Abstract
Background
We investigated the use of deep brain stimulation (DBS) of the ventral capsule/ventral striatum (VC/VS) for treatment refractory depression.
Methods
Fifteen patients with chronic, severe, highly refractory depression received open-label DBS at three collaborating clinical sites. Electrodes were implanted bilaterally in the VC/VS region. Stimulation was titrated to therapeutic benefit and the absence of adverse effects. All patients received continuous stimulation and were followed for a minimum of 6 months to longer than 4 years. Outcome measures included the Hamilton Depression Rating Scale—24 item (HDRS), the Montgomery-Asberg Depression Rating Scale (MADRS), and the Global Assessment of Function Scale (GAF).
Results
Significant improvements in depressive symptoms were observed during DBS treatment. Mean HDRS scores declined from 33.1 at baseline to 17.5 at 6 months and 14.3 at last follow-up. Similar improvements were seen with the MADRS (34.8, 17.9, and 15.7, respectively) and the GAF (43.4, 55.5, and 61.8, respectively). Responder rates with the HDRS were 40% at 6 months and 53.3% at last follow-up (MADRS: 46.7% and 53.3%, respectively). Remission rates were 20% at 6 months and 40% at last follow-up with the HDRS (MADRS: 26.6% and 33.3%, respectively). The DBS was well-tolerated in this group.
Conclusions
Deep brain stimulation of the VC/VS offers promise for the treatment of refractory major depression.
Keywords: Deep brain stimulation, efficacy, major depression, ventral capsule/ventral striatum
Depression, a leading cause of disability in developed countries, imposes substantial suffering and health burden worldwide (1). Suicide is a major complication, and patients with the greatest degree of treatment resistance have poor long-term prognoses (2,3). Notably, even electroconvulsive therapy (ECT), the most effective acute treatment, produces less response in this group (4). Even with effective antidepressant medication or ECT treatment, the majority of these patients relapse over a relatively short period of time (5).
Given the failure of conventional therapies in treatment-resistant individuals, neurosurgical alternatives have remained an option in selected cases of both obsessive-compulsive disorder (OCD) and major depressive disorder (MDD). Ablative procedures performed for MDD include anterior cingulotomy (6), anterior capsulotomy (7), subcaudate tractotomy (8), and limbic leucotomy (9). Approximately one-third to two-thirds of patients undergoing these procedures demonstrate benefit (10). Neural systems affected by these procedures overlap, because they target connections in the cortico-striato-thalamo-cortical (CSTC) networks involving the orbital and medial prefrontal cortex, basal ganglia, and/or anterior cingulate (11).
These same circuits have been implicated in the pathophysiology of OCD (12–14). Research using deep brain stimulation (DBS) to affect these pathways in OCD has been undertaken as an alternative to ablative procedures (15,16). Several different groups have reported improvement in OCD symptomatology with DBS in treatment refractory patients (15–19). The target used in the studies by Nuttin et al. (15) and Greenberg et al. (16) was the ventral anterior internal capsule/ventral striatum (VC/VS), guided in part by studies of gamma-knife capsulotomy (20). Benefit was seen not only for OCD symptoms but also for depressive symptoms. These observations in OCD, along with lesioning studies targeting the same cortico-basal circuitry, led to our exploration of this target for the treatment of MDD.
One report of DBS for depression, targeting white matter adjacent to subgenual cingulate cortex (Cg25), found benefit in four of six patients (21). That study tested a hypothesis developed from neuroimaging research suggesting that the subgenual cingulate is a key node in circuitry mediating depressive mood (22). Earlier studies reporting positive emotional responses and reduced anxiety associated with acute brain stimulation in this same region were also consistent with this theory (23,24).
Here, we report long-term outcomes of VC/VS stimulation in treatment-resistant MDD, performed at three study centers. Multiple clinical domains were assessed including severity of depressive symptoms, global functionality, and adverse events. Description of long-term outcomes is particularly important, given the nature of the intervention and the natural history of the illness in this refractory subgroup.
Methods and Materials
This investigation was a collaborative effort between the departments of Psychiatry and Neurosurgery at the Cleveland Clinic (CC), Butler Hospital/Brown Medical School (BH), and the Massachusetts General Hospital (MGH), following published guidelines for the conduct of psychiatric neurosurgery (25). The work was informed by prior CC and BH experience with DBS for intractable OCD at the same stimulation target (16). Institutional review board and Food and Drug Administration approvals (Investigational Device Exemptions) were obtained at all sites and were based upon a shared protocol. A data and safety monitoring board was in place at MGH. Starting at BH in January 2003, 15 patients received implantation over a period of 45 months and were followed continuously. Patient accrual at the three centers is reflected in the last follow-up times reported. Data monitoring was conducted at all sites by independent clinical personnel employed by Medtronic.
Patient Selection
Patient selection was led by a psychiatrist at each site (DM, BG, or DD). Referrals came from treating psychiatrists both within and outside study centers. All available records were obtained and reviewed in detail, treating psychiatrists and therapists were interviewed, and patients underwent multidisciplinary on-site evaluations. Resulting data were reviewed by separate committees that determined study eligibility. Informed consent was obtained after meeting with both the psychiatrist and neurosurgeon at each site.
Inclusion Criteria
Inclusion criteria were comparable at all sites. Patients were required to be 18–55 years old with at least a 5-year history of chronic or recurrent depression (2 or more years in current episode) by clinical and Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-IV) assessments (26), be in good general health, have a stable regimen of psychotropic medications for at least 6 weeks before study entry, and be able to comply with study requirements that included providing written informed consent. Patients were also required to score at least 21 on a 24-item Hamilton Depression Rating Scale (HDRS) (27). This threshold was chosen to allow inclusion of patients partially responsive to their current treatment. Previous treatment attempts must have included: 1) adequate trials (>6 weeks at maximum recommended or tolerated dose) of primary antidepressant drugs from at least three different classes; 2) adequate trials (>4 weeks) of augmentation/combination strategies using a primary antidepressant with at least two other different agents; 3) at least one adequate trial of ECT (six or more bilateral treatments); and 4) an adequate trial of psychotherapy (at least 20 sessions with an experienced therapist).
