Abstract
Benzothiazinones (BTZ) are a new class of drug candidates to combat tuberculosis that inhibit decaprenyl-phosphoribose epimerase (DprE1), an essential enzyme involved in arabinan biosynthesis. Using the checkerboard method and cell viability assays, we have studied the interaction profiles of BTZ043, the current lead compound, with several antituberculosis drugs or drug candidates against Mycobacterium tuberculosis strain H37Rv, namely, rifampin, isoniazid, ethambutol, TMC207, PA-824, moxifloxacin, meropenem with or without clavulanate, and SQ-109. No antagonism was found between BTZ043 and the tested compounds, and most of the interactions were purely additive. Data from two different approaches clearly indicate that BTZ043 acts synergistically with TMC207, with a fractional inhibitory concentration index of 0.5. TMC207 at a quarter of the MIC (20 ng/ml) used in combination with BTZ043 (1/4 MIC, 0.375 ng/ml) had a stronger bactericidal effect on M. tuberculosis than TMC207 alone at a concentration of 80 ng/ml. This synergy was not observed when the combination was tested on a BTZ-resistant M. tuberculosis mutant, suggesting that DprE1 inhibition is the basis for the interaction. This finding excludes the possibility of synergy occurring through an off-target mechanism. We therefore hypothesize that sub-MICs of BTZ043 weaken the bacterial cell wall and allow improved penetration of TMC207 to its target. Synergy between two new antimycobacterial compounds, such as TMC207 and BTZ043, with novel targets, offers an attractive foundation for a new tuberculosis regimen.
INTRODUCTION
With 8.8 million incident cases of tuberculosis (TB) worldwide and around 1.5 million deaths in 2010, Mycobacterium tuberculosis infection is one of the most important causes of death from an infectious disease (14). The spread of multidrug-resistant TB (MDR-TB), namely, resistance to both isoniazid (INH) and rifampin (RIF), poses additional challenges to treatment with currently available anti-TB drugs. The situation is exacerbated by the increasing emergence of extensively drug-resistant (XDR) strains of M. tuberculosis, which cause diseases essentially untreatable with existing compounds. Greater effort is required to find more efficacious combinations of molecules in order to meet the desired goals of killing both active and persistent tubercle bacilli while decreasing treatment duration. The TB drug development pipeline now comprises several candidates that are in clinical trials or soon will be (2, 12). The recommendations of the Global TB Alliance for Drug Development are to aim for a completely new TB chemotherapy with innovative molecules in combination in order to decrease the risk of emergence of drug resistance (3). Here, benzothiazinones (BTZ) are an extremely potent class of novel antimycobacterials that act by blocking the synthesis of decaprenyl-phospho-arabinose, the precursor of the arabinans in the mycobacterial cell wall (5). BTZ043, the current lead compound of this class, displays similar activity against all clinical isolates of M. tuberculosis yet tested, including MDR and XDR strains (8). The nanomolar activity and the strong bactericidal effect of BTZ043 make it a promising drug candidate against TB. However, BTZ, like other mycobacterial cell wall inhibitors, appear to be poorly active on nonreplicating bacteria and require association with molecules that target this reservoir of latent bacilli (11). Further experiments are urgently required in preclinical trials with BTZ043 to establish the best BTZ-containing combination against M. tuberculosis where no antibacterial compounds adversely interact with each other.
In this study, we investigated the in vitro efficacy of BTZ043 in combination with current TB drugs or drug candidates against log-phase mycobacteria in order to elucidate potential synergistic, antagonistic, or additive interactions.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
M. tuberculosis H37Rv and an isogenic BTZ-resistant strain (H37Rv-BTZ-R) that carries a point mutation in dprE1 (C387S) were routinely grown at 37°C in 7H9 broth (Difco) supplemented with Middlebrook albumin-dextrose-catalase (ADC) enrichment, 0.2% glycerol, and 0.05% Tween 80 or on solid Middlebrook 7H10 medium (Difco) supplemented with 0.5% glycerol and Middlebrook oleic acid-albumin-dextrose-catalase (OADC).
Drugs and chemicals.
