Abstract
The objective of this study was to evaluate the antibacterial activities of joint fluids of patients undergoing total-knee arthroplasty (TKA). Thirty patients who were scheduled for primary cemented TKA were enrolled in the study. The patients were grouped on the basis of whether the cement was without antibiotic loading (control group) or loaded with oxacillin (oxacillin group) or vancomycin (vancomycin group). Cefazolin was administered to every patient as the perioperative prophylactic antibiotic. Samples of joint fluids were collected from the knee joints at 8, 16, 24, 32, 40, and 48 h after prosthesis implantation. We assessed the bioactivities of the joint fluids against methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant S. aureus (MRSA). The antibiotic contents of the joint fluid samples were further evaluated by using high-performance liquid chromatography. Against MSSA, all joint fluid samples exhibited at least 24 h of bacterial inhibition activity. The oxacillin (43.2 h ± 2 h) and vancomycin (40.8 h ± 1.8 h) groups exhibited significantly longer durations of antibacterial activities than the control group (28 h ± 1.3 h; P < 0.05). However, antibacterial activity against MRSA was observed only in the vancomycin group. In conclusion, cefazolin, which was administered as a prophylactic antibiotic in TKA, exhibited good ability for knee joint penetration and was sufficient to inhibit MSSA during its administration. The use of antibiotic-loaded cement can prolong the antibacterial activity of joint fluid in TKA. Further, vancomycin-loaded cement had antibacterial activity against MRSA superior to that of cement loaded with oxacillin or without antibiotic loading.
INTRODUCTION
Deep infection is one of the most devastating complications of total-joint arthroplasty. Systemic prophylactic antibiotics have been widely accepted as effective agents for reducing the rate of deep infection (22). Cefazolin, a first-generation cephalosporin, is commonly used for prophylaxis during joint replacement because of its broad-spectrum activity, favorable pharmacokinetics, good safety profile, and good ability for bone penetration. However, despite its use, a 1 to 2% prevalence of deep infection was noted in most of the large case series reporting on total-joint arthroplasty (12, 18).
The use of antibiotic-loaded polymethylmethacrylate (PMMA) bone cement has been shown to be effective in the reduction of the rate of early to intermediate deep infection after total-joint arthroplasty of both the hip (14) and the knee (5). Unlike total-hip arthroplasty, total-knee arthroplasty (TKA) is mostly performed using bone cement. The use of antibiotic-loaded bone cement has been recommended for prophylaxis against infection acquired during TKA in patients with immune-compromising comorbidities (6). However, the in vivo behavior of antibiotic-loaded bone cement in TKA has not been sufficiently investigated.
Staphylococcus aureus is the most common organism implicated in the infection of the skeletal system (3, 8, 11). Oxacillin, a penicillinase-resistant β-lactam similar to methicillin, is widely used clinically to treat penicillin-resistant S. aureus infection (2). Approximately 20% of S. aureus isolates collected in Europe are reported to be methicillin-resistant S. aureus (MRSA); the prevalence ranges from 33% to 55% in U.S. hospitals (1). Vancomycin, a glycopeptide antibiotic, remains the standard therapeutic agent used in most MRSA infections. Both oxacillin (16) and vancomycin (3) have been shown to be stable in PMMA and are released in a microbiologically active form. Therefore, the use of oxacillin- or vancomycin-loaded bone cement may be an effective method to prevent S. aureus infection in TKA.
To our knowledge, no study has been conducted on the bacterial-eradication abilities of joint fluid in TKA fixed with bone cement with or without antibiotic loading. Further, data on the comparison of the in vivo characteristics of bone cement loaded with oxacillin and that loaded with vancomycin are also unavailable. The aim of this study was to measure the antibiotic contents of the joint fluid of patients undergoing cemented TKA using bone cement with or without antibiotic (oxacillin or vancomycin) loading and to compare the antibacterial activities of the fluid samples against methicillin-susceptible S. aureus (MSSA) and MRSA.
MATERIALS AND METHODS
Patients and operations.