Exclusion Criteria
These criteria were also comparable at all sites and included: 1) significant comorbid neurological or medical illness; 2) clinically significant abnormal preoperative magnetic resonance imaging; 3) significant comorbid psychiatric illness that would impact safety or compliance during the study; 4) presence of psychosis outside of a depressive episode; 5) active or unstably remitted substance abuse or dependence; 6) surgical contraindications to DBS; 7) imminent risk of suicide; or 8) pregnancy. Women of childbearing potential were required to use effective contraception during the study. Bipolar patients were excluded at CC and MGH but allowed at BH.
Surgical Procedure
Electrodes were implanted bilaterally in the VC/VS with frame-based, magnetic resonance imaging-guided stereotactic technique. Targeting was based upon prior experience with DBS for OCD (28). Thirteen of the 15 patients were implanted bilaterally with Model 3387 IES leads (Medtronic, Minneapolis, Minnesota). Each lead is 1.27 mm in diameter with four 3-mm long electrode contacts, separated from adjacent contacts by 4 mm. The contacts are numbered from 0 (deepest) to 3 (most superficial) and are independently programmable. Early in the series, one patient (BH3) was implanted bilaterally with Model 3387 leads, which have four contacts of 1.5-mm length and spacing of 1.5 mm, in order to investigate more finely controlled spatial stimulation within the ventral target region. Another patient (CC6) had a 3387 IES lead implanted on the right side and a 3387 lead on the left due to a device malfunction at implantation.
Leads were implanted following the dorso-ventral trajectory of the anterior limb of the internal capsule (AIC) (Figure 1). The target for contact 0 was the ventral striatum (VS) below the level of the anterior commissure. The intention was for contact 1 to be near the junction of the VS and ventral capsule (VC) with contacts 2 and 3 located more dorsally within the AIC. Nominal target coordinates for the electrode tip were: 6–7 mm lateral to midline (X), 1–2 mm anterior to the posterior border of the anterior commissure (Y), and 3–4 mm inferior to the anterior commissure–posterior commissure line (Z). Microelectrode recording was performed at CC and MGH but was not used for identifying ultimate target location. Final targeting in all cases was based on individual anatomical landmarks and intra-operative responses to test stimulation. The surgical target was not modified over the course of the study. Postoperative imaging was conducted to verify lead placement. Leads were later connected via subcutaneous extensions to implantable neurostimulators (Soletra or Kinetra; Medtronic) placed bilaterally in an infraclavicular location with the patient under general anesthesia.
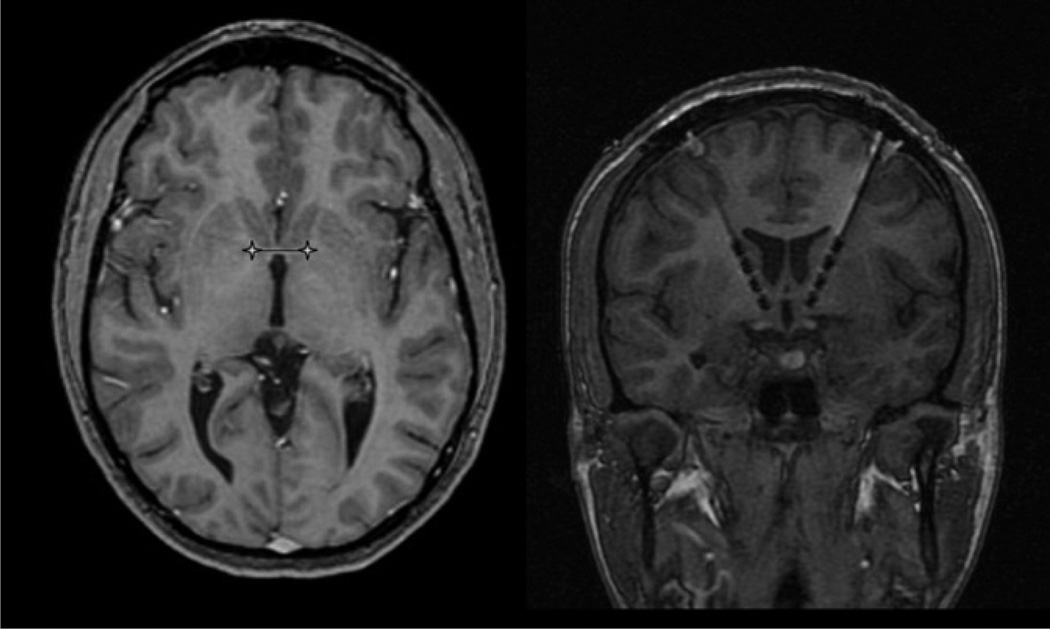
Figure 1.
Magnetic resonance images from a representative patient showing preoperative targeting (left) and postoperative deep brain stimulation (DBS) lead position (right). Image distortion due to artifact from the metal components of the DBS lead causes the size of the lead to appear larger than actual.
Intra-operative test stimulation was performed after lead implantation, with the patient awake and able to respond to questions. The goal of test stimulation was to identify contact locations that produced acute improvements in mood and reductions in anxiety, without significant adverse effects. Common observations during intra-operative stimulation included acute mood improvement, spontaneous smiling, reduced anxiety, and increased energy and awareness. Adverse effects included tachycardia, increased anxiety, a sense of warmth/sweating, speech perseveration, and facial motor effects, the latter of which were reported previously (29). Qualitative symptom improvement and/or lack of adverse effects to stimulation of at least one contact were felt to be indicative of proper targeting. Lead position was altered, on the basis of intra-operative testing, in only two cases. In one patient, one lead was retracted 1.5 mm after significant anxiety was noted during stimulation of the most ventral contacts. In another case, a device malfunction required a second lead to be implanted along the same trajectory as the original lead.