Compounds tested in drug susceptibility assays were pharmaceutical standard (moxifloxacin [MXF] [Avelox] tablets and meropenem [MER] powder) or came from Sigma-Aldrich (INH, RIF, streptomycin, clavulanate, ethambutol). The beta-lactam antibiotic meropenem was used alone or in combination with a beta-lactamase inhibitor (clavulanate [10 μg/ml]). Experimental drugs were provided by K. Andries (TMC207), H. Boshoff and C. E. Barry (SQ-109, PA-824), and V. Makarov (BTZ043).
MICs.
Determination of compound MIC using the resazurin reduction microplate assay (REMA) was performed as previously described (7). Briefly, bacterial stocks of M. tuberculosis H37Rv were generated from mid-log cultures and frozen at −80°C to standardize the inoculum. Two-fold serial dilutions of each test compound (starting from 1× the MIC) were prepared in 24- or 48-well plates. Frozen aliquots of tubercle bacilli were thawed and diluted to an optical density at 600 nm (OD600) of 0.0025 and added to the plates to obtain a total volume of 500 (24-well plate) or 300 (48-well plate) μl. Plates were incubated for 6 days at 37°C before the addition of resazurin (0.025% [wt/vol] to 1/10 of well volume). After overnight incubation, fluorescence of the resazurin metabolite resorufin was determined (by excitation at 560 nm and emission at 590 nm, measured by using a TECAN infinite M200 microplate reader). The MIC was defined visually as the last concentration preventing resazurin turnover from blue to pink and confirmed by the level of fluorescence measured by the microplate reader.
Determination of compound interactions using a REMA checkerboard assay.
In order to assess if compound combinations act synergistically, antagonistically, or additively, a checkerboard assay was initially employed using REMA as a viability marker (10). Fractional inhibitory concentrations (FICs) were calculated by use of the following formula: FIC (X + Y) = [MIC of compound X in combination with Y]/[MIC of X alone]. The fractional inhibitory index (ΣFIC) was calculated as FIC of compound X + FIC of compound Y to evaluate interaction profiles. ΣFICs of ≤0.5 designate synergistic activity, ΣFICs of ≥4.0 indicate antagonism, and values in between correspond to additivity, as outlined in previous work on antibacterial combination studies (6, 9).
Evaluation of compound interactions using CFU determination.
As described above, bacteria were incubated in the presence of combinations of compounds at their respective MICs (concentrations used in the checkerboard assays) or fractions thereof. As an alternative readout for bacterial viability, bacteria were plated on solid medium (supplemented 7H10) and CFU counts were determined after 3 weeks of incubation at 37°C.
RESULTS
Compound interactions assessed by checkerboard assay.
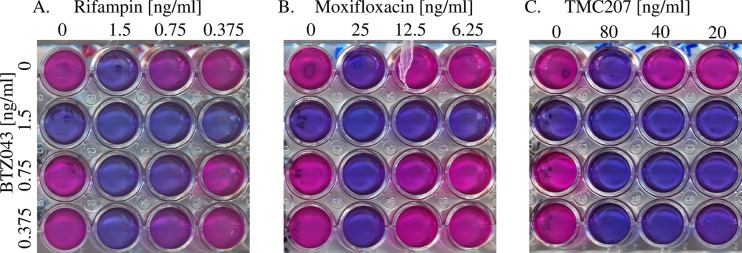
To evaluate the impact of compound combinations on H37Rv, first the MIC of each individual compound was determined by REMA and found to be consistent with previously reported MIC values (Table 1). Second, using the checkerboard assay (as illustrated in Fig. 1), compound interactions were evaluated by growing H37Rv in sub-MIC fractions of BTZ043 in combination with sub-MIC fractions of various front-line and experimental anti-TB compounds. The ΣFIC for each combination was calculated and is summarized in Table 1. Checkerboard experiments were performed twice, and results were consistent between duplicates. The data indicate that in combination with the majority of the compounds, BTZ043 acts additively, meaning that each compound exerts its normal activity without affecting the other (ΣFIC between 0.5 and 4). Interestingly, however, the combination of BTZ043 and TMC207 gave a ΣFIC of 0.5, indicating that the drugs act synergistically against M. tuberculosis H37Rv (consistent between biological triplicates). This finding is clearly seen in Fig. 1, where it is evident that one-quarter the MIC of BTZ043 added to one-quarter the MIC of TMC207 prevents resazurin turnover (Fig. 1C). No antagonistic interaction was found between BTZ043 and the compounds tested.
Table 1.
MICs of selected antituberculosis compounds against M. tuberculosis H37Rv and corresponding interaction profiles with BTZ043 assessed by REMA checkerboard
| Compound | MIC (μg/ml) by REMA | Interaction profile with BTZ043 |
|
|---|---|---|---|
| ΣFIC | Outcome | ||
| BTZ043 | 0.0015 | ||
| Rifampin | 0.0015 | 1.0 | Additive |
| Isoniazid | 0.2 | 1.0 | Additive |
| Ethambutol | 0.6 | 1.0 | Additive |
| TMC207 | 0.08 | 0.5 | Synergistic |
| PA-824 | 0.15 | 1.0 | Additive |
| Moxifloxacin | 0.025 | 1.0 | Additive |
| Meropenem | 2 | 0.75 | Additive |
| Meropenem plus clavulanate | 0.5 | 0.75 | Additive |
| SQ-109 | 0.175 | 1.0 | Additive |
Fig 1.
Representative results from the resazurin checkerboard assay. Depicted are the wells containing H37Rv growing in MIC, one-half the MIC, and one-quarter the MIC of BTZ043 (vertical) in combination with MIC, one-half the MIC, and one-quarter the MIC of rifampin (A), moxifloxacin (B), and TMC207 (C). Viability is determined by resazurin (blue), which is converted to pink (resorufin) by viable bacteria. Images clearly show that combinations of one-quarter the MIC of BTZ043 with one-quarter the MIC of rifampin or moxifloxaxin allows bacterial growth, while no resazurin turnover is seen with one-quarter the MIC of BTZ043 and one-quarter the MIC of TMC207.
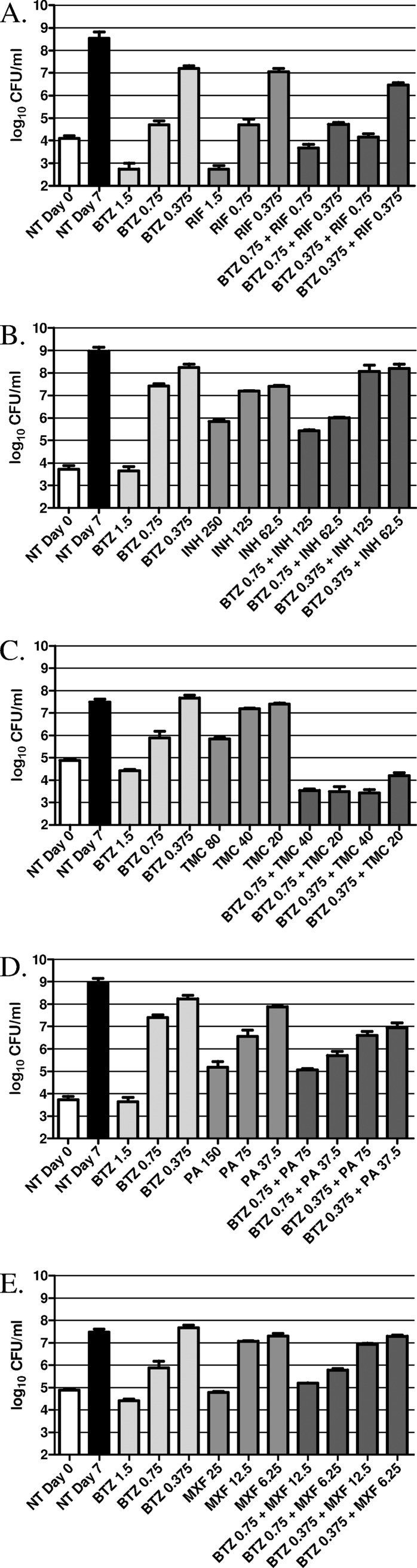
Compound interactions assessed by CFU counts.