The study protocol was approved by the institutional review board, and written informed consent was provided by all participants. Between May 2011 and September 2011, 30 patients diagnosed with end stage knee osteoarthritis and scheduled for primary TKA were asked for their consent to participate in this study. Patients were excluded from the study if they had diabetes mellitus, peripheral arterial occlusive disease, psoriasis, prior knee surgery, any type of lower-extremity infection, osteomyelitis, or malignant tumor; had received immunosuppressive agents; or had a history of cephalosporin, oxacillin, or vancomycin allergy. The study population included 21 women (21 knees) and 9 men (9 knees), with a mean age of 68.7 ± 9.2 years (range, 52 to 84 years). Patients were randomly grouped on the basis of whether PMMA was without antibiotic loading (control group; systemic cefazolin) or loaded with oxacillin (oxacillin group; systemic cefazolin plus oxacillin-loaded PMMA) or vancomycin (vancomycin group; systemic cefazolin plus vancomycin-loaded PMMA). In all cases, arthroplasty was performed with a midline incision and through a midvastus approach. A NexGen LPS-Flex fixed-bearing knee system (Zimmer Inc., Warsaw, IN) or a P.F.C. Sigma rotating-platform knee system (DePuy Orthopedics, Inc., Warsaw, IN) was used according to the surgeon's usual practice. All components were fixed with cement (Simplex P; Howmedica, Rutherford, NJ). All the patients were administered 1 g cefazolin (Chi Sheng, Taipei, Taiwan) intravenously 30 min before the operation and every 8 h after the operation for 24 h as the prophylactic antibiotic. The demographic characteristics of the enrolled patients are summarized in Table 1. The 3 groups did not show any significant differences with regard to gender, age, body mass index (BMI), amount of drainage, amount of implanted cement, and brand of prosthesis (Table 1).
Table 1.
Characteristics of patients in different groups
| Characteristic | Valuea |
P value | ||
|---|---|---|---|---|
| Control | Oxacillin | Vancomycin | ||
| Age (yr) | 69.6 (55–82) | 73.3 (57–84) | 63.3 (55–73) | 0.052 |
| Sex ratio (male/female) | 3/7 | 5/5 | 1/9 | 0.449 |
| BMI | 28.5 (22.2–36.6) | 28.4 (22.7–32.1) | 27.4 (20.3–5.8) | 0.783 |
| Serum creatinine (mg/dl) | 0.8 (0.5–1.3) | 0.9 (0.5–1.3) | 0.8 (0.4–1.2) | 0.519 |
| eGFRb (ml/min/1.73 m2) | 83.8 (52–126) | 76.8 (41–127) | 84.7 (46–147) | 0.856 |
| Prosthesis (Zimmerc/DePuyd) | 6/4 | 5/5 | 5/5 | 1.000 |
| Amt of drainage (ml) at (h): | ||||
| 8 | 126.5 (90–190) | 215 (50–320) | 237 (90–450) | 0.210 |
| 16 | 101.5 (50–210) | 147 (40–350) | 99 (40–210) | 0.552 |
| 24 | 83.5 (35–160) | 107.5 (35–240) | 95 (60–150) | 0.636 |
| 32 | 78 (20–170) | 93 (140–60) | 77 (50–110) | 0.304 |
| 40 | 39 (10–110) | 74 (25–130) | 64.5 (20–280) | 0.056 |
| 48 | 44 (20–80) | 66 (20–150) | 46 (20–70) | 0.684 |
| Total amt of drainage (ml) | 472.5 (310–660) | 702.5 (345–1010) | 618.5 (350–030) | 0.089 |
| Amt of cement (g) | 19.4 (11–32) | 21.5 (12–32) | 18.7 (12–35) | 0.535 |
Values shown are means, with ranges in parentheses.
eGFR, estimated glomerular filtration rate.
NexGen LPS-Flex fixed-bearing knee system (Zimmer Inc.).
P.F.C. Sigma rotating-platform knee system (DePuy Orthopedics, Inc.).
For the preparation of bone cement, 40 g of methyl-methacrylate polymer was thoroughly mixed with or without 1 g of vancomycin (Gentle Pharmaceutical Co., Yulin, Taiwan) or oxacillin (Union Chemical and Pharmaceutical Co., New Taipei, Taiwan). Then, liquid monomer was added and mixed. The weight of the implantation cement was recorded. After implantation, 10-cm3 aliquots of the drainage were collected under sterile conditions at 8, 16, 24, 32, 40, and 48 h. The drainage container was changed after each collection. Along with the collection of the first drainage sample, 10-ml specimens of peripheral venous blood and midstream urine were also collected. The specimens were subsequently stored at −80°C until further analysis. Preliminary data had shown that the degradation of cefazolin, vancomycin, or oxacillin was less than 1% when it was stored at −80°C for 3 months. Hence, −80°C was considered the optimal temperature for the storage of the specimens.
Determination of antibiotic concentrations.
The concentrations of the antibiotics were determined by high-performance liquid chromatography (HPLC). Chromatography was carried out on a model ALC 717 chromatograph (Waters Associates, Milford, MA) with a stainless steel column (RP18 column [100 mm by 4.6 mm; 5-μm particle size] for vancomycin and oxacillin; C18 column [300 mm by 3.9 mm; 10-μm particle size] for cefazolin). The mobile phase consisted of water-acetonitrile-100 mM ammonium formate (composite ratio, 78/12/10), acetonitrile-0.5% ammonium phosphate (composite ratio, 34/66), and 50% methanol-50% double-distilled H2O (composite ratio, 50/50) for vancomycin, oxacillin, and cefazolin, respectively. The HPLC system had sensitivities of 0.5 μg/ml for vancomycin and 0.1 μg/ml for oxacillin and cefazolin. The concentrations of antibiotics in the drainage samples were then determined by comparison with the peak areas of standard curves prepared daily.