Stimulation Procedures
After a postoperative recovery phase (2–4 weeks), patients underwent outpatient stimulation parameter titration for several hours over multiple days, as described elsewhere for OCD (16). All electrodes were tested first in a monopolar configuration to determine the effects elicited at each contact, followed by assessment of selected bipolar contact pairs, with patients blind to stimulation settings. The total time required for these surveys was approximately 5–8 hours. Chronic stimulation parameters were selected on the basis of positive mood benefit and absence of adverse effects. Other effects were seen that helped inform optimal parameters. These included increased eye contact, facial expressiveness, and interpersonal spontaneity. Effects were very similar to those described earlier for intra-operative testing. Once appropriate settings were identified, subjects entered the chronic stimulation phase. During this phase, they returned at least monthly for rating assessments and device interrogation. Different individuals were responsible for programming changes and clinical ratings. The raters were aware of stimulation status (due to open-label nature) but not to particular stimulation settings. Modifications to stimulation settings, most commonly to amplitude or pulse width, were allowed during this phase to mitigate adverse effects and to optimize efficacy.
Outcome Measures
Multiple instruments were selected, on the basis of the pilot nature of this investigation, to characterize the effects on different domains of depressive symptoms as well as functional outcomes. These included the HDRS (primary measure), the Montgomery-Asberg Depression Rating Scale (MADRS), and the Global Assessment of Function Scale (GAF), which were collected and analyzed as continuous outcomes. The DBS treatment effects were also assessed categorically, with response defined as a 50% reduction of the depression rating scales from pre-operative baseline for each individual patient. Remission was defined as a score of 10 or lower for both the MADRS and HDRS. Response and remission rates were determined separately for each rating scale.
Cognition was assessed in multiple domains before implantation and at approximately 6 months of chronic DBS (30) (Supplement 1).
For all continuous outcomes (MADRS, HDRS, and GAF), repeated measures assessment was performed with generalized estimating equations (GEE). The GEE analyses use an exchangeable working correlation matrix, normal distribution, and identity link function. Model effects for treatment (df = 1) were evaluated conservatively with score statistics reported for Type 3 results and did not include imputed values. Treatment effects were estimated as differences, either in absolute scores or percentage of change, between baseline and the DBS treatment period. Analyses were performed with SAS (v9.1; SAS, Cary, North Carolina).
Data were collected at pre-surgical baseline and during monthly visits. Imputation of data was performed for major time points with last-observation carried forward only if data were available from the calendar month before a missing visit.
Results
Patient Demographic Data
Table 1 summarizes clinical features of the 15 patients. Fourteen met DSM-IV criteria for chronic or recurrent major depression, and 1 had recurrent bipolar depression. This patient was included because of reports of successful treatment of bipolar depression with lesion procedures targeting the same neural systems (31). The mean age at the onset of depressive illness was 25.3 (± 10.5) years, mean duration of illness was 21.0 (± 10.9) years, and mean age at implant was 46.3 (± 10.8) years. All patients were in a current depressive episode of at least 2 years’ duration, and all exceeded the number of treatment attempts required for study inclusion. In the current depressive episode, patients had an average of 6.1 (± 2.6) antidepressant trials and 6.1 (± 1.8) augmentation/combination trials. The average number of lifetime ECT treatments was 30.5 (± 26.3). Of the 15 patients, 13 had an adequate trial of ECT in the current depressive episode. Two patients were refractory to Vagus Nerve Stimulation. Personality disorders present on evaluation were not felt to represent an excessive risk of noncompliance or safety.
Table 1.
Patient Demographic Data
| Center/Patient | Gender | Age at Implant | Age at Onset | Primary Diagnosis | Secondary Diagnosis (Axis I and II) |
|---|---|---|---|---|---|
| Butler Hospital | |||||
| BH1 | M | 52 | 18 | MDD | Polysubstance dependence in remission |
| BH2 | M | 59 | 15 | Bipolar I, depressed | Polysubstance dependence in remission |
| BH3 | F | 51 | 33 | MDD | Dysthymia |
| BH4 | F | 51 | 41 | MDD | Borderline PD |
| BH5 | F | 43 | 38 | MDD | None |
| Cleveland Clinic | |||||
| CC1 | F | 37 | 20 | MDD | Borderline traits |
| CC2 | F | 50 | 22 | MDD | Mixed PD |
| CC3 | F | 27 | 16 | MDD | Dependent PD |
| CC4 | F | 53 | 38 | MDD | None |
| CC5 | M | 54 | 38 | MDD | Alcohol dependence in remission |
| CC6 | F | 53 | 34 | MDD | PTSD in remission |
| Mass General Hospital | |||||
| MGH1 | F | 33 | 14 | MDD | Agoraphobia w/o Panic; Anorexia Nervosa |
| MGH2 | F | 25 | 11 | MDD | Binge Eating disorder |
| MGH3 | F | 52 | 21 | MDD | Atypical Anxiety disorder, Mixed PD Traits |
| MGH4 | M | 55 | 20 | MDD | Panic disorder, Alcohol Dependence in Remission, PTSD |
BH, Butler Hospital; MDD, major depressive disorder; CC, Cleveland Clinic; PTSD, posttraumatic stress disorder; MGH, Massachusetts General Hospital; PD, personality disorder.
The longest follow-up period was 51 months with a mean last follow-up of 23.5 (± 14.9) months. The cumulative treatment period amounted to 353 patient months of experience with DBS. Antidepressant regimens were held stable over the first 6 months of stimulation in 11 of 15 patients. Of the other four patients, two had a change in their primary antidepressant (one to bupropion and one to duloxetine), another started lithium augmentation, and the fourth had a discontinuation of paroxetine.
