Abstract
Perturbation of hydroxyl radical accumulation by subinhibitory concentrations of 2,2′-bipyridyl plus thiourea protects Escherichia coli from being killed by 3 lethal antimicrobial classes. Here, we show that 2,2′-bipyridyl plus thiourea delays and/or reduces antimicrobial killing of Staphylococcus aureus by daptomycin, moxifloxacin, and oxacillin. While the protective effect of 2,2′-bipyridyl plus thiourea varied among strains and compounds, the data support the hypothesis that hydroxyl radical enhances antimicrobial lethality.
TEXT
The growing problem of antimicrobial resistance has become increasingly difficult to counter by developing new agents for two reasons. First, most large pharmaceutical companies have shelved their antimicrobial development programs due to financial, regulatory, and legal considerations (9, 10). Second, expectations that novel genomic/proteomic approaches would quickly produce many new antimicrobials may not be realistic, since no antimicrobial has materialized from such approaches, despite more than 10 years of effort (8). As an alternative to finding new antimicrobial classes, we have been seeking ways to enhance the efficacy of existing agents. Recent studies indicate that reactive oxygen species (ROS) contribute to antimicrobial-mediated killing of Escherichia coli for three important drug classes (5, 11). If the lethal action of many antimicrobials is stimulated by ROS with many pathogens, it may be possible to develop broad-spectrum, small-molecule enhancers of multiple antimicrobial classes by identifying novel, bacterium-specific targets within oxidative stress pathways. Since work to date has focused primarily on E. coli (only norfloxacin has shown a correlation between killing and hydroxyl radical surge with Staphylococcus aureus [5]), the relevance of ROS-mediated lethality to antimicrobial treatment of Gram-positive bacteria is largely unknown. Below, we describe the effects of inhibitors of ROS accumulation on the ability of three agents to kill two strains of Staphylococcus aureus under laboratory conditions.
Two methicillin-susceptible S. aureus strains (RN450 [7] and ATCC 25923) were grown at 37°C on Mueller-Hinton agar or in Mueller-Hinton broth with shaking at 250 rpm. Bacterial growth was measured by diluting an overnight culture into fresh Mueller-Hinton broth by 100-fold, followed by turbidity determinations at 600 nm with a spectrophotometer. The MIC and minimal bactericidal concentration (MBC) were determined by broth microdilution according to CLSI guidelines (1) for daptomycin (Cubist Pharmaceuticals, Lexington, MA), moxifloxacin (Bayer AG, West Haven, CT), and oxacillin (Sigma, St. Louis, MO) in the presence or absence of subinhibitory concentrations (one-half of the MIC) of 2,2′-bipyridyl plus thiourea, two compounds that interfere with antimicrobial-mediated hydroxyl radical accumulation (5, 11). Concentration-kill curves and killing kinetics were measured by incubating exponentially growing cells with daptomycin, moxifloxacin, or oxacillin in the presence or absence of subinhibitory concentrations of 2,2′-bipyridyl plus thiourea for various times at a fixed antimicrobial concentration or at various antimicrobial concentrations for a fixed time. After incubation with a drug, samples were diluted and plated on drug-free agar. The number of viable cells was determined after incubation for 24 h, and percent survival was calculated relative to viable count in samples taken at the time the drug was added. Calcium chloride was added to the growth medium at 1.25 mM (50 mg/liter Ca2+) to assay MIC and lethality for daptomycin (1, 4). Intracellular hydroxyl radical was measured in cultures of strain RN450 by pulse labeling with hydroxyphenyl fluorescein (HPF; Invitrogen, Carlsbad, CA), a hydroxyl radical-specific fluorescent dye, for 10 min, followed by flow cytometry analysis using an Accuri C6 flow cytometer (BD Accuri Cytometers, Ann Arbor, MI).