Bioassay of antibiotic activity.
The bioactivity of joint fluid was determined using an agar disk diffusion bioassay with a MSSA strain (ATCC 25923) and a MRSA strain (ATCC 43300). The technique was based on the inhibitory activity of the disks (PDM Diagnostic Discs; AB Biodisk, Sweden) containing 35 μl of test samples and a standardized concentration of cefazolin, oxacillin, or vancomycin. The disks were placed on MSSA- or MRSA-seeded agar and incubated overnight at 37°C. The diameters of the inhibition zones were measured using a caliper (4). All samples were tested in triplicate.
Statistical analysis.
The antimicrobial concentrations of joint fluid samples obtained from different patients at each time point were sampled and tested in triplicate. The results are presented as means and standard deviations. The Kruskal-Wallis test was used to determine the intergroup differences in the continuous variables, including age, BMI, amount of drainage, amount of implanted cement, and concentrations of antibiotics. Fisher's exact test was used to determine the statistical differences of the categorical variables, including gender and type of prosthesis. Kaplan-Meier survival analysis was used to compare the groups for the durations of joint fluid antibacterial activities. The Kruskal-Wallis test was used to compare the statistical differences in the elution efficacies of PMMA loaded with oxacillin or vancomycin. Spearman's rank correlation was used to determine the correlation between the amounts of implanted cements and the amounts of antibiotic released from the bone cements. A P value of <0.05 was considered significant.
RESULTS
Antibiotic concentrations in joint fluid.
Cefazolin, which was administered as the prophylactic antibiotic in this study, was detected in the joint fluid of each enrolled patient, irrespective of whether the bone cement was loaded with an antibiotic. Further, no significant intergroup difference was noted in the cefazolin concentrations in the joint fluid samples at any time of collection (Fig. 1A). All the samples collected within 24 h of prosthesis implantation contained cefazolin (Fig. 1A), and the concentrations were 48.3 ± 19.5 μg/ml, 42 ± 17.5 μg/ml, and 39.2 ± 16 μg/ml for the joint fluid samples obtained at 8 h, 16 h, and 24 h, respectively (Fig. 1A). However, cefazolin was detected in only 53% (16 of 30) and 20% (6 of 30) of the joint fluid samples obtained at 32 h and 40 h after the prosthesis implantation, and the concentrations were 12 ± 16 μg/ml and 1.8 ± 6.3 μg/ml, respectively. Forty-eight hours after the prosthesis implantation, cefazolin could not be detected in the joint fluid of any patient (Fig. 1A). Cefazolin was mainly excreted through the urine, and the urinary concentration of cefazolin was 780 ± 238 μg/ml.