Patient Outcomes
Continuous Measures of Response
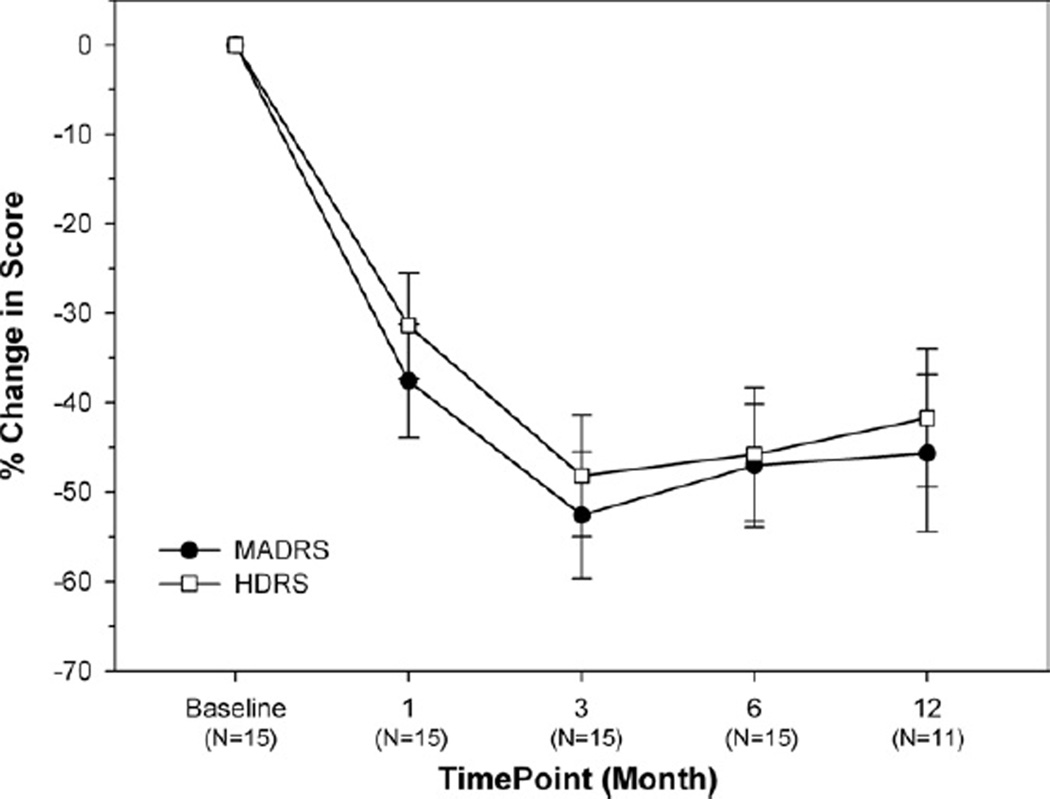
The mean pre-implantation MADRS score for the 15 subjects was 34.8 ± 7.3; mean baseline on the HDRS was 33.1 ± 5.5. Table 2 provides individual MADRS and HDRS scores over time. All patients were followed for at least 6 months after DBS began; the last follow-up time points vary, due to staggered enrollment. Figure 2 shows the mean percentage change in MADRS and HDRS scores for the cohort. Scores for both measures decreased with DBS treatment (repeated measures overall GEE for treatment: χ2 = 11.98, p = .0005 for the MADRS; and χ2 = 11.6, p = .0007 for the HDRS). A sustained reduction in scores was observed over time, with good agreement between these two measures. The maximum reduction on both scales (approximately 50%) was attained at 3 months and was sustained out to 12 months. A 16.6 ± 2.2-point decrease in average MADRS score was observed between baseline and treatment phase, which corresponded to an average reduction of 46.6%. Mean HDRS ratings decreased by 14.4 ± 2.0 (41.9%). Self-report measures, the Inventory for Depressive Symptoms-SR and Patient Global Impressions-Severity of Illness were assessed at 6 months. Inventory for Depressive Symptoms scores showed significant improvement from 47.47 at baseline to 33.27 at 6 months (p = .008). Patient Global Impressions-Severity of Illness scores improved from 5.27 to 3.87, which was also significant (p = .006). No significant difference in change was seen between patients with comorbid personality disorders and the rest of the subject group on the HDRS, MADRS, or GAF (p > .05). Even though subjects who had medication changes within the first 6 months experienced less overall benefit from DBS, this was not statistically significant (p > .05).
Table 2.
Montgomery-Asberg Depression Rating Scale and Hamilton-24 Depression Rating Scale Scores for Each Subject at Baseline and Multiple Time Points During DBS Treatment, Including LFU
| MADRS | HDRS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patienta | Baseline | Month 1 |
Month 3 |
Month 6 |
Month 12 |
LFU (Months) | Baseline | Month 1 |
Month 3 |
Month 6 |
Month 12 |
LFU (Months) |
| BH1 | 50 | 26 | 13 | 20 | 19 | 22 (51) | 37 | 25 | 11 | 14 | 17 | 24 (51) |
| BH2 | 29 | 16 | 17 | 32 | 30 | 5 (41) | 24 | 21 | 20 | 27 | 26 | 8 (41) |
| BH3 | 43 | 21 | 20 | 20 | 9 | 26 (40) | 41 | 21 | 16 | 24 | 13 | 25 (40) |
| BH4 | 28 | 26 | 15 | 18 | 14 | 11 (36) | 31 | 22 | 19 | 23 | 15 | 13 (36) |
| BH5 | 33 | 25 | 4 | 10 | 10 | 1 (36) | 24 | 23 | 8 | 14 | 13 | 1 (36) |
| CC1 | 37 | 30 | 37 | 23 | 19 | 36 (32) | 34 | 30 | 30 | 24 | 26 | 28 (32) |
| CC2 | 28 | 22 | 23 | 22 | 18 | 16 (25) | 27 | 31 | 26 | 25 | 22 | 17 (25) |
| CC3 | 32 | 23 | 23 | 22 | 27 | 19 (23) | 37 | 20 | 23 | 26 | 24 | 16 (23) |
| CC4 | 29 | 8 | 2 | 7 | 3 | 3 (16) | 34 | 11 | 2 | 3 | 4 | 2 (16) |
| CC5 | 30 | 26 | 18 | 21 | — | 15 (10) | 35 | 25 | 20 | 18 | — | 10 (10) |
| CC6 | 26 | 1 | 8 | 2 | — | 2 (6) | 26 | 8 | 15 | 2 | — | 2 (6) |
| MGH1 | 44 | 18 | 19 | 21 | 25 | 25 (12) | 40 | 20 | 15 | 20 | 22 | 22 (12) |
| MGH2 | 33 | 28 | 25 | 23 | 29 | 29 (11) | 35 | 24 | 25 | 15 | 22 | 22 (11) |
| MGH3 | 44 | 27 | 7 | 7 | — | 4 (8) | 37 | 29 | 6 | 7 | — | 4 (8) |
| MGH4 | 36 | 27 | 10 | 21 | — | 21 (6) | 34 | 23 | 13 | 20 | — | 20 (6) |
MADRS, Montgomery-Asberg Depression Rating Scale; HDRS, Hamilton-24 Depression Rating Scale; LFU, last follow-up; DBS, deep brain stimulation; other abbreviations as in Table 1.
