude of renal elimination. However, Satlin et al. reported a modest microbiologic clearance rate in patients receiving polymyxin B for treatment of carbapenem-resistant Klebsiella pneumoniae bacteriuria (16). The low recovery in urine, combined with the limited efficacy reported for treatment of urinary tract infections (16), suggests that polymyxin B may not be predominantly eliminated via the kidneys. To further substantiate this observation, we compared the pharmacokinetics of polymyxin B in rats pre- and post-renal insufficiency. Renal insufficiency did not appear to have a considerable impact on the rate of elimination. The total drug exposure was elevated only by approximately 30%, which was not deemed to be clinically significant to justify the drastic dose reduction commonly used in clinical practice (between 50 and 87%) (18). These findings are consistent with our previous observation in a patient with renal insufficiency (10). Collectively, these results suggest that clearance of polymyxin B may not be highly sensitive to renal function changes. Therefore, drastic dose reduction may not be necessary in case of renal insufficiency.
In summary, our data suggest that polymyxin B appears to have preferential accumulation and prolonged residence in renal tissue, which could be correlated to its nephrotoxicity potential. Furthermore, a mechanism(s) other than renal excretion could be involved in the elimination of polymyxin B. A limitation to our study was that we did not examine the metabolism of polymyxin B. Further investigations on the mechanisms of renal cellular uptake of polymyxin B, its biodistribution, and metabolism are ongoing. This could provide further insights into strategies to minimize the toxicity potential of polymyxin B. Clinical data are warranted to validate our findings as well as the current dosing recommendations for polymyxin B.
ACKNOWLEDGMENT
This work was supported by grant R15AI089671-01 from the National Institutes of Health.
Footnotes
Published ahead of print 20 August 2012
REFERENCES
- 1. Abdelraouf K, et al. 2012. Characterization of polymyxin B-induced nephrotoxicity: implications for dosing regimen design. Antimicrob. Agents Chemother. 56:4625–4629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. D'Argenio DZ, Schumitzky A. 1997. ADAPT II user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles, CA [Google Scholar]
- 3. Elias LS, Konzen D, Krebs JM, Zavascki AP. 2010. The impact of polymyxin B dosage on in-hospital mortality of patients treated with this antibiotic. J. Antimicrob. Chemother. 65:2231–2237 [DOI] [PubMed] [Google Scholar]
- 4. Giacomini KM, Roberts SM, Levy G. 1981. Evaluation of methods for producing renal dysfunction in rats. J. Pharm. Sci. 70:117–121 [DOI] [PubMed] [Google Scholar]
- 5. He J, et al. 2010. Variability of polymyxin B major components in commercial formulations. Int. J. Antimicrob. Agents 35:308–310 [DOI] [PubMed] [Google Scholar]
- 6. Hoeprich PD. 1970. The polymyxins. Med. Clin. North Am. 54:1257–1265 [PubMed] [Google Scholar]
- 7. Ko KS, et al. 2007. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J. Antimicrob. Chemother. 60:1163–1167 [DOI] [PubMed] [Google Scholar]
- 8. Kunin CM. 1970. Binding of antibiotics to tissue homogenates. J. Infect. Dis. 121:55–64 [DOI] [PubMed] [Google Scholar]
- 9. Kunin CM, Bugg A. 1971. Binding of polymyxin antibiotics to tissues: the major determinant of distribution and persistence in the body. J. Infect. Dis. 124:394–400 [DOI] [PubMed] [Google Scholar]
- 10. Kwa AL, Abdelraouf K, Low JG, Tam VH. 2011. Pharmacokinetics of polymyxin B in a patient with renal insufficiency: a case report. Clin. Infect. Dis. 52:1280–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwa AL, et al. 2008. Pharmacokinetics of polymyxin B1 in patients with multidrug-resistant Gram-negative bacterial infections. Diagn. Microbiol. Infect. Dis. 60:163–167 [DOI] [PubMed] [Google Scholar]
- 12. Kwa AL, Tam VH, Falagas ME. 2008. Polymyxins: a review of the current status including recent developments. Ann. Acad. Med. Singapore 37:870–883 [PubMed] [Google Scholar]
- 13. Landman D, Bratu S, Alam M, Quale J. 2005. Citywide emergence of Pseudomonas aeruginosa strains with reduced susceptibility to polymyxin B. J. Antimicrob. Chemother. 55:954–957 [DOI] [PubMed] [Google Scholar]
- 14. Orwa JA, et al. 2001. Isolation and structural characterization of polymyxin B components. J. Chromatogr. A 912:369–373 [DOI] [PubMed] [Google Scholar]
- 15. Ouderkirk JP, Nord JA, Turett GS, Kislak JW. 2003. Polymyxin B nephrotoxicity and efficacy against nosocomial infections caused by multiresistant gram-negative bacteria. Antimicrob. Agents Chemother. 47:2659–2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Satlin MJ, et al. 2011. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob. Agents Chemother. 55:5893–5899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tam VH, Cao H, Ledesma KR, Hu M. 2011. In vitro potency of various polymyxin B components. Antimicrob. Agents Chemother. 55:4490–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuan Z, Tam VH. 2008. Polymyxin B: a new strategy for multidrug-resistant Gram-negative organisms. Expert Opin. Investig. Drugs 17:661–668 [DOI] [PubMed] [Google Scholar]
- 19. Zavascki AP, et al. 2008. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin. Infect. Dis. 47:1298–1304 [DOI] [PubMed] [Google Scholar]