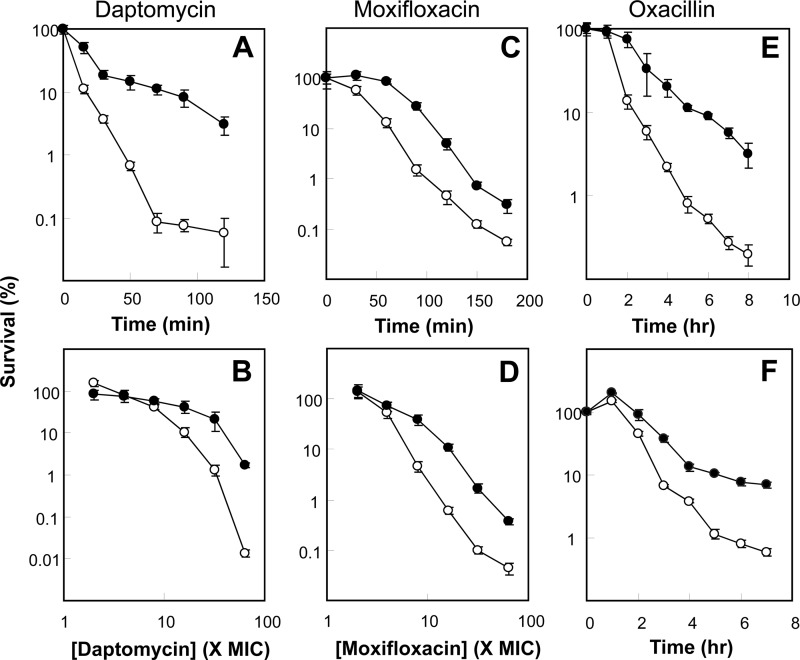
The presence of 2,2′-bipyridyl plus thiourea had little (e.g., no more than 2-fold) effect on MIC and MBC for the three antimicrobials tested (Table 1), as previously observed with E. coli (5, 11). In contrast, 2,2′-bipyridyl plus thiourea (or thiourea alone in the case of daptomycin) reduced rapid killing by all three antimicrobials with strain RN450 (Fig. 1). The protective effect of 2,2′-bipyridyl plus thiourea was evident in both killing kinetics and concentration-kill curves for the concentration-dependent killers, daptomycin and moxifloxacin (Fig. 1A to D); protection by 2,2′-bipyridyl plus thiourea was also seen with killing kinetics for the time-dependent killer, oxacillin (Fig. 1E). The quantitative effect of 2,2′-bipyridyl plus thiourea differed among the antimicrobials, reducing lethality by about 100-, 20-, and 10-fold for daptomycin, oxacillin, and moxifloxacin, respectively (Fig. 1). These differences may reflect differing contributions of hydroxyl radical accumulation to the lethal activity of a given compound, as reported previously for several quinolones that kill E. coli (12).
Table 1.
Effects of blocking hydroxyl radical accumulation on MIC and MBC of antistaphylococcus agents
| Compound | MIC/MBC (μg/ml) under indicated conditionsa in strain: |
|||
|---|---|---|---|---|
| RN450 |
ATCC 25923 |
|||
| −BT | +BT | −BT | +BT | |
| Daptomycin | 0.06/0.24 | 0.03/0.12b | 0.12/0.96 | 0.06/0.48b |
| Moxifloxacin | 0.06/0.24 | 0.06/0.24 | 0.03/0.24 | 0.03/0.24 |
| Oxacillin | 0.25/0.5 | 0.125/0.5 | 0.25/1.0 | 0.125/0.5 |
−BT, MIC and MBC were determined in the absence of 2,2′-bipyridyl plus thiourea; +BT, MIC and MBC were determined in the presence of 2,2′-bipyridyl plus thiourea (or thiourea alone, as indicated), both at one-half of the MIC. With strain RN450, the MICs for 2,2′-bipyridyl and thiourea were 1.5 and 200 mM, respectively; with strain ATCC 25923, the MICs were 1.0 and 100 mM for 2,2′-bipyridyl and thiourea, respectively. Little growth inhibition was observed when 2,2′-bipyridyl plus thiourea, both at one-half of the MIC, were added to the S. aureus culture.