During the DBS treatment period, the following patients had missed visits or no data was available: CC1: 3 and 6 months; MGH2: 12 months; and MGH3: 6 months. For these time points, MADRS and HDRS scores were imputed with prior calendar month values.
Figure 2.
Change in Montgomery-Asberg Depression Rating Scale (MADRS) and Hamilton Depression Rating Scale (HDRS) over time for the subject population.
Table 3 summarizes the mean values from baseline to last follow-up for depression severity (MADRS, HDRS) and global functioning (GAF). Preoperative baseline MADRS and HDRS scores indicated severe illness. Mean GAF scores were consistent with serious impairment. There was a notable reduction in depressive symptoms reported in this highly treatment-resistant cohort over the course of study. In parallel, mean GAF scores increased significantly for the group as a whole. After 3 months of stimulation, the mean GAF improved from 43.4 ± .7 (baseline) to 58.4 ± 2.2, with the same level of improvement maintained at 12 months. At last follow-up an 18.4-point improvement in average GAF score was observed, with a number of patients demonstrating marked improvement in function (range 50–95) compared with baseline (range 40–50) (χ2 = 10.93, p = .0009). Eight patients (53.3%) scored 60 or higher at last follow-up compared with no patients exhibiting this functional level at baseline. On average, a 12.9 ± 2.0-point increase in GAF scores was observed between baseline and treatment phase (χ2 = 10.93; p < .0009). Not unexpectedly, there was a strong trend between improvement on the GAF and reduction in MADRS (R2 = .67) and HDRS (R2 = .63) percent change scores assessed at last observation.
Table 3.
Summary of Depression and Functional Rating Scales Showing Mean (± SD) and Range at Baseline and Multiple Time Points During DBS Treatment, Including LFU
| Time Pointa | ||||||
|---|---|---|---|---|---|---|
| Measuresb | Baseline | Month 1 | Month 3 | Month 6 | Month 12 | LFU |
| MADRS | 34.8 (7.3) | 21.6 (8.0) | 16.1 (9.2) | 17.9 (7.9) | 18.5 (8.8) | 15.7 (11.0) |
| 26–50 | 1–30 | 2–37 | 2–32 | 3–30 | 1–36 | |
| HDRS | 33.1 (5.5) | 22.2 (6.2) | 16.6 (7.8) | 17.5 (8.2) | 18.5 (6.8) | 14.3 (9.3) |
| 24–41 | 8–31 | 2–30 | 2–27 | 4–26 | 1–28 | |
| GAF | 43.4 (2.8) | 53.1 (8.6) | 58.4 (8.4) | 55.5 (11.2) | 58.4 (12.9) | 61.8 (13.1) |
| 40–50 | 41–70 | 45–75 | 41–85 | 45–95 | 50–95 | |
GAF, Global Assessment of Function Scale; other abbreviations as in Table 2.
During the DBS treatment period, the following patients had missed visits or no data were available: CC1: 3 and 6 months; MGH2: 12 months; and MGH3: 6 months. For these time points, scores were imputed with prior calendar month values.
All 15 patients in the sample had 6 months of follow-up. At 12 months, scores are reported for 11 patients.
Categorical Measures of Response
After 1 month of active DBS, 4 of the 15 patients (26.7%) met the 50% or greater reduction in MADRS score criterion for clinical response, with 3 (20%) meeting the corresponding criterion on the HDRS. Response and remission rates on both scales were similar over time, although slightly lower for the HDRS. For the 15 patients, responder rates at 3 months, 6 months, and last follow-up were 53.3%, 46.7%, and 53.3%, respectively, on the MADRS and 46.7%, 40%, and 53.3%, respectively, on the HDRS. Figure 3 shows the relative difference in response over time between responders and nonresponders categorized at 6 months.
Figure 3.
Change in Montgomery-Asberg Depression Rating Scale (MADRS) and Hamilton Depression Rating Scale (HDRS) over 6 months in responders versus nonresponders.
The number of patients meeting the absolute criterion for remission, compared with the relative criterion for response, varied somewhat more between the two scales. Remission rates for the MADRS were 33.3% at 3 months, 26.6% at 6 months, and 33.3% at last observation. Corresponding remission rates assessed with the HDRS were 20% at 3 and 6 months and 40% at last follow-up.
Neuropsychological Testing
Results from detailed cognitive assessment were available for all but one CC and BH patients (n = 10) and revealed no deleterious effects on measures of general intellectual ability, language, processing speed, executive function, learning, and memory (Supplement 1).
Stimulation Parameters
The majority of patients had the most distal electrodes (0, 1, or both) programmed as a cathode (−) and the neurostimulator case or electrode 3 configured as the anode (+). Stimulation frequency was either 100 or 130 Hz, and pulse width was typically 90 or 210 µS. Frequency was lowered to 100 Hz in some patients as an attempt to improve battery longevity but did not seem to lessen response. Changes in pulse width were important to patient response. However, symptom improvement could be seen with an increase or decrease in pulse width, depending on the patient and contacts used. Thus, individual assessment of pulse width was important. Similarly, amplitude changes brought about very individual responses and required titration. Mean stimulation parameters at last follow-up were: amplitude 6.7 (± 1.8) volts; pulse width 113.0 (± 45.0) µS, and frequency 127.0 (± 11.1) Hz. Mean amplitudes were lower in the patients that met response criterion (6.2 V) than nonresponders (7.3 V), although not statistically significant (p = .23). Stimulation levels remained below the recommended charge density limit of 30 µC/cm2 in all patients. Battery replacements occurred in 12 patients, with an average time to replacement of 10.6 months (range 6–17 months). Often, batteries were replaced proactively to prevent any interruption of DBS therapy.