Fig 1.
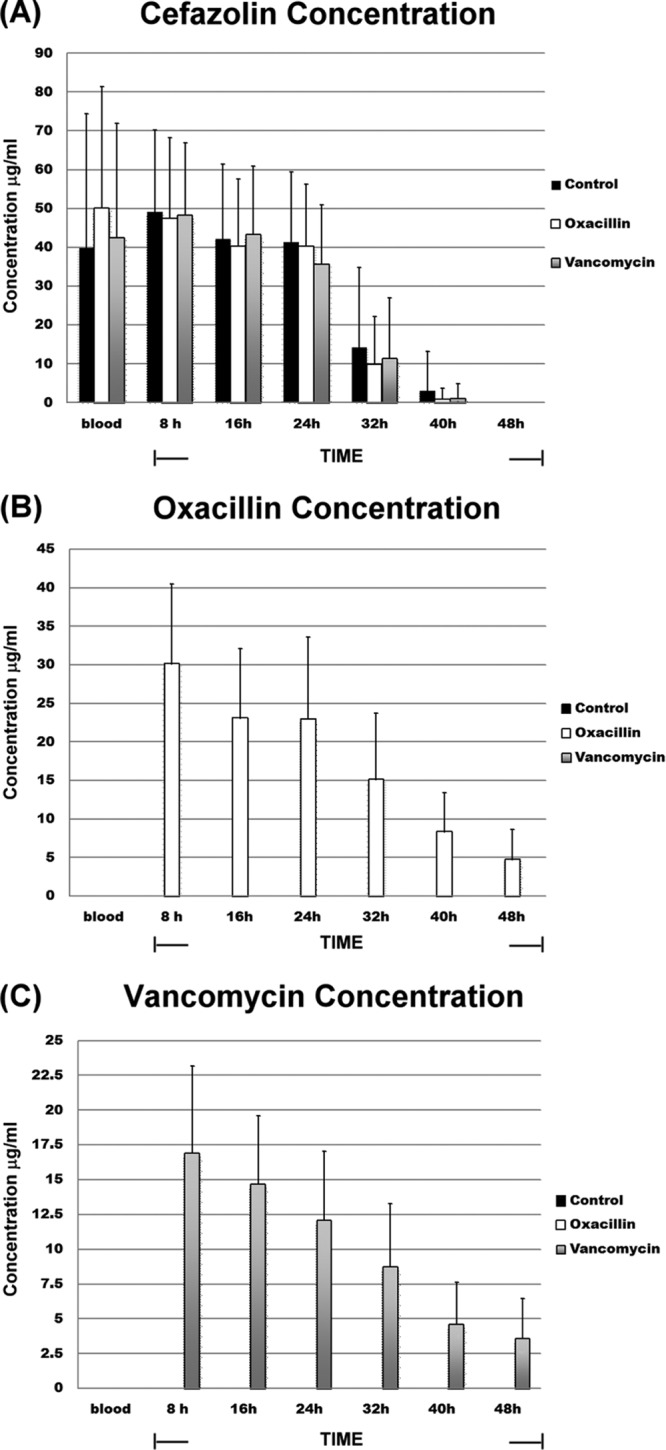
Antibiotic contents in joint fluid samples at each collection time point. Shown are the concentrations of cefazolin (A), oxacillin (B), and vancomycin (C) in the different groups. All the patients were administered 1 g cefazolin intravenously 30 min before the operation and every 8 h after the operation for 24 h as the prophylactic antibiotic. The control group received systemic cefazolin; the oxacillin group received systemic cefazolin plus oxacillin-loaded PMMA; the vancomycin group received systemic cefazolin plus vancomycin-loaded PMMA. The data are expressed as means and standard errors of the mean (SEM) for triplicate specimens assessed for each group.
Oxacillin and vancomycin were detected in the joint fluid samples of the oxacillin group and the vancomycin group, respectively. PMMA powder (40 g) loaded with 1 g of oxacillin or vancomycin released the antibiotic for up to 48 h (Fig. 1B and C). In the oxacillin group, the concentrations of oxacillin were 30.2 ± 10.3 μg/ml, 23.22 ± 8.9 μg/ml, 23 ± 10.6 μg/ml, 15.2 ± 8.5 μg/ml, 8.4 ± 5 μg/ml, and 5.9 ± 3.8 μg/ml for the joint fluid samples collected at 8 h, 16 h, 24 h, 32 h, 40 h, and 48 h, respectively (Fig. 1B). Correspondingly, the concentrations of vancomycin in the vancomycin group were 16.9 ± 6.3 μg/ml, 14.7 ± 4.9 μg/ml, 12.7 ± 5 μg/ml, 10.1 ± 4.5 μg/ml, 8.6 ± 3 μg/ml, and 2.6 ± 2.9 μg/ml, respectively (Fig. 1C). The concentrations of oxacillin and vancomycin in the urine and peripheral venous blood samples were too low to be detected by our HPLC system. During the 48 h of the sample collection period, oxacillin exhibited significantly better release efficacy (total amount of oxacillin release, 14,170 ± 7,138 μg) than vancomycin (total amount of vancomycin release, 7,750 ± 6,621 μg; P < 0.05). Spearman's rank correlation test indicated no statistical correlation between the amount of implanted cement and the release efficacy of antibiotics in either the oxacillin or the vancomycin group (data not shown).
Bioassay of antibiotic activity.
Both the staphylococcal strains, MSSA and MRSA, were found to be susceptible to different concentrations of the test antibiotics (Fig. 2). In the disk diffusion assay, the disk loaded with vancomycin exhibited a significantly smaller inhibitory zone against MSSA than that loaded with the same concentration of cefazolin or oxacillin (Fig. 2A). With regard to MRSA, the inhibitory zone was observed at a concentration as low as 8 μg/ml in the case of vancomycin (Fig. 2B) but could be observed only at concentrations higher than 32 μg/ml and 64 μg/ml of oxacillin and cefazolin, respectively.
Fig 2.
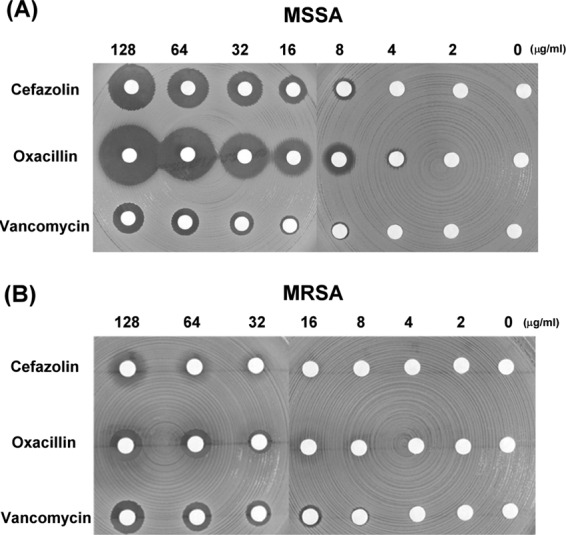
Different concentrations of antibiotics exhibited inhibitory effects against bacteria. The data are presented in terms of the inhibition of the test organisms, namely, MSSA (A) and MRSA (B), as determined by agar disk diffusion bioassay.
The joint fluid samples from all the enrolled patients exhibited antibacterial activity, irrespective of whether the cement was loaded with antibiotics. However, the joint fluid samples exhibited intergroup differences in the durations of antibacterial activities (Fig. 3). The oxacillin group (43.2 h ± 2 h) and the vancomycin group (40.8 h ± 1.8 h) exhibited significantly longer durations of antibacterial activity against MSSA than the control group (28 h ± 1.3 h; P < 0.001) (Fig. 3A). With regard to MRSA, the joint fluid samples from the control and oxacillin groups did not exhibit antibacterial activity, and only those of the vancomycin group showed inhibitory zones on the disk diffusion assay (Fig. 3B). Figure 4 presents the antibacterial activities of the joint fluids against MSSA (Fig. 4A) and MRSA (Fig. 4B) in the disk diffusion assay.
Fig 3.
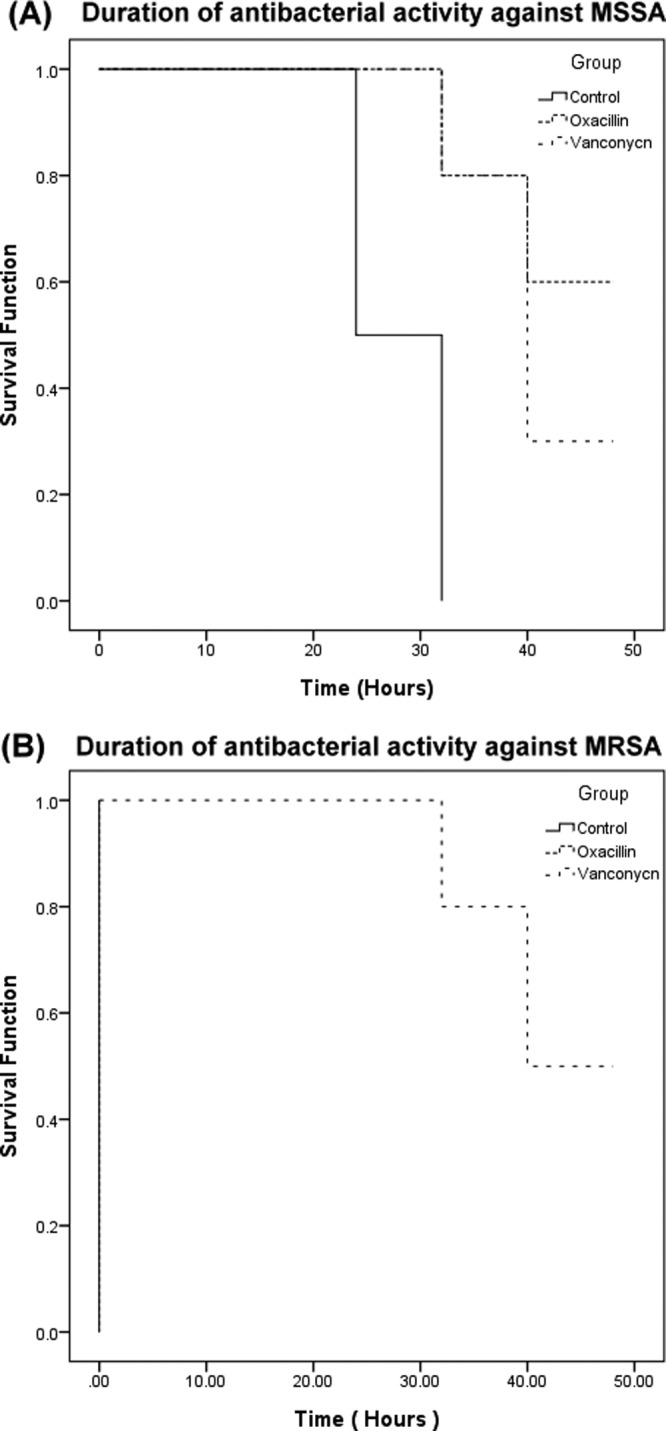
Kaplan-Meier survival analysis of the duration of antibacterial activities of joint fluid samples in different groups. The data are presented as the duration of inhibitory function against the test organisms, MSSA (A) and MRSA (B), as determined by agar disk diffusion bioassay.
Fig 4.
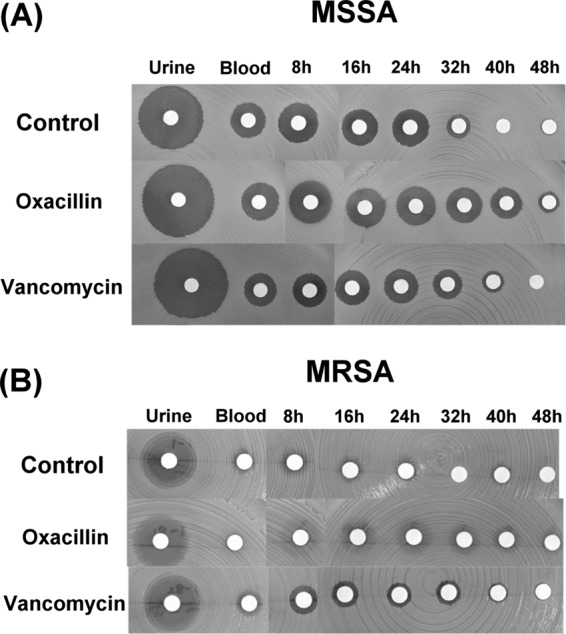
The antibacterial activities of urine, peripheral venous blood, and joint fluid samples obtained at different time points. The data are presented in terms of the inhibition of the test organisms, namely, MSSA (A) and MRSA (B), as determined by the agar disk diffusion bioassay.
DISCUSSION
Antibiotic-loaded bone cements have been widely used in total-joint arthroplasty, particularly for patients at high risk of infection (6, 10, 17, 23). On the basis of 2 prospective randomized trials, Chiu et al. reported that cefuroxime-impregnated bone cement was effective in reducing the rate of deep knee infection after TKA (5), particularly in the case of diabetic patients (6). The antibiotic elution characteristics of antibiotic-loaded bone cements have been widely investigated. However, the majority of these studies have been based on in vitro models (3, 15). In vitro studies of specimens prepared under laboratory conditions do not necessarily address actual clinical circumstances. Such studies do not account for the quantity and flow of body fluids, limb mobility, host response, and antibiotic stability in vivo. The in vivo antibiotic release characteristics of antibiotic-loaded PMMA bone cement in TKA have not yet been investigated.
Since cephalosporins are microbiologically active against most of the common pathogenic organisms, they are currently the commonest antibiotics used for prophylaxis in surgery. Compared to other cephalosporins, such as cephalothin or cephradine, cefazolin has been shown to exhibit a higher peak concentration and a longer duration of antimicrobial activity in bones for the same dose level (7). Because of these superior characteristics, cefazolin is frequently used as the prophylactic antibiotic in orthopedic surgeries, including total-joint arthroplasty (7). In this study, cefazolin, which was administered intravenously as a prophylactic antibiotic in TKA, exhibited good ability for knee joint penetration. When 1 g of cefazolin was used, the concentration of cefazolin in the joint fluid was sufficient to inhibit MSSA during the administration period. However, the antibacterial activity declined dramatically after the discontinuation of cefazolin.
In this study, the concomitant use of antibiotic-loaded bone cement, along with an intravenous prophylactic antibiotic, in TKA was found to prolong the antibacterial activity of joint fluid after the intravenous drug administration. A longer duration of antibacterial activity may contribute to a lower infection rate in total-joint arthroplasty with antibiotic-loaded cement (6, 10, 17, 23). In addition, the administration of prophylactic antibiotics both systemically and in the bone cement has been proven to provide excellent results in the prevention of infection in total-joint arthroplasty (9). Dual-antibiotic prophylaxis has also been shown to reduce the incidence of infection, particularly MRSA infection, in total-joint arthroplasty (21).
The use of antibiotic-loaded bone cement as a prophylactic measure requires the administration of low doses of antibiotics to avoid adverse mechanical effects on the cement used for the fixation of the prosthesis. In general, low-dose loading of antibiotic in the cement is defined as ≤1 g of powdered antibiotic per 40 g of PMMA bone cement (13). However, the details of the in vivo antibiotic elution of bone cement loaded with low doses of antibiotics are still unknown. In this study, bone cement loaded with low-dose oxacillin or vancomycin (1 g antibiotic in 40 g PMMA) released the antibiotic for up to 48 h, thereby retaining its antibacterial activity after the implantation. These results indicate that bone cement loaded with low-dose oxacillin or vancomycin could release the antibiotic effectively in an in vivo environment. Our findings also suggest that oxacillin and vancomycin not only were stable under the high temperatures applied during PMMA polymerization, but also retained their bioactivities after exposure to body temperature (37°C) for up to 48 h. When antibiotics are used in cement, the release efficacy is the critical point determining the antibacterial activities. Our findings revealed that oxacillin-loaded cement provided a significantly better antibiotic release efficacy than vancomycin-loaded cement. This superior release efficacy may have contributed to the longer duration of antibacterial activity against MSSA in the oxacillin group than in the vancomycin group, although the difference was not significant.
The increasing resistance of S. aureus to antibiotics has become a major concern in the treatment of orthopedic infections (4, 9, 12). A multihospital study conducted in England revealed that S. aureus is responsible for 50% of surgical-site infections (19) and that 59% of the S. aureus isolates were methicillin resistant. At our institute, 51% of the clinical isolates of S. aureus obtained in 2011 were MRSA. Thus, it appears that S. aureus is the primary bacterium toward which antibiotic-loaded bone cement should be directed (20). In this study, only the joint fluid samples of the vancomycin group exhibited antibacterial effects against MRSA on the disk diffusion assay. This anti-MRSA activity was not observed in the joint fluid samples obtained from the oxacillin group or the control group. It may be speculated that using parenteral cefazolin with or without oxacillin-loaded PMMA bone cement is not sufficient to inhibit MRSA infection in TKA. Considering MRSA infection prophylaxis, vancomycin-loaded PMMA bone cement is a better choice than cement loaded with oxacillin or without antibiotic. However, using vancomycin as a prophylactic antibiotic in bone cement triggers several concerns, including the potential risks of toxicity, allergic reactions, and drug-resistant organisms. Therefore, its use is recommended only for revisions after primary arthroplasty because of the prevalence of antibiotic resistance in association with such revisions (13) or the high prevalence of MRSA infection or colonization at the medical institute (21).
Our study has certain limitations. First, due to the small sample sizes and short follow-up period, we could not determine the intergroup differences in infection rates. Second, since the mechanical strength of bone cement may be compromised by mixing it with antibiotics, we do not know the impact of this alteration in mechanical strength on the fixation strength of the cement and the survival of the prosthesis. Third, only 1 type of bone cement and 1 method of preparation were used in this study. Fourth, only resistant or nonresistant strains of S. aureus were selected as test organisms in the experiment. We do not know the bioactivities of specimens against other organisms, particularly biofilm-forming organisms. Fifth, 70% (21 of 30) of enrolled patients were female in the current study. Even though gender did not seem to contribute to the differences between groups in the current study, it was possibly because of the small size of the study, which would not make it powerful enough to confirm even large differences (e.g., 90% of the vancomycin group was female). Since the specimens in this study were all prepared and tested in a uniform and reproducible manner, these results should provide useful information applicable to clinical practice.
The use of antibiotic-loaded bone cement has been shown to be effective in the reduction of the rate of deep infection after total-joint arthroplasty (14, 15). The primary concerns regarding antibiotic-loaded bone cement include the potential for detrimental effects on the mechanical structural characteristics of PMMA when antibiotics are admixed, systemic toxicity related to high antibiotic levels eluted from the cement, allergic reactions to the specific antibiotic used, development of drug-resistant bacteria, and cost (13). The issue of whether to use antibiotic-loaded bone cement routinely for prophylaxis against deep periprosthetic infection when performing primary or aseptic revision total-joint arthroplasty is still controversial.
In conclusion, cefazolin, which was used as a prophylactic antibiotic in TKA, was sufficient to inhibit MSSA during its administration. The use of antibiotic-loaded PMMA bone cement can prolong antibacterial protection after the discontinuation of the intravenous prophylactic antibiotic. With regard to MRSA, vancomycin-loaded PMMA bone cement provided antibacterial activity superior to that of cement with or without oxacillin loading.
ACKNOWLEDGMENTS
This work was supported by the Chang Gung Memorial Hospital (grant no. BMRP 982).
We thank Chee Jen Chang (Chang Gung University, Taoyuan, Taiwan) for his assistance with the statistical analysis.
Footnotes
Published ahead of print 13 August 2012
REFERENCES
- 1. Appelbaum PC. 2006. MRSA—the tip of the iceberg. Clin. Microbiol. Infect. 12(Suppl. 2):3–10 [DOI] [PubMed] [Google Scholar]
- 2. Austin TW, Wallace JF. 1973. Staphylococcus aureus bacteremia: a critical review of its treatment and association with infective endocarditis. Infection 1:214–217 [DOI] [PubMed] [Google Scholar]
- 3. Chang Y, et al. 2011. In vitro activities of daptomycin-, vancomycin-, and teicoplanin-loaded polymethylmethacrylate against methicillin-susceptible, methicillin-resistant, and vancomycin-intermediate strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 55:5480–5484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang Y, et al. 2010. The concentration of antibiotic in fresh-frozen bone graft. J. Bone Joint Surg. Br. 92:1471–1474 [DOI] [PubMed] [Google Scholar]
- 5. Chiu FY, Chen CM, Lin CF, Lo WH. 2002. Cefuroxime-impregnated cement in primary total knee arthroplasty: a prospective, randomized study of three hundred and forty knees. J. Bone Joint Surg. Am. 84-A:759–762 [PubMed] [Google Scholar]
- 6. Chiu FY, Lin CF, Chen CM, Lo WH, Chaung TY. 2001. Cefuroxime-impregnated cement at primary total knee arthroplasty in diabetes mellitus. A prospective, randomised study. J. Bone Joint Surg. Br. 83:691–695 [DOI] [PubMed] [Google Scholar]
- 7. Cunha BA, Gossling HR, Pasternak HS, Nightingale CH, Quintiliani R. 1977. The penetration characteristics of cefazolin, cephalothin, and cephradine into bone in patients undergoing total hip replacement. J. Bone Joint Surg. Am. 59:856–859 [PubMed] [Google Scholar]
- 8. Davis JS. 2005. Management of bone and joint infections due to Staphylococcus aureus. Intern. Med. J. 35(Suppl. 2):S79–S96 [DOI] [PubMed] [Google Scholar]
- 9. Engesaeter LB, et al. 2003. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0–14 years in the Norwegian Arthroplasty Register. Acta Orthop. Scand. 74:644–651 [DOI] [PubMed] [Google Scholar]
- 10. England SP, Stern SH, Insall JN, Windsor RE. 1990. Total knee arthroplasty in diabetes mellitus. Clin. Orthop. Relat. Res. 260:130–134 [PubMed] [Google Scholar]
- 11. Goldenberg DL. 1998. Septic arthritis. Lancet 351:197–202 [DOI] [PubMed] [Google Scholar]
- 12. Hill C, Flamant R, Mazas F, Evrard J. 1981. Prophylactic cefazolin versus placebo in total hip replacement. Report of a multicentre double-blind randomised trial. Lancet i:795–796 [DOI] [PubMed] [Google Scholar]
- 13. Jiranek WA, Hanssen AD, Greenwald AS. 2006. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J. Bone Joint Surg. Am. 88:2487–2500 [DOI] [PubMed] [Google Scholar]
- 14. Josefsson G, Gudmundsson G, Kolmert L, Wijkstrom S. 1990. Prophylaxis with systemic antibiotics versus gentamicin bone cement in total hip arthroplasty. A five-year survey of 1688 hips. Clin. Orthop. Relat. Res. 253:173–178 [PubMed] [Google Scholar]
- 15. Kuechle DK, Landon GC, Musher DM, Noble PC. 1991. Elution of vancomycin, daptomycin, and amikacin from acrylic bone cement. Clin. Orthop. Relat. Res. 264:302–308 [PubMed] [Google Scholar]
- 16. Marks KE, Nelson CL, Lautenschlager EP. 1976. Antibiotic-impregnated acrylic bone cement. J. Bone Joint Surg. Am. 58:358–364 [PubMed] [Google Scholar]
- 17. Meding JB, et al. 2003. Total knee replacement in patients with diabetes mellitus. Clin. Orthop. Relat. Res. 416:208–216 [DOI] [PubMed] [Google Scholar]
- 18. Norden CW. 1985. Prevention of bone and joint infections. Am. J. Med. 78:229–232 [DOI] [PubMed] [Google Scholar]
- 19. Ridgeway S, et al. 2005. Infection of the surgical site after arthroplasty of the hip. J. Bone Joint Surg. Br. 87:844–850 [DOI] [PubMed] [Google Scholar]
- 20. Sendi P, Zimmerli W. 2011. Challenges in periprosthetic knee-joint infection. Int. J. Artif. Organs 34:947–956 [DOI] [PubMed] [Google Scholar]
- 21. Sewick A, et al. 2012. Does dual antibiotic prophylaxis better prevent surgical site infections in total joint arthroplasty? Clin. Orthop. Relat. Res., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walenkamp GH. 2001. Gentamicin PMMA beads and other local antibiotic carriers in two-stage revision of total knee infection: a review. J. Chemother. 13(Spec. No. 1):66–72 [DOI] [PubMed] [Google Scholar]
- 23. Yang K, Yeo SJ, Lee BP, Lo NN. 2001. Total knee arthroplasty in diabetic patients: a study of 109 consecutive cases. J. Arthroplasty 16:102–106 [DOI] [PubMed] [Google Scholar]