To confirm the results found using the checkerboard assay, we also analyzed compound interactions using the number of CFU as a viability readout (Fig. 2). In agreement with the findings of the checkerboard assay, CFU determination confirmed that BTZ in combination with RIF (Fig. 2A), INH (Fig. 2B), PA-824 (Fig. 2D), and MXF (Fig. 2E) acts additively, with the combinations giving only slightly better inhibition of bacterial viability than the compounds alone. The combinations of BTZ043 with TMC207 (Fig. 2C), however, confirm the clear synergy between these compounds. The data show that the combination of 0.375 ng/ml of BTZ043 (one-quarter the MIC) and 20 ng/ml TMC207 (one-quarter the MIC), each of which has no impact on bacterial growth alone, shows clear bactericidal activity (0.5 log decrease compared to activity on day 0). Correspondingly, the other sub-MIC combinations of BTZ043 and TMC207 showed similar synergy.
Fig 2.
Evaluation of compound combinations on bacterial viability as determined by CFU counts. Here H37Rv was grown in the presence of different concentrations (ng/ml) of BTZ043 alone or in combination with decreasing concentrations (ng/ml) of RIF (A), INH (B), TMC207 (TMC) (C), PA-824 (PA) (D), and MXF (E). Following 7 days of culture, bacteria were plated to determine CFU counts. The nontreated bacteria (NT) were also plated on day 0 and on day 7.
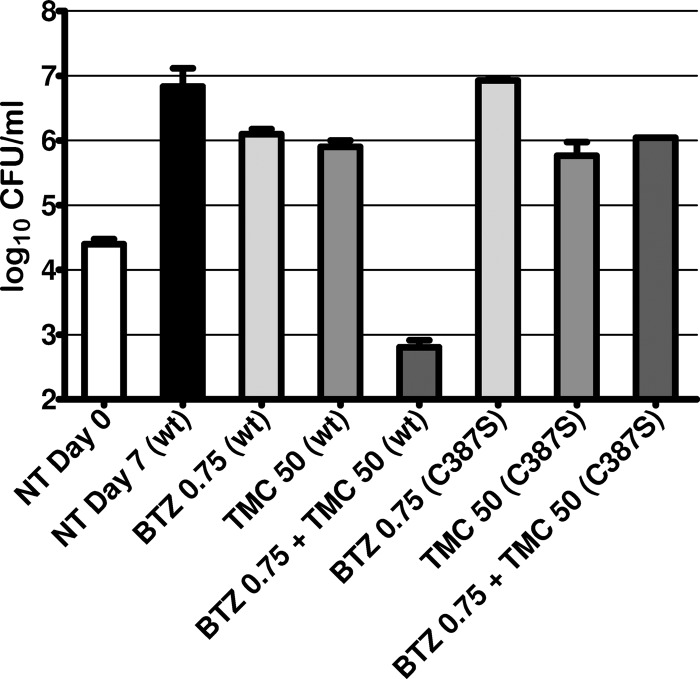
The mechanism of synergy observed between BTZ043 and TMC207 could be due to on- or off-target effects. To assess if the synergy was caused by the activity of BTZ043 on its target, DprE1, the synergistic compound interaction was reanalyzed by performing the CFU assay with an H37Rv mutant (dprE1_C387S) that is fully resistant to BTZ043 (Fig. 3). The data clearly show that under these conditions there is no longer any synergy between BTZ043 and TMC207, suggesting that inhibition of DprE1 is essential for the interaction.
Fig 3.
Impact of DprE1 inhibition on the synergy between BTZ043 and TMC207. To determine if synergy between BTZ043 and TMC207 was due to the inhibition of DprE1 by BTZ043 or to an off-target effect, the activities of combinations of BTZ043 and TMC207 were tested on wild-type H37Rv (wt) and on BTZ043-resistant H37Rv which carried a point mutation in DprE1 (C387S). Compound activity was determined by CFU counting. Values represent compound concentrations in ng/ml.
DISCUSSION
TB chemotherapy requires treatment with a multidrug combination for at least 6 months; it is therefore mandatory to study the in vitro activities of the new drug candidates when combined with other common or experimental TB drugs in preclinical studies. Some in vivo studies have already questioned whether the standard TB regimen displays the optimal interaction. Pharmacokinetic antagonism occurs in mice in the initial phase of TB chemotherapy between INH and RIF-PZA (4). A coherent methodology for the development of a new TB regimen is to assess the interactions of a drug candidate with other drugs in preclinical trials, in order to predict which combinations would have the best impact in the clinic.
In the present study, we set out to determine how BTZ043 interacts with other currently used anti-tuberculosis compounds in vitro. This report clearly shows that at least at the level of the bacterium itself, no antagonism was observed between BTZ043 and the tested compounds and that most of these interactions were purely additive. Interestingly, data using two different approaches clearly indicate that BTZ043 acts synergistically with TMC207. TMC207 at one-quarter the MIC (20 ng/ml) used in combination with BTZ043 (1/4 MIC 0.375 ng/ml) had a stronger bactericidal effect on M. tuberculosis than TMC207 at a concentration of 80 ng/ml. Interestingly, this dramatic synergy is not observed when the compound combination is tested on a BTZ043-resistant M. tuberculosis mutant. Not only does this finding exclude the possibility of synergy occurring through an off-target mechanism, but it also illustrates that BTZ043 must inhibit DprE1 to improve the activity of TMC207, thereby allowing synergy to occur. As DprE1 is involved in the synthesis of arabinan, an integral component of the bacterial cell wall, we hypothesize that sub-MICs of BTZ043 weaken the bacterial cell wall and allow improved penetration of TMC207 to its target, ATP synthase (1). In vitro synergy has also been reported recently between TMC207 and the ethylenediamine SQ-109, though the mechanism of this interaction was not elucidated (10). Taken in combination with the data presented here, and the very recent finding that SQ-109 affects translocation of mycolic acids to the cell wall by inhibiting MmpL3 (13), we suggest that the nature of this synergy is also likely to be due to weakening of the cell wall that improves TMC207 penetration.
Our in vitro results now justify studying BTZ043 in combination with TMC207 in animal models of TB in order to establish whether synergy also occurs in vivo. The discovery of such synergy would be most encouraging for the development of a new TB regimen for use in humans.
ACKNOWLEDGMENTS
We thank K. Andries, H. Boshoff, C.E. Barry, and V. Makarov for providing drugs.
Benoit Lechartier is the recipient of a grant from the Fondation Jacqueline Beytout. The research leading to these results has received funding from the European Community's Seventh Framework Programme ([FP7/2007-2013]) under grant agreement no. 260872.
Footnotes
Published ahead of print 27 August 2012
REFERENCES
- 1. Andries K, et al. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227 [DOI] [PubMed] [Google Scholar]
- 2. Cole ST, Riccardi G. 2011. New tuberculosis drugs on the horizon. Curr. Opin. Microbiol. 14:570–576 [DOI] [PubMed] [Google Scholar]
- 3. Ginsberg A. 2011. The TB Alliance: overcoming challenges to chart the future course of TB drug development. Future Med. Chem. 3:1247–1252 [DOI] [PubMed] [Google Scholar]
- 4. Grosset J, Truffot-Pernot C, Lacroix C, Ji B. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob. Agents Chemother. 36:548–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Makarov V, et al. 2009. Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. Science 324:801–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 7. Palomino JC, et al. 2002. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 46:2720–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pasca MR, et al. 2010. Clinical isolates of Mycobacterium tuberculosis in four European hospitals are uniformly susceptible to benzothiazinones. Antimicrob. Agents Chemother. 54:1616–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rand KH, Houck HJ, Brown P, Bennett D. 1993. Reproducibility of the microdilution checkerboard method for antibiotic synergy. Antimicrob. Agents Chemother. 37:613–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reddy VM, Einck L, Andries K, Nacy CA. 2010. In vitro interactions between new antitubercular drug candidates SQ109 and TMC207. Antimicrob. Agents Chemother. 54:2840–2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sala C, et al. 2010. Simple model for testing drugs against nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 54:4150–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sala C, Hartkoorn RC. 2011. Tuberculosis drugs: new candidates and how to find more. Future Microbiol. 6:617–633 [DOI] [PubMed] [Google Scholar]
- 13. Tahlan K, et al. 2012. SQ109 targets MmpL3, a membrane transporter of trehalose monomycolate involved in mycolic acid donation to the cell wall core of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 56:1797–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. WHO 2011. Global Tuberculosis Control 2011. World Health Organization, Geneva, Switzerland [Google Scholar]