Adverse Events
Adverse events were categorized according to Food and Drug Administration definitions and classification methods (Title 21 CFR Part 312)1. Serious adverse events (SAEs) were classified as related to DBS therapy (surgical procedure, device, or stimulation); unrelated to DBS (e.g., underlying disorder); or unknown. There were a total of 25 SAEs reported in six patients over a period equal to 353 patient months of experience (Table 4). Four of these were identified as related to DBS and included one case of occipital pain associated with location of the extension and one case of a lead fracture, both of which required revision. There were two incidents of hypomania in the bipolar patient; both resolved after modification of stimulation parameters and medications. Three additional SAEs were categorized as unknown relatedness, including two syncopal episodes in one patient. The DBS was discontinued for a period of time during neurological and syncope evaluation, which was inconclusive. Seizures were considered a possible cause, anticonvulsants were started, and driving was restricted for 6 months. The patient continues on active DBS therapy and anticonvulsant medication. One incident of worsening of depression, accompanied by disinhibition and impulsivity, occurred in the bipolar patient and was addressed with changes in medication and stimulation parameters. The remaining 18 of the 25 SAEs were unrelated to DBS therapy.
Table 4.
Summary of SAEs
| Event | No. Patients (%) |
No. SAEs (%) |
Related |
|---|---|---|---|
| Surgical and Procedural | |||
| Pain or discomfort at incision/implant sites | 1 (6.7) | 1 (4.0) | Yes |
| Device | |||
| Lead fracture | 1 (6.7) | 1 (4.0) | Yes |
| Mood and Anxiety | |||
| Suicidality | 2 (13.3) | 11 (44.0) | No |
| Suicidality/increased depression | 2 (13.3) | 2 (8.0) | No |
| Hypomania | 1 (6.7) | 2 (8.0) | Yes |
| Mixed bipolar state | 1 (6.7) | 1 (4.0) | Unknown |
| Increased depression | 1 (6.7) | 1 (4.0) | No |
| Autonomic | |||
| Syncope (medication or DBS) | 1 (6.7) | 2 (8.0) | Unknown |
| Syncope (activity/dehydration) | 1 (6.7) | 1 (4.0) | No |
| Other | |||
| Cancer | 1 (6.7) | 1 (4.0) | No |
| Lung calcification | 1 (6.7) | 1 (4.0) | No |
| Shortness of breath | 1 (6.7) | 1 (4.0) | No |
| Total | 6 (40.0%) | 25 (100%) | — |
SAEs, serious adverse events; DBS, deep brain stimulation.
The most common non-serious adverse events were increased depression and/or suicidal ideation (compared with prior ratings, not with baseline), insomnia, and hypomania. Increased depression was noted on several occasions to be associated with cessation of stimulation due to neurostimulator battery depletion (four patients) or accidental deactivation (three patients). Hypomania was reported in two of the earliest patients (including the bipolar patient) and was resolved by adjustment of stimulation parameters.
Discussion
The results of this study suggest that DBS of the VC/VS can provide benefit in highly treatment-refractory patients with depression. Efficacy was demonstrated in both categorical measures of response and remission and by a significant, sustained improvement in mean depression measures. Although follow-up durations varied, at last observation, five patients met accepted MADRS criterion for remission and eight met accepted MADRS criterion for clinical response. Mean MADRS scores were reduced by 52.6% at 3 months, 47.0% at 6 months, 45.7% at 1 year, and 56.4% at last follow-up with similar results for the HDRS. Improvements were also noted in measures of global functioning. These symptomatic and functional improvements were seen despite the highly refractory illness that these patients experienced.
Despite the invasive nature of DBS treatment, patients tolerated both the surgical procedure and stimulation well. Acute changes in mood and anxiety were frequently seen during programming sessions. Undesirable changes were rapidly reversed through stimulation parameter changes. Interestingly, the quality or intensity of these acute observations did not necessarily predict how patients would do with chronic stimulation. No adverse effects on cognitive functioning were noted on the basis of an extensive battery of neuropsychological measures. Importantly, none of the adverse events resulted in removal of the DBS system or withdrawal from the study. All patients were continuing on DBS therapy at last reported follow-up.
Stimulation parameter titration was a time-consuming process. Similar to the experience with DBS in movement disorders (32), electrode configurations and stimulation parameters resulting in optimal response varied among patients. Testing a variety of different settings was therefore necessary to optimize response for individual patients. Variation in stimulation settings is expected and might arise from a variety of factors. These include slight differences in targeting on the basis of individual anatomy, variations in sensitivity to stimulation, and anatomical variation of fiber pathways. Consistent with results observed with DBS in OCD (28), higher amplitudes were used in nonresponders in an attempt to attain therapeutic benefit.
Stimulation amplitudes were higher than those used in the treatment of movement disorders. This is not surprising, given the different structure and tissue impedance profile of the mixed VC/VS target compared with gray matter nuclear targets typical of movement disorder DBS therapies. It should also be noted that the larger contacts of the VC/VS lead have twice the surface area of standard leads, resulting in charge densities comparable to those used in other DBS applications. These factors all contribute to the need for more frequent battery replacements in these patients. The use of rechargeable devices, common in other neurostimulation therapies, will enhance acceptability.
Close monitoring of patients is a significant consideration with DBS therapies. Implanted stimulators can be turned off accidentally (e.g., theft detectors) or batteries can become depleted before replacement surgery. Depressive symptoms returned quickly in some patients when this occurred, resulting in a situation that required intervention. Even though symptoms did not typically worsen beyond preoperative levels, patients that had improved felt quite distressed by depressive symptom return. This risk during DBS treatment can be mitigated by the use of available devices that allow patients to monitor neurostimulator status.
A single bipolar patient, who experienced only one previous manic episode, was included in the study. This patient’s clinical course was the most variable. Although the patient had periods of depressive symptom improvement lasting weeks—not previously achieved after aggressive treatment including bilateral ECT and VNS—these were followed by hypomanic episodes. Hypomania reversed rapidly when stimulation was stopped. Another patient (MGH3) was noted to have had manic-like symptoms over 10 years before study enrollment. These symptoms occurred with the initiation of antidepressant treatment and quickly resolved. Subsequent antidepressant treatment failed to elicit this same response. This patient achieved stable remission with DBS. Although caution is indicated in interpreting these observations, nevertheless, the potential for DBS-induced affective instability in Bipolar I patients merits particular attention. Other patient variables such as personality or anxiety disorders might also influence patient response. The small number of subjects in this study precludes definitive evaluation of this. However, no significant differences were noted in patients with or without comorbid personality disorders.
This study had several limitations in addition to patient variables. These include a variable duration of patient follow-up and an open-label design. Although there was a wide range of follow-up periods, all patients had a minimum of 6 months of active stimulation and over two-thirds had 1 year of follow-up. Documenting long-term outcomes of DBS is especially important in this patient population. There could have been an effect of medication changes within the first 6 months in four subjects. Not surprisingly, these changes occurred in subjects with less overall improvement, although this was not statistically significant. An argument could be made to report only those patients without medication changes for a defined time period. This would eliminate these influences as possible confounding factors. However, this would only serve to improve the results, whereas our intention is to provide all data in order to inform future study design.
Due to the open-label nature of this study, the possible influence of rater bias and placebo response cannot be ruled out. The rate of response to placebo cannot be determined, but is expected to be low for several reasons. Patients enrolled had already undergone numerous interventions for their illness, including ECT. Individual treatment history far exceeded the stringent entry criteria. It is plausible that this level of refractoriness would reduce placebo response compared with less-refractory patients. The actual placebo response rate in such patients is unknown, in part because they are overwhelmingly excluded from treatment trials. In addition, some patients with minimal acute stimulation benefit eventually reached responder status, whereas others with positive acute changes ultimately were nonresponders, inconsistent with a placebo effect.
Data on placebo response are available from a study of VNS in a group of MDD patients with highly refractory illness (33). Only 10% of patients responded to sham stimulation over a 10-week period. On average, these subjects had far fewer medication trials than our patients and were not required to have undergone ECT. It seems reasonable to expect that our patient cohort would have a placebo response rate at or below what was observed in that study.
Additional observations during the study, including the sustained response in many patients for periods of 1 year and greater, also support our conclusion of benefit from active stimulation. When patients were blind to the presence, absence, or type of stimulation during initial titration, individual responses were quite consistent to repeated testing of the same parameter settings over several days. Second, during chronic DBS, patients noted symptom worsening when stimulation was interrupted by either accidental shut-off or battery expiration, even though they were unaware of device status. These observations suggest that active DBS was responsible for the sustained symptomatic and functional improvements seen.
Another study of DBS for depression reported results from six patients stimulated in the subgenual cingulate region (21). Although it is difficult to draw direct comparisons, results from that 6-month pilot study and those reported here seem qualitatively similar. Experience with DBS for movement disorders has shown that it is possible to treat a variety of related disorders through stimulation of different nodes of the cortico-striatal-thalamic-cortical circuit controlling motor function (32). It is also possible that multiple DBS targets might similarly exist within the networks influencing mood and affective state. Neuroimaging studies of patients treated with VC/VS stimulation for OCD have demonstrated modulation of neural structures within this network, including orbital frontal cortex, basal ganglia, and subgenual cingulate (34,35). These results are consistent with neuroanatomical studies in nonhuman primates, which suggest that fiber pathways connecting medial and orbital frontal cortex to thalamus coalesce within this region (11,36). In addition, the VS in this area has complex architecture and includes structures such as the bed nucleus of the stria terminalis and the nucleus accumbens, regions believed to be involved with stress-related and reward-motivation components of depression (37,38).
Finally, Schlaepfer et al. (39) have reported improvement in depressive symptoms and anhedonia with DBS of the nucleus accumbens. The stereotactic target in their study is close in location to ours. However, the more dorsal contacts are much less proximate due to differences in surgical trajectory and model of DBS lead used. Long-term benefit in these patients is not yet known, and it remains unclear whether acute benefit results in continued response.
Overall, the results of this multicenter investigation of DBS of the VC/VS region provide encouraging preliminary evidence of a sustained therapeutic effect in an otherwise highly treatment-resistant population. These results are consistent with the findings of prior studies of DBS in treatment-resistant OCD and depression and provide additional support for the therapeutic potential of DBS in individuals suffering from these chronic, severe psychiatric disorders unresponsive to conventional treatments.
Acknowledgments
This study was funded by Medtronic. We wish to thank: Bart Nuttin, Volker Sturm, and Robert Coffey for consultation on neurosurgical issues; Loes Gabriels for consultation on psychiatric issues; Patty St. Marie, Natalie Sykuta, Jenna Stump, and Rouba Youssef for help with data management; Rees Cosgrove, Paul Summergrad, and Naomi Simon for data and safety monitoring-board roles; and Richard Marsland and Steven Zella for assistance in patient care.
Dr. Malone has received research funding and is a consultant to Medtronic. He has received speaking honoraria from Lilly and Bristol-Myers-Squibb. Dr. Dougherty has received research support from Medtronic, Cyberonics, Northstar Neuroscience, Pfizer, Lilly, Forest, and McNeil. He has received honoraria from Cyberonics and McNeil. Dr. Rezai holds the following financial interests in Intelect Medical: Founder and Director, Patent Holder, Chair of the Scientific Advisory Board, and Chief Scientific Officer. He has also received research funding and is a consultant to Medtronic as well as a stockholder of Surgivision. Dr. Carpenter has received research support from Pfizer, UCB Pharma, and Sepracor; consulting fees from Abbott, Bristol-Myers-Squibb, Cyberonics, Medtronic, Novartis, Pfizer, Sepracor, and Wyeth; honoraria from AstraZeneca, Cyberonics, Pfizer, and Wyeth; and travel support from Neuronetics. Dr. Friehs has received research funding from Medtronic. Dr. Eskandar has received research funding from Medtronic. Dr. Rauch has received research funding from Cephalon, Cyberonics, and Medtronic; honoraria from Cyberonics, Neurogen, Novartis, and Sepracor; and consulting fees from Novartis. Dr. Rasmussen has received research funding and consulting fees from Medtronic. Dr. Machado has received research funding from Medtronic and is a shareholder in Intelect Medical. Dr. Kubu has received research funding from Medtronic. Dr. Tyrka has received grant support from Cephalon, Cyberonics, Medtronic, Pfizer, Sepracor, and UCB Pharma. Dr. Price has received research support from Sepracor, Pfizer, and UCB Pharma; consulting fees from Bio Vid, Gerson Lehrman, Oxford Univ Press, Springer, and Wiley; and honoraria from AstraZeneca. Drs. Stypulkowski, Giftakis, and Rise are employees and shareholders of Medtronic. Dr. Salloway has received research funding from Cephalon, Elan, Neurochem, and Voyager; research support and honoraria from Athena Diagnostics, Eisai, Forest, Johnson & Johnson, Myriad, and Pfizer; and consulted to Merck and Sanofi-Aventis. Dr. Greenberg has received research funding and honoraria for presentations from Medtronic. He is a consultant for Northstar Neuroscience and Jazz Pharmaceuticals and is on the latter’s Speakers’ Bureau.
