Abstract
Preclinical evaluation of drug-like molecules requires their oral administration to experimental animals using suitable vehicles. We studied the effect of oral dosing with corn oil, carboxymethyl cellulose, dimethyl sulfoxide, and polysorbate-80 on the progression of Mycobacterium tuberculosis infection in mice. Infection was monitored by physical (survival time and body weight) and bacteriological (viable counts in lungs) parameters. Compared with water, corn oil significantly improved both sets of parameters, whereas the other vehicles affected only physical parameters.
TEXT
Every year, progressive infection with Mycobacterium tuberculosis leads to approximately 8 million new tuberculosis (TB) cases and 3 million deaths worldwide. Furthermore, the spread of multidrug-resistant (MDR) TB has become a major threat to global TB control programs. This alarming situation emphasizes the need for new drugs against TB (28, 31).
Evaluation of the in vivo efficacy of a new drug candidate in a suitable animal model is a critical step in determining whether it will enter the preclinical and clinical development phases (10). When infected with a high number of CFU of M. tuberculosis by the intravenous route, the lung of a mouse harbors a bacillary population that is similar in number and in metabolic state to that present in the lungs of TB patients (10). Thus, the mouse model can provide information on lead molecule activity that can be extrapolated to humans (8, 22). Outbred mice, particularly Swiss mice, are used for in vivo assays for anti-TB activity because of their heterogeneity, which is akin to that of the human population (3).
For in vivo evaluations, a lead molecule is preferably administered as a solution or suspension by the oral route (1), which is an important requirement for its successful development as a new drug. Water is the universally accepted “ideal” vehicle for oral dosing, having no chemical, biochemical, immunological, or pharmacological effects on the test molecule, the host, or the pathogen (2). However, if the molecule is highly hydrophobic, then edible oils (such as corn or peanut oil) are used as vehicles (21). For moderately hydrophobic molecules, certain solubilizing agents, such as carboxymethyl cellulose (CMC), dimethyl sulfoxide (DMSO), and polysorbate-80 (Tween 80), can be added to the aqueous dosing vehicle (23, 24).
The influence of dosing vehicles on the progression of disease in animal models, if any, is an important consideration for in vivo evaluation of test molecules. There is hardly any information available on whether these vehicles themselves could modulate the disease process, thereby influencing the outcome of the evaluation of the drug candidates. This is particularly important in cases of chronic infections, such as TB, where treatments are given to the animals for long periods of time (6, 12, 19). We therefore studied the effect that some of the vehicles themselves could exert on the progression of M. tuberculosis infection in mice. Five vehicles—water, corn oil, CMC, DMSO, and Tween 80—were comparatively studied. Study parameters were physical, i.e., mean survival time (MST) and body weight, as well as bacteriological, i.e., CFU recovered from the lung.
Corn oil was administered undiluted, whereas dilutions of CMC (0.5%), Tween 80 (0.05%) and DMSO (10%) were made in sterile water. Female outbred Swiss mice (16 to 18 g), obtained from the Laboratory Animal Division of the Central Drug Research Institute, Lucknow, India, were infected intravenously (2 × 107 bacilli/mouse) with M. tuberculosis H37Rv (ATCC 27294) and caged in groups of 8 to 10 animals. Each group was given water or the other vehicles separately (0.2 ml/mouse, by oral gavage, once daily, for 30 days). The progress of infection was monitored in the control (untreated) mice by determining numbers of viable bacilli (CFU) on day 1 postinfection and then at weekly intervals (Fig. 1). For this, serial dilutions of lung homogenates (1 g tissue/ml) in physiological saline were spread on Middlebrook 7H11 agar medium (containing OADC [oleic acid-albumin-dextrose-catalase] supplement). The plates were incubated at 37°C, and CFU were counted after 3 to 4 weeks (4). Numbers of CFU in the lungs of mice from each experimental (vehicle-treated) group were determined on day 30.
Fig 1.
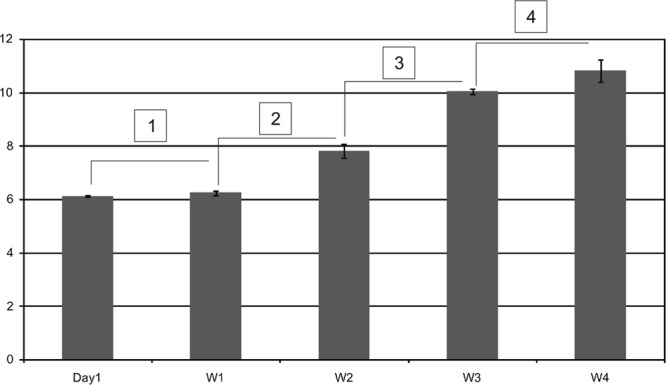
Progression of M. tuberculosis infection in Swiss mice infected with 2 × 107 bacilli/mouse without any oral dosing. Each bar represent log10 CFU (mean ± SD) in lungs of three mice each on day 1 and at weekly intervals up to 4 weeks (W4) postinfection. Statistical significance is indicated by numbers in boxes: 1, P = 0.073; 2, P = 0.0006; 3, P = 0.0001; 4, P = 0.035.
The corn oil-fed mice showed far less decline in body weight (weight on day 0 − weight on day 21) than the water-fed animals (0.50 ± 1.00 g and 4.67 ± 3.06 g, respectively [Table 1]). This difference was statistically significant (P = 0.047 [Table 2]). More importantly, oil-fed animals also exhibited a >1-log reduction in lung CFU compared with the water-fed animals (Table 1). This difference was also statistically significant (P = 0.0016) (Table 2). However, a significant difference was not observed between MST (mean survival time of all animals in a group) of the water-fed (24.75 ± 4.50 days) and oil-fed (25.25 ± 3.69 days) animals (Table 1).
Table 1.
Survival, average body weight, and bacterial load in lungs of M. tuberculosis-infected mice treated with different vehicles
| Vehicle | MST on day 30 | Net decline (from day 0 to 21) in avg body wt (g)a | Bacterial load in lung (log10 CFU/g tissue)a |
|---|---|---|---|
| Distilled water | 24.75 ± 4.50 | 4.67 ± 3.06 | 11.44 ± 0.212 |
| Corn oil | 25.25 ± 3.69 | 0.50 ± 1.00 | 10.36 ± 0.122 |
| CMC | 28.00 ± 2.31 | 3.00 ± 1.15 | 11.84 ± 0.242 |
| DMSO | >30 | 1.50 ± 1.00 | 11.75 ± 0.316 |
| Tween 80 | 28.75 ± 2.50 | 2.00 ± 1.63 | 11.80 ± 0.451 |
Values are means ± standard deviations.
Table 2.
Comparison of changes brought in the assessment parameters with experimental vehicles
| Comparison |
P value fora: |
|
|---|---|---|
| Net decline in avg body wt | Bacterial load in lungs | |
| Distilled water vs oil | 0.0471* | 0.0016** |
| CMC vs distilled water | NS | NS |
| CMC vs oil | 0.017* | 0.0007# |
| DMSO vs distilled water | NS | NS |
| DMSO vs oil | NS | 0.0021** |
| Tween 80 vs distilled water | NS | NS |
| Tween 80 vs oil | NS | 0.0060** |
NS, not significant;
, statistically significant;
, very significant;
, extremely significant.
Among the three aqueous vehicles, administration of DMSO resulted in “healthier” physical parameters. The MST with DMSO was >30 days (no deaths up to day 30) compared with 28.00 ± 2.31 days with CMC and 28.75 ± 2.50 days with Tween 80 (Table 1). All three MSTs were higher than those observed with water and oil. As none of the mice in DMSO group died, it was not possible to calculate statistical significance of the differences in MST. The decline in body weight with all three vehicles was also less than that with water (Table 1), though the differences were not significant (P > 0.05). With respect to oil, only CMC showed a significantly greater decline in the body weight (3.00 ± 1.15 g, P = 0.0176) (Table 2). Finally, the numbers of CFU in lungs with all aqueous vehicles (Table 1) were close to that obtained with water (P > 0.05) but significantly higher than that obtained with oil (P = 0.0007 versus CMC, 0.0021 versus DMSO, and 0.0060 versus Tween 80) (Table 2).
The apparent beneficial effects of corn oil could be due to the presence of 86% unsaturated fatty acids, of which two-thirds is linoleic acid and one-third is oleic acid (15). Linoleic acid has been found to inhibit mycobacteria in vitro (15, 29); thus, it could also produce the same effect in vivo. The three aqueous vehicles (DMSO, CMC, and Tween 80) caused some apparent, though not significant, modulation of only the physical parameters, particularly MST. Among the three, administration of DMSO resulted in much improved physical parameters. This may influence the general body physiology and/or metabolism, which may in turn modulate the handling of the pathogen by the infected host (5, 7, 9, 11, 13, 14, 17, 18, 25–27, 30).
Measurement of survival time or body weight of animals is an appropriate, cost- and time-effective criterion to assess the progression of infection and to evaluate new drugs against M. tuberculosis in vivo (10, 16, 20). Our results, however, show that weight loss and MST may not be reliable criteria and that determination of bacterial load in the infected organs is a true measure of infection. Moreover, in a previous screening for TB-induced weight loss, some active compounds or drugs possessing anabolic properties could have affected the results (20). In conclusion, the vehicles used for oral dosing of test molecules can influence physical as well as bacteriological parameters in mice infected with M. tuberculosis.
ACKNOWLEDGMENT
We are grateful to Director, Central Drug Research Institute, Lucknow, India, for providing necessary facilities and support to carry out the work.
This paper has CDRI communication number 8299.
Footnotes
Published ahead of print 27 August 2012
REFERENCES
- 1. Aldwell FE, Tucker IG, de Lisle GW, Buddle BM. 2003. Oral delivery of Mycobacterium bovis BCG in a lipid formulation induces resistance to pulmonary tuberculosis in mice. Infect. Immun. 71:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amolsmalpani, Waghere P, Nbelorkar 2009. Aqueous solubility: measurement and prediction tools. Latest Rev. 7:5–13 [Google Scholar]
- 3. Brayton C. 2007. Spontaneous diseases in commonly used mouse strains, p 623–718 In Fox JG, et al. (ed), The mouse in biomedical research diseases. American College of Laboratory animal medicine series, vol. 2, 2nd ed Academic Press, New York, NY [Google Scholar]
- 4. Canetti G, Grumbach F, Grosset J. 1960. Studies of bacillary populations in experimental tuberculosis of mice treated with isoniazid. Am. Rev. Respir. Dis. 82:295–313 [DOI] [PubMed] [Google Scholar]
- 5. Chang CY, Simon E. 1968. The effect of dimethyl sulfoxide (DMSO) on cellular systems. Proc. Soc. Exp. Biol. Med. 128:60–66 [DOI] [PubMed] [Google Scholar]
- 6. Cross ML, Lambeth MR, Aldwell FE. 2009. An oral Mycobacterium bovis BCG vaccine for wildlife produced in the absence of animal-derived reagents. Clin. Vaccine Immunol. 16:1378–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhariwal KR, Venkitasubramanian TA. 1978. Effect of Tween 80 on lipids of Mycobacterium phlei ATCC 354. Experientia 34:303–304 [DOI] [PubMed] [Google Scholar]
- 8. Dharmadhikari AS, Nardell EA. 2008. What animal models teach humans about tuberculosis. Am. J. Respir. Cell Mol. Biol. 39:503–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dozier MM, Ratajczakt HV, Sothern J, Thomas PT. 1997. The influence of vehicle gavage on seasonality of immune system parameters in the B6C3F1 mouse. Fundamental Appl. Toxicol. 38:116–122 [DOI] [PubMed] [Google Scholar]
- 10. Global Alliance for Tuberculosis Drug Development 2001. Scientific blueprint for TB drug development. Tuberculosis (Edinburgh) 81(Suppl. 1):1–52 [DOI] [PubMed] [Google Scholar]
- 11. Gordeliy VI, Kiselev KA, Lesieur P, Pole AV, Teixeira J. 1998. Lipid membrane structure and interactions in dimethylsulphoxide/water mixtures. Biophys. J. 75:2343–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gruppo V, et al. 2006. Rapid microbiologic and pharmacologic evaluation of experimental compounds against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 50:1245–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jagannath C, Reddy VM, Gangadharam PR. 1995. Enhancement of drug susceptibility of multi-drug resistant strains of Mycobacterium tuberculosis by ethambutol and dimethylsulphoxide. J. Antimicrob. Chemother. 35:381–390 [DOI] [PubMed] [Google Scholar]
- 14. Kataoka T, Yamamoto S, Yamamoto T, Tokunaga T. 1990. Immunotherapeutic potential in guinea-pig tumor model of deoxyribonucleic acid from Mycobacterium bovis BCG complexed with poly-l-lysine and carboxymethylcellulose. Jpn. J. Med. Sci. Biol. 43:171–182 [DOI] [PubMed] [Google Scholar]
- 15. Layton HW, Youmans GP. 1965. Effect of dietary factors upon the resistance of albino mice to experimental infection with Mycobacterium tuberculosis. J. Bacteriol. 90:958–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lenaerts AJM, Gruppo V, Brooks JV, Orme IM. 2003. Rapid in vivo screening of experimental drugs for tuberculosis using gamma interferon gene-disrupted mice. Antimicrob. Agents Chemother. 47:783–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li X, et al. 2011. Polysorbates as novel lipid-modulating candidates for reducing serum total cholesterol and low-density lipoprotein levels in hyperlipidemic C57BL/6J mice and rats. Eur. J. Pharmacol. 660:468–475 [DOI] [PubMed] [Google Scholar]
- 18. Masaki S, Sugimori G, Okamoto A, Imose J, Hayashi Y. 1990. Effect of Tween 80 on the growth of Mycobacterium avium complex. Microbiol. Immunol. 34:653–663 [DOI] [PubMed] [Google Scholar]
- 19. Neurmberger E, Grosset J. 2004. Pharmacokinetic and pharmacodynamic issues in the treatment of mycobacterial infections. Eur. J. Clin. Microbiol. Infect. Dis. 23:243–255 [DOI] [PubMed] [Google Scholar]
- 20. Nikonenko BV, Samala R, Einck L, Nacy CA. 2004. Rapid, simple in vivo screen for drugs active against Mycobacterium tuberculosis. Antimicrobial Agents Chemother. 48:4550–4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Driscoll CM, Griffin BT. 2008. Biopharmaceutical challenges associated with drugs with low aqueous solubility—the potential impact of lipid-based formulations. Adv. Drug Deliv. Rev. 60:617–624 [DOI] [PubMed] [Google Scholar]
- 22. Orme IM. 2003. The mouse as a useful model of tuberculosis. Tuberculosis (Edinburgh) 83:112–115 [DOI] [PubMed] [Google Scholar]
- 23. Sastry SV, Nyshadham JR, Joseph AF. 2000. Recent technological advances in oral drug delivery—a review. Pharmaceut. Sci. Technol. Today 3:138–145 [DOI] [PubMed] [Google Scholar]
- 24. Strickley RG. 2004. Solubilizing excipients in oral and injectable formulations. Pharmaceut. Res. 21:201–230 [DOI] [PubMed] [Google Scholar]
- 25. Topolev VV, Krishtalik LI. 1999. Adsorption of dimethylsulphoxide on proteins. Biofyzika 44:992–995 [PubMed] [Google Scholar]
- 26. van Boxtel RM, Lambrecht RS, Collins MT. 1990. Effect of polyoxyethylene sorbate compounds (Tweens) on colonial morphology, growth, and ultrastructure of Mycobacterium paratuberculosis. APMIS 98:901–908 [DOI] [PubMed] [Google Scholar]
- 27. Wood DC, Wood J. 1975. Pharmacologic and biochemical considerations of dimethylsulphoxide. Ann. N. Y. Acad. Sci. 243:7–19 [DOI] [PubMed] [Google Scholar]
- 28. World health organization 2012. Guidelines for the programmatic management of drug resistant tuberculosis, 2011 update. World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- 29. Youmans AS, Youmans GP. 1954. Studies on the metabolism of Mycobacterium tuberculosis. The effect of fatty acids on the growth of M. tuberculosis var. hominis. J. Bacteriol. 67:731–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu ZW, Quinn PJ. 1998. The modulation of membrane structure and stability by dimethylsulphoxide. Mol. Membrane Biol. 15:59–68 [DOI] [PubMed] [Google Scholar]
- 31. Zumla A, Hafner R, Lienhardt C, Hoeischer M, Nunn A. 2012. Advancing the development of tuberculosis therapy. Nat. Rev. Drug Discov. 11:171–172 [DOI] [PubMed] [Google Scholar]