Fig 1.
Effects of inhibitors of hydroxyl radical accumulation on lethality of daptomycin, moxifloxacin, and oxacillin. Exponentially growing S. aureus strain RN450 was treated with a fixed concentration of an antimicrobial for the indicated times (A, C, E, and F) or with the indicated concentrations of an antimicrobial for a fixed time (B and D) in the absence (empty circles) or presence (filled circles) of one-half of the MIC of 2,2′-bipyridyl plus thiourea. (A) Concentration of 1.9 μg/ml (32-fold MIC) daptomycin for the indicated times. (B) Indicated concentrations of daptomycin for 1 h. (C) Concentration of 0.9 μg/ml (15-fold MIC) moxifloxacin for the indicated times. (D) Indicated concentrations of moxifloxacin for 2 h. (E) Concentration of 12.5 μg/ml (50-fold MIC) oxacillin for the indicated times. (F) A noninhibitory concentration (e.g., one-half of the MIC) of glutathione replaced the 2,2′-bipyridyl plus thiourea used for the experiment whose results are shown in panel E. All experiments were repeated 3 times with similar results; error bars indicate standard deviations.
For moxifloxacin and oxacillin, strain-to-strain variation may be important, as the effect of 2,2′-bipyridyl plus thiourea was far less with strain ATCC 25923 (see Fig. S1 in the supplemental material). These data point to the need to examine large numbers of isolates before assessing the clinical relevance of ROS on antimicrobial action.
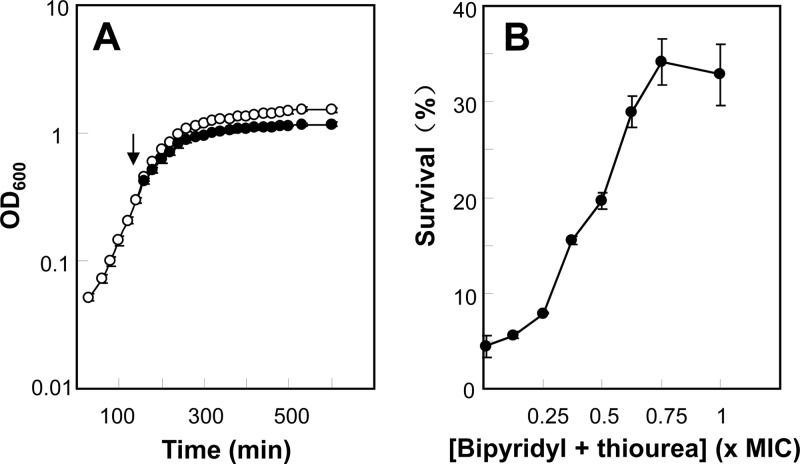
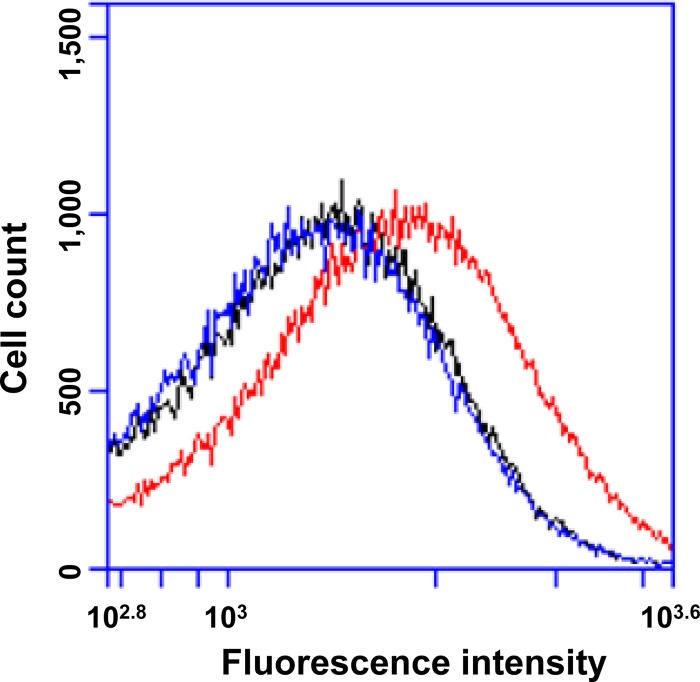
Several lines of evidence indicated that suppression of intracellular hydroxyl radical accumulation rather than inhibition of growth by 2,2′-bipyridyl plus thiourea accounted for the observed reduction of antimicrobial killing. First, 2,2′-bipyridyl plus thiourea, both at half the MIC, showed no inhibition of growth rate and only a slight reduction in culture yield (Fig. 2A). Second, protection from moxifloxacin-mediated killing by 2,2′-bipyridyl plus thiourea was observed at concentrations as low as one-fourth of the MIC, and the protection was concentration dependent between one-fourth and three-fourths of the MIC of 2,2′-bipyridyl plus thiourea (Fig. 2B). Third, glutathione, a natural antioxidant used by bacterial cells to counter oxidative stress, provided protection against oxacillin-mediated killing (Fig. 1F) at a level similar to that observed with 2,2′-bipyridyl plus thiourea (Fig. 1E). This result provides further support for the generality of previous work that ties ROS to antimicrobial killing (5, 6, 11). Fourth, a moxifloxacin-stimulated surge in intracellular hydroxyl radical accumulation (Fig. 3, indicated by a shift of fluorescence intensity to the right) was blocked by 2,2′-bipyridyl plus thiourea. These data indicate that antimicrobial-mediated accumulation of hydroxyl radical contributes to the lethal action of several classes of antistaphylococcal agent.
Fig 2.
Effects of fractional MIC of 2,2′-bipyridyl plus thiourea on bacterial growth and moxifloxacin lethality. (A) Effect of 2,2′-bipyridyl plus thiourea on bacterial growth. S. aureus strain RN450 was grown to exponential phase (empty circles). At the indicated time (arrow), 2,2′-bipyridyl and thiourea at one-half of the MIC were added into the growing culture (filled circles). Culture turbidity was measured as absorption (optical density [OD]) at 600 nm. (B) Effect of fractional MIC of 2,2′-bipyridyl plus thiourea on bacterial killing by moxifloxacin. Exponentially growing S. aureus strain RN450 was treated with 0.9 μg/ml (15-fold MIC) moxifloxacin for 90 min in the presence of the indicated concentrations of 2,2′-bipyridyl plus thiourea. All experiments were repeated 3 times with similar results; error bars indicate standard deviations.
Fig 3.
Effect of moxifloxacin and 2,2′-bipyridyl plus thiourea on intracellular hydroxyl radical accumulation. Exponentially growing S. aureus strain RN450 was treated with 0.9 μg/ml (15-fold MIC) moxifloxacin in the presence or absence of one-half of the MIC of 2,2′-bipyridyl plus thiourea for 35 min. Hydroxyphenyl fluorescein (HPF) was added to both treated samples and an untreated control to a final concentration of 5 μM. Cells were further incubated for 10 min at 37°C in the dark before they were subjected to fluorescence-activated cell sorting (FACS) analysis. In total, 100,000 cells were analyzed for each sample. y axis indicates fluorescence-labeled-cell numbers and x axis represents fluorescence intensity of HPF-labeled cells. A peak shift to the right indicates an increase in hydroxyl radical production/accumulation. Black, untreated control sample; red, moxifloxacin-treated sample; blue, moxifloxacin- and 2,2′-bipyridyl–plus–thiourea-cotreated sample.
The presence of only a minor effect of 2,2′-bipyridyl plus thiourea on MBC (Table 1), a parameter that involves a long incubation time, and the parallel nature of the slope of moxifloxacin killing kinetics (Fig. 1C) suggested that 2,2′-bipyridyl plus thiourea may only delay antimicrobial killing rather than reduce its extent. As a further test, we measured the killing kinetics for moxifloxacin with strain RN450 over 7 h. A delay was indeed observed (see Fig. S2 in the supplemental material). Thus, with S. aureus, some compounds appear to speed killing through ROS, with little change in the extent of killing.
A caveat to the present work, as well as to all previous work using 2,2′-bipyridyl to study antimicrobial lethality (2, 3, 5, 6, 11, 12), is that potential effects of this agent on iron-dependent processes other than hydroxyl radical formation have not been identified, evaluated, and excluded as alternative explanations for the data. Moreover, work to date has been with only a few strains, and strain-to-strain variation clearly exists with compounds such as moxifloxacin and oxacillin. Thus, additional work is required before clinical relevance is clear, even though 2,2′-bipyridyl–plus–thiourea-mediated effects occur at antimicrobial concentrations that are therapeutically achievable.
In conclusion, the results described above with a Gram-positive bacterium, plus earlier work with a Gram-negative organism, indicate that a contribution of hydroxyl radical to antimicrobial lethality may be common to many bacterial species and antimicrobial classes. A better understanding of oxidative stress pathways may help identify targets whose inactivation will stimulate intracellular ROS accumulation and enhance antimicrobial lethality.
Supplementary Material
ACKNOWLEDGMENTS
We thank Karl Drlica and an anonymous reviewer for critical comments on the manuscript.
The work was supported by grants from National Natural Science Foundation of China (grant 30973596 to X.W.) and from the National Institutes of Health (grant 1-DP2-OD007423 to X.Z.).
Footnotes
Published ahead of print 4 September 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Clinical and Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed CLSI publication M07-A8. CLSI, Wayne PA [Google Scholar]
- 2. Davies BW, et al. 2009. Hydroxyurea induces hydroxyl radical-mediated cell death in Escherichia coli. Mol. Cell 36:845–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. 2012. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science 336:315–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fuchs PC, Barry AL, Brown SD. 2000. Daptomycin susceptibility tests: interpretive criteria, quality control, and effect of calcium on in vitro tests. Diagn. Microbiol. Infect. Dis. 38:51–58 [DOI] [PubMed] [Google Scholar]
- 5. Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810 [DOI] [PubMed] [Google Scholar]
- 6. Kohanski MA, Dwyer DJ, Wierzbowski J, Cottarel G, Collins JJ. 2008. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell 135:679–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kornblum JS. 1987. Some findings concerning methicillin resistance in S. aureus and analysis of countertranscript RNAs of several pT181 copy mutants. Ph.D. thesis New York University, New York, NY [Google Scholar]
- 8. Livermore DM. 2011. Discovery research: the scientific challenge of finding new antibiotics. J. Antimicrob. Chemother. 66:1941–1944 [DOI] [PubMed] [Google Scholar]
- 9. Projan SJ. 2003. Why is big Pharma getting out of antibacterial drug discovery? Curr. Opin. Microbiol. 6:427–430 [DOI] [PubMed] [Google Scholar]
- 10. Projan SJ, Shlaes DM. 2004. Antibacterial drug discovery: is it all downhill from here? Clin. Microbiol. Infect. 10(Suppl 4):18–22 [DOI] [PubMed] [Google Scholar]
- 11. Wang X, Zhao X. 2009. Contribution of oxidative damage to antimicrobial lethality. Antimicrob. Agents Chemother. 53:1395–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang X, Zhao X, Malik M, Drlica K. 2010. Contribution of reactive oxygen species to pathways of quinolone-mediated bacterial cell death. J. Antimicrob. Chemother. 65:520–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.