Footnotes
An adverse event (AE) is defined as any unfavorable and unintended sign including an abnormal laboratory finding, symptom or disease associated with the use of a medical treatment or procedure, regardless of whether it is considered related to the medical treatment or procedure, that occurs during the course of the study. A serious adverse event is any untoward medical occurrence that results in death, is life threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability/incapacity, or is a congenital anomaly/birth defect.
Dr. Malloy reports no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Murray C, Lopez AD. The Global Burden of Disease. Cambridge, Massachusetts: Harvard University Press; 1996. [Google Scholar]
- 2.Dunner DL, Rush AJ, Russell JM, Burke M, Woodard S, Wingard P, Allen J. Prospective, long-term, multicenter study of the naturalistic outcomes of patients with treatment-resistant depression. J Clin Psychiatry. 2006;67:688–695. doi: 10.4088/jcp.v67n0501. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 4.Prudic J, Sackeim HA, Devanand DP. Medication resistance and clinical response to electroconvulsive therapy. Psychiatry Res. 1990;31:287–296. doi: 10.1016/0165-1781(90)90098-p. [DOI] [PubMed] [Google Scholar]
- 5.Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM, et al. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: A randomized controlled trial. JAMA. 2001;285:1299–1307. doi: 10.1001/jama.285.10.1299. [DOI] [PubMed] [Google Scholar]
- 6.Dougherty DD, Weiss AP, Cosgrove GR, Alpert NM, Cassem EH, Nierenberg AA, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J Neurosurg. 2003;99:1010–1017. doi: 10.3171/jns.2003.99.6.1010. [DOI] [PubMed] [Google Scholar]
- 7.Herner T. Treatment of mental disorders with frontal stereotactic thermo-lesions: A follow-up of 116 cases. Acta Psychiatr Scand Suppl. 1961;36:941–967. [Google Scholar]
- 8.Strom-Olsen R, Carlisle S. Bi-frontal stereotactic tractotomy. A follow-up study of its effects on 210 patients. Br J Psychiatry. 1971;118:141–154. doi: 10.1192/bjp.118.543.141. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell-Heggs N, Kelly D, Richardson A. Stereotactic limbic leucotomy: A follow-up at 16 months. Br J Psychiatry. 1976;128:226–240. doi: 10.1192/bjp.128.3.226. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg BD, Price LH, Rauch SL, Friehs G, Noren G, Malone D, et al. Neurosurgery for intractable obsessive-compulsive disorder and depression: Critical issues. Neurosurg Clin N Am. 2003;14:199–212. doi: 10.1016/s1042-3680(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 11.Rauch SL. Neuroimaging and neurocircuitry models pertaining to the neurosurgical treatment of psychiatric disorders. Neurosurg ClinN Am. 2003;14:213–223. vii–viii. doi: 10.1016/s1042-3680(02)00114-6. [DOI] [PubMed] [Google Scholar]
- 12.Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry Suppl. 1998:26–37. [PubMed] [Google Scholar]
- 13.Baxter LR, Jr, Schwartz JM, Mazziotta JC, Phelps ME, Pahl JJ, Guze BH, Fairbanks L. Cerebral glucose metabolic rates in nondepressed patients with obsessive-compulsive disorder. AmJ Psychiatry. 1988;145:1560–1563. doi: 10.1176/ajp.145.12.1560. [DOI] [PubMed] [Google Scholar]
- 14.Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- 15.Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354:1526. doi: 10.1016/S0140-6736(99)02376-4. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 17.Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005;57:510–516. doi: 10.1016/j.biopsych.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 18.Aouizerate B, Cuny E, Martin-Guehl C, Guehl D, Amieva H, Benazzouz A, et al. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J Neurosurg. 2004;101:682–686. doi: 10.3171/jns.2004.101.4.0682. [DOI] [PubMed] [Google Scholar]
- 19.Anderson D, Ahmed A. Treatment of patients with intractable obsessive-compulsive disorder with anterior capsular stimulation. Case report. J Neurosurg. 2003;98:1104–1108. doi: 10.3171/jns.2003.98.5.1104. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen S, Greenberg B, Mindus P, Friehs G, Noren G. Neurosurgical approaches to intractable obsessive-compulsive disorder. CNS Spectr. 2000;5:23–34. doi: 10.1017/s1092852900021891. [DOI] [PubMed] [Google Scholar]
- 21.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 23.Laitinen LV. Stereotactic lesions in the knee of the corpus callosum in the treatment of emotional disorders. Lancet. 1972;1:472–475. doi: 10.1016/s0140-6736(72)90124-9. [DOI] [PubMed] [Google Scholar]
- 24.Laitinen LV. Emotional responses to subcortical electrical stimulation in psychiatric patients. Clin Neurol Neurosurg. 1979;81:148–157. doi: 10.1016/0303-8467(79)90002-7. [DOI] [PubMed] [Google Scholar]
- 25.OCD-DBS Collaborative Group. Deep brain stimulation for psychiatric disorders. Neurosurgery. 2002;51:519. [PubMed] [Google Scholar]
- 26.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition. New York: New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 27.Hamilton M. Rating Scale for Depression. J Neurol Neurosurg Psychiatry. 1960;23:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenberg BD, Gabriels LA, Malone DA, Jr, Rezai AR, Friehs GM, Okun MS, et al. Deep brain stimulation of the ventral capsule/ventral striatum for obsessive-compulsive disorder: Worldwide experience. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.55. [published online ahead of print May 20]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okun MS, Bowers D, Springer U, Shapira NA, Malone D, Rezai AR, et al. What’s in a “smile?” Intra-operative observations of contralateral smiles induced by deep brain stimulation. Neurocase. 2004;10:271–279. doi: 10.1080/13554790490507632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubu CS, Greenberg B, Malone D, Rasmussen S, Friehs G, Machