Abstract
Since cations have been reported as essential regulators of biofilm, we investigated the potential of the broad-spectrum antimicrobial and cation-chelator nitroxoline as an antibiofilm agent. Biofilm mass synthesis was reduced by up to 80% at sub-MIC nitroxoline concentrations in Pseudomonas aeruginosa, and structures formed were reticulate rather than compact. In preformed biofilms, viable cell counts were reduced by 4 logs at therapeutic concentrations. Complexation of iron and zinc was demonstrated to underlie nitroxoline's potent antibiofilm activity.
TEXT
Biofilm formation on epithelia and foreign devices poses a severe clinical challenge, as bacteria embedded in these structures are protected from antimicrobial therapy and host immune responses (6, 8, 13). Maintenance of these complex bacterial communities is a dynamic process that has previously been shown to be regulated by various divalent cations, including Fe2+, Zn2+, Mg2+, and Ca2+ (3, 5, 21, 22). Currently, attempts are under way to identify therapeutically applicable compounds that would show strong chelating properties without concomitant immunogenicity or toxicity (1, 18, 19). The antimicrobial effects of the urinary antibiotic nitroxoline have long been known to depend on its ability to chelate bivalent and trivalent cations (9, 20). We thus examined the influence of nitroxoline on biofilm formation by the well-established laboratory model strain PAO1 and by three clinical P. aeruginosa aeruginosa isolates from different sources (see Table 1 for a description of strains).
Table 1.
Strain descriptions and MICsa
| Strain | Source | MIC (μg/ml) |
|---|---|---|
| P. aeruginosa PA01 | Laboratory strain | 64 |
| P. aeruginosa UR5586-10 | UTI | 16 |
| P. aeruginosa BK6695-10 | Blood culture | 32 |
| P. aeruginosa VA9057-10 | Wound infection | 32 |
| Klebsiella pneumoniae DSMZ 30104 | Laboratory strain | 4 |
| Escherichia coli ATCC 25922 | Laboratory strain | 2 |
| Proteus mirabilis DSMZ 4479 | Laboratory strain | 8 |
Strain descriptions and MICs determined according to CLSI recommendations (4). MICs for Enterobacteriaceae strains are shown for comparison. The relatively high MICs of P. aeruginosa make it an ideal candidate to discriminate specific effects on biofilm from simple growth inhibition.
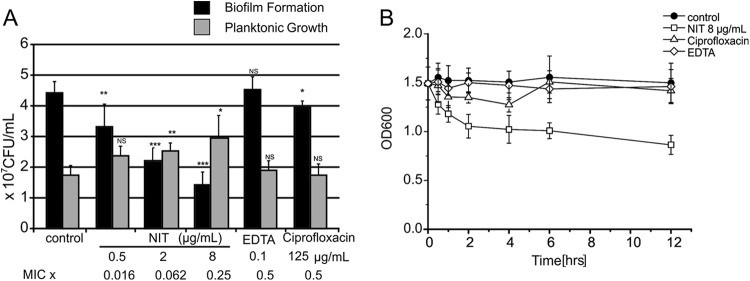
As shown in Fig. 1A, exposure to sub-MICs of nitroxoline inhibited de novo biofilm mass synthesis on polystyrene tubes by up to 80% in a concentration-dependent manner. Planktonic growth inhibition was excluded by measurement of turbidity in the supernatant (Fig. 1B). In one strain with a particularly favorable ratio of sessile versus planktonic growth (BK6695-10), even a significant increase of cell density in the supernatant was noted. This effect was also seen when preformed biofilms of BK6695-10 were exposed to subinhibitory concentrations (Fig. 2A) and is reminiscent of the previously described rapid dispersion of P. aeruginosa PAO1 from preformed biofilms induced by EDTA in flowthrough systems (1). It thus appears that nitroxoline might induce a shift of bacteria from biofilm to the planktonic compartment. In comparison, treatment with one-half of the MIC of EDTA had no effect and ciprofloxacin had only a mild effect on preformed biofilms of BK6695-10. Longer incubation periods for up to 12 h revealed the steepest decline in biofilm to occur between 30 min and 2 h, with an altogether decrease in biofilm mass of about 40% (compare Fig. 2B).
Fig 1.
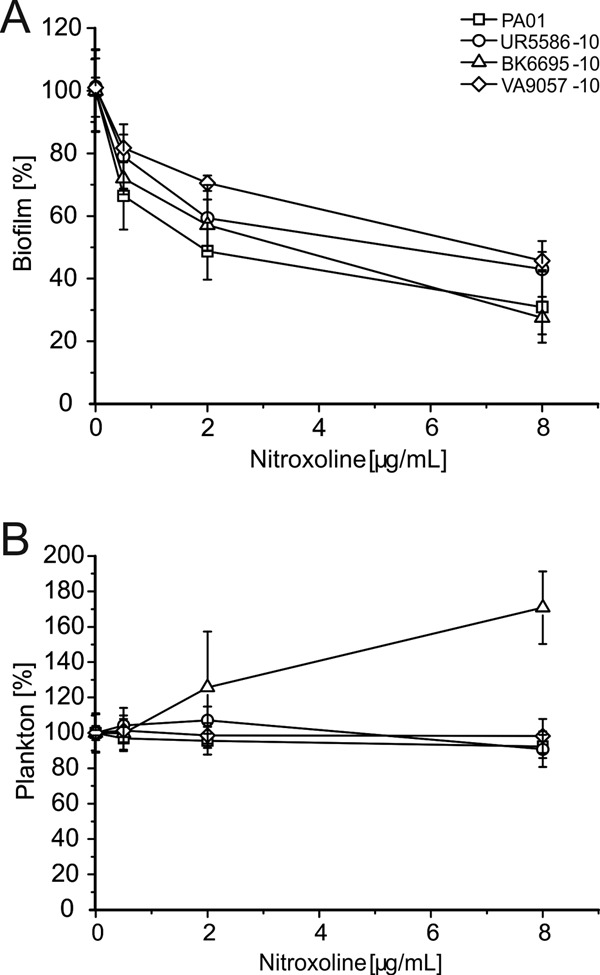
Dosage-dependent effects of sub-MICs of nitroxoline on biofilm mass (A) and planktonic cell density (B) in various P. aeruginosa isolates. Bacteria were grown for 18 h in tryptic soy broth (TSB) medium with or without indicated nitroxoline concentrations. (A) Biofilm formed was stained with crystal violet, measured at 600 nm, and depicted as a percentage of control in medium alone. (B) Density of the planktonic population was determined in parallel by turbidity measurement of the supernatant at 620 nm. Data shown represent respective means ± standard deviations (SD) of three independent experiments, with three replicate tubes per experiment.
Fig 2.
Dispersal of mature biofilms by sub-MICs of nitroxoline (NIT). (A) Dosage dependence. Biofilms of strain BK6695-10 were grown in polystyrol tubes (1-ml bathing volume exposed to approximately 5-cm2 tube surface, CELLSTAR polystyrene tubes; Greiner Bio-One GmbH, Kremsmünster, Austria) washed and subsequently exposed to the indicated agents or phosphate-buffered saline (PBS) alone (control) for 1.5 h. Viable cell numbers in the supernatant and, after dislodgment by sonication (17), in the biofilm layer were determined by serial dilution and plating. Data represent means ± SD of five independent experiments, and significant differences to controls are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001). (B) Kinetics of dispersal. Biofilms were exposed over a period of 12 h and subsequently stained with crystal violet. Results are representative of three independent experiments, with three replicate tubes per experiment.
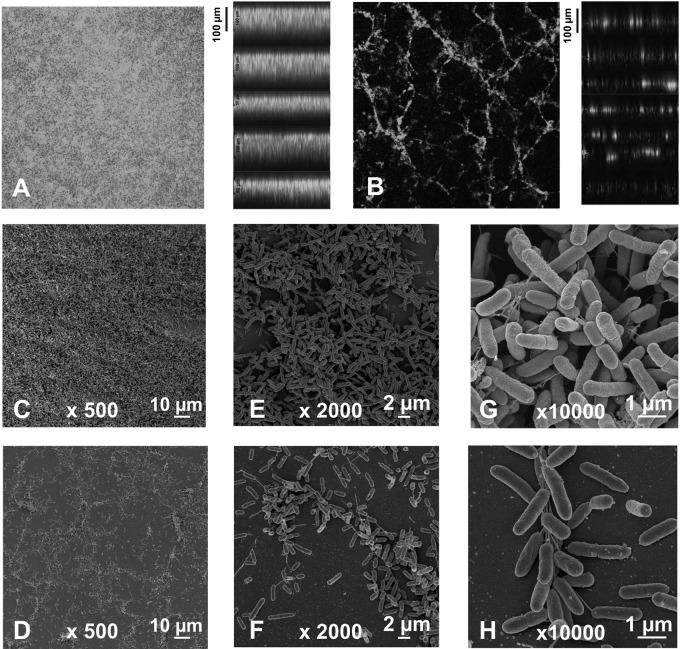
To visualize the morphological changes that nitroxoline might induce in biofilm structure, acridine orange-stained biofilms were examined by confocal laser scanning microscopy (CLSM) or glutaraldehyde fixed layers were studied by scanning electron microscopy (SEM). Whereas control biofilms appeared as confluent layers of densely packed cell clusters (Fig. 3A, C, E, and G), the presence of subinhibitory amounts of nitroxoline resulted in the formation of reticulate structures with distinctively reduced surface coverage and significantly lower thickness (about half of control biofilms) (Fig. 3B, D, F, and H). At sub-MICs, nitroxoline (8 μg/ml or 40 μM) had no significant effect on surface coverage of already established biofilms, but the thickness of nitroxoline-treated layers was reduced by approximately 40% as determined by the measurement of vertical CLSM optical sections (Fig. 4A to D). Interestingly, the basal half of the biofilm layer appeared to remain unaffected by nitroxoline. Similar results were previously reported by O'May et al., who demonstrated a substantial mass but not a surface reduction by exposure of mature PAO1 biofilms to high dosages of the iron chelator 2,29-dipyridyl (about a two-third decrease in thickness at 2,500 μM) (19).
Fig 3.
Effect of sub-MIC nitroxoline on biofilm morphology. Biofilms were grown under static conditions on tissue culture flasks for 18 h in the absence (A, C, E, and G) or presence of 8 μg/ml nitroxoline (B, D, F, and H). (A and B) CLSM images at 100-fold original magnification of acridine orange-stained biofilm. Horizontal optical sections from the midpoint of the biofilms are shown on the left and vertical optical sections on the right. SEM images at 500-fold (C and D), 2,000-fold (E and F) and 10,000-fold (G and H) original magnifications.
Fig 4.
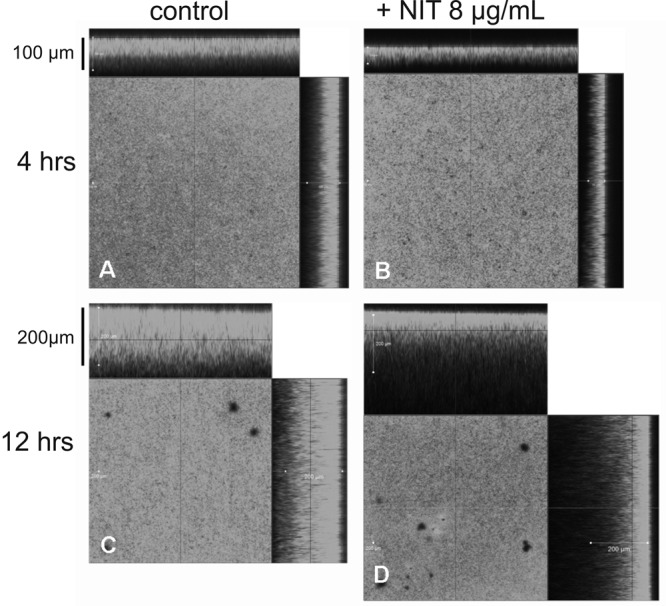
Effect of sub-MIC nitroxoline on preexisting biofilms. CLSM images of acridine-stained biofilm that was treated with 8 μg/ml nitroxoline for 4 h (B) and 12 h (D) in comparison to PBS with 2% TSB alone (A and C).
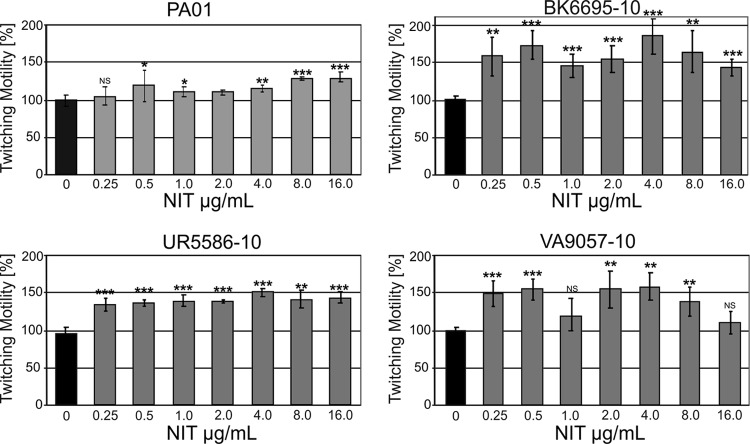
Biofilm formation in P. aeruginosa is a well-studied, complex process that proceeds in defined steps tightly regulated by quorum sensing (for a recent review, see reference 8). Independent of the system used to generate the biofilm, it always consists of two clearly distinguishable subpopulations: a basal layer (in flowthrough systems the “stalks” of the mushroom-like structures) which forms from sessile cells growing via microcolonies into a confluent coating, and a luminal layer (in flowthrough systems constituting the “caps”) which is recruited from the planktonic phase upon attachment of mobile cells to accumulated extracellular DNA (eDNA). It was previously shown that iron chelation by lactoferrin prevents microcolony formation due to an enhancement of type IV pilus-mediated twitching motility in surface-attached cells (11, 21). As binding of the luminal layer is also mediated by type IV pili, and as type IV pilus expression is generally regulated by iron (12, 14–16, 19, 23), it is intriguing to speculate that iron chelation by nitroxoline could interfere with the function of this system. We thus examined twitching motility by employing the stab inoculation method (7). Inclusion of nitroxoline in the LB agar layer (0.25 to 16 μg/ml) enhanced twitching motility in all strains examined (Fig. 5). Interestingly, no simple dose dependence was observed, but rather the response tended to be biphasic.
Fig 5.
Effect of nitroxoline on twitching motility. Strains were stab inoculated through a thin agar layer containing the indicated nitroxoline concentration onto plastic petri dishes, and the hazy zone forming at the interface between agar and polystyrene surface was measured. Results are presented as the mean percentage change ± SD in twitching zone diameter compared to LB agar alone (control, twitching zone taken as 100% ± SD).
To further establish the role of iron chelation in the antibiofilm activity of nitroxoline, M9 minimal medium (with 1% wt/vol KNO3 and 0. 4% vol/vol glycerol), supplemented with defined concentrations of the respective cations [2 mM MgSO4, 0.1 mM CaCl2, 10 μM ZnCl2, 20 μM (NH4)2Fe(SO4)], was incubated with or without 200 μg/ml nitroxoline. After complex formation, nitroxoline and its chelates were removed by chloroform extraction. As shown in Fig. 6, biofilm mass as related to planktonic growth was decreased by about 70% when P. aeruginosa PAO1 was grown in nitroxoline-pretreated medium compared to the chloroform-extracted control medium. Biofilm formation was reconstituted by resupplementation with iron and zinc, whereas further addition of magnesium and calcium was ineffective. This proves that the antibiofilm activity of nitroxoline is indirect, not requiring the presence of the compound in the medium, and is indeed mediated by the removal of free iron and, to a lesser degree, zinc.
Fig 6.
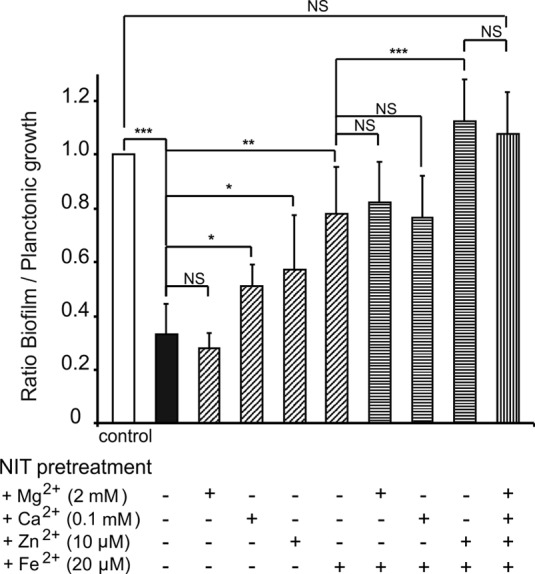
Relative growth of P. aeruginosa PAO1 biofilms in dependence of defined cation concentrations. M9 medium fully supplemented with the respective cations at the concentrations indicated was incubated overnight with or without 200 μg/ml nitroxoline. Pretreated and control media were subsequently chloroform extracted (6 by 50 ml) to remove nitroxoline and its chelates. Biofilm growth of PAO1 in control medium and in nitroxoline-pretreated medium and in pretreated medium resupplemented with the indicated cations was examined. Biofilm growth as determined by crystal violet staining was related to planktonic growth (turbidity measurement at 620 nm), with the growth ratio in the control medium taken as 1. Data represent means ± SD of three independent experiments, and significant differences to controls are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
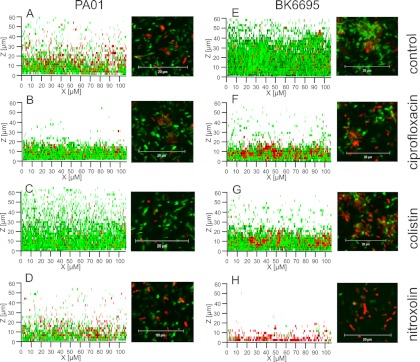
To confirm the possible clinical relevance of the observed effects, preformed biofilms were exposed to concentrations actually maintained in urine during oral treatment regimens (200 μg/ml of nitroxoline or ciprofloxacin) (2, 10). Nitroxoline treatment resulted in a reduction of viable cells by 4 logs after 6 h (in comparison to 2 logs by ciprofloxacin and about 1 log by colistin) (Fig. 7). The results were similar for PAO1 and BK6695-10 and confirmed by live and dead staining in CLSM (Fig. 8).
Fig 7.
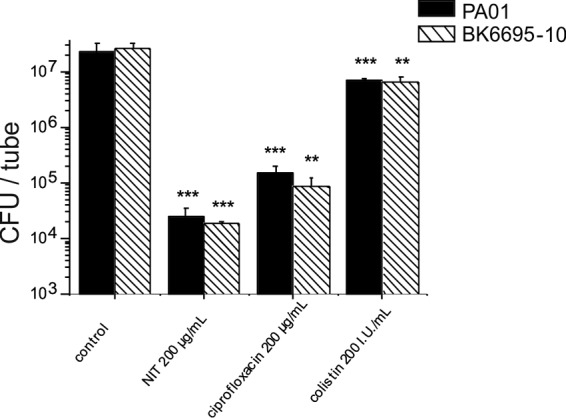
Effect of urinary concentrations of nitroxoline on the survival of biofilm cells. Biofilms were grown anaerobically in 1% KNO3 supplemented TSB (PAO1) as previously described (19) or aerobically in TSB (BK6695-10) for 20 h, washed, and subsequently exposed to the indicated agents or PBS with 1% TSB alone (control) for 6 h. Viable cell numbers in the biofilm layer were determined by serial dilution and plating after dislodgment by sonication (17). Data represent means ± SD of three independent experiments, and significant differences to controls are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Colonized tube surface corresponding to approximately 15 cm2.
Fig 8.
Effect of physiological concentrations of nitroxoline, colistin, and ciprofloxacin on preexisting biofilm. Biofilms of strains PAO1 (A to D) and BK6695-10 (E to H) were grown in x-well tissue culture coverglass chambers (Sarstedt) for 18 h, treated with the compounds indicated for 6 h, and dyed by LIVE/DEAD BacLight bacterial viability kit (Invitrogen). CLSM was performed as z-stacks of 70 μm, with 28 layers each. Images are shown as superposition in the x-z plane (left) and as sections of approximately 30 m2 (basal layers; 6 μm above the glass surface) (right). Living cells are colored green (SYTO 9), and dead cells are colored red (propidium iodide).
In summary, nitroxoline, which has been in clinical use in Europe for half a century, was found to be a very effective agent against P. aeruginosa biofilms. Its activity is comparable to that reported for EDTA (1, 19), but it is orally applicable, less toxic, and the antibiofilm effect was obtained at concentrations that are in the range achieved in vivo during oral nitroxoline treatment (plasma peak levels of approximately 6 mg/liter and persistent urine levels around 300 mg/liter) (2). In contrast to lactoferrin and the related egg white-derived conalbumin, it is a synthetic, relatively cheap compound, without additional immunomodulating functions and far lesser allergenic potential. Moreover, its activity was found superior to that reported for pure iron chelators like 2,29-dipyridyl, deferoxamine mesylate, or diethylenetriaminepentaacetic acid (19). Therefore, nitroxoline constitutes a very promising candidate for therapeutic application in the context of biofilm-related infections.
ACKNOWLEDGMENTS
We thank Guido V. Bloemberg for critical revision and discussion of the manuscript. We are also grateful to Claudia Ranke for technical assistance with sample preparation for scanning electron microscopy.
This work was supported by a grant from the Paul Ehrlich foundation and by the Federal Ministry of Education and Research (BMBF), Germany (grants FKZ 01EO1002 and FKZ 01KI1204).
Footnotes
Published ahead of print 27 August 2012
REFERENCES
- 1. Banin E, Brady KM, Greenberg EP. 2006. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl. Environ. Microbiol. 72:2064–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergogne-Berezin E, Berthelot G, Muller-Serieys C. 1987. Present status of nitroxoline. Pathol. Biol. 35:873–878 (In French.) [PubMed] [Google Scholar]
- 3. Chen X, Stewart PS. 2002. Role of electrostatic interactions in cohesion of bacterial biofilms. Appl. Microbiol. Biotechnol. 59:718–720 [DOI] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2008. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, eighth edition. Approved standard M7-A8. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Conrady DG, et al. 2008. A zinc-dependent adhesion module is responsible for intercellular adhesion in staphylococcal biofilms. Proc. Natl. Acad. Sci. U. S. A. 105:19456–19461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 7. Darzins A. 1993. The pilG gene product, required for Pseudomonas aeruginosa pilus production and twitching motility, is homologous to the enteric, single-domain response regulator CheY. J. Bacteriol. 175:5934–5944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies D. 2003. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2:114–122 [DOI] [PubMed] [Google Scholar]
- 9. Gale EF. 1949. The assimilation of amino-acids by bacteria: trace metals on glutamic acid assimilation and their inactivation by 8-hydroxyquinoline. J. Gen. Microbiol. 3:369–384 [DOI] [PubMed] [Google Scholar]
- 10. Gilbert DN. 2006. Urinary tract infections in patients with chronic renal insufficiency. Clin. J. Am. Soc. Nephrol. 1:327–331 [DOI] [PubMed] [Google Scholar]
- 11. Glick R, et al. 2010. Increase in rhamnolipid synthesis under iron-limiting conditions influences surface motility and biofilm formation in Pseudomonas aeruginosa. J. Bacteriol. 192:2973–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klausen M, et al. 2003. Biofilm formation by pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 48:1511–1524 [DOI] [PubMed] [Google Scholar]
- 13. Lewis K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liles MR, Viswanathan VK, Cianciotto NP. 1998. Identification and temperature regulation of Legionella pneumophila genes involved in type IV pilus biogenesis and type II protein secretion. Infect. Immun. 66:1776–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luke NR, Howlett AJ, Shao J, Campagnari AA. 2004. Expression of type IV pili by Moraxella catarrhalis is essential for natural competence and is affected by iron limitation. Infect. Immun. 72:6262–6270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matilla MA, et al. 2007. Temperature and pyoverdine-mediated iron acquisition control surface motility of Pseudomonas putida. Environ. Microbiol. 9:1842–1850 [DOI] [PubMed] [Google Scholar]
- 17. Monsen T, Lövgren T, Widerström M, Wallinder L. 2009. In vitro effect of ultrasound on bacteria and suggested protocol for sonication and diagnosis of prosthetic infections. J. Clin. Microbiol. 47:2496–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Musk DJ., Jr 2010. Zinc fingered: new compounds that thwart gram-positive biofilm formation by sequestering zinc. Chembiochem 11:758–760 [DOI] [PubMed] [Google Scholar]
- 19. O'May CY, Sanderson K, Roddam LF, Kirov SM, Reid DW. 2009. Iron-binding compounds impair Pseudomonas aeruginosa biofilm formation, especially under anaerobic conditions. J. Med. Microbiol. 58:765–773 [DOI] [PubMed] [Google Scholar]
- 20. Pelletier C, Prognon P, Bourlioux P. 1995. Roles of divalent cations and pH in mechanism of action of nitroxoline against Escherichia coli strains. Antimicrob. Agents Chemother. 39:707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh PK, Parsek MR, Greenberg EP, Welsh MJ. 2002. A component of innate immunity prevents bacterial biofilm development. Nature 417:552–555 [DOI] [PubMed] [Google Scholar]
- 22. Turakhia MH, Characklis WG. 1989. Activity of Pseudomonas aeruginosa in biofilms: effect of calcium. Biotechnol. Bioeng. 33:406–414 [DOI] [PubMed] [Google Scholar]
- 23. Zaini PA, et al. 2008. The iron stimulon of Xylella fastidiosa includes genes for type IV pilus and colicin V-like bacteriocins. J. Bacteriol. 190:2368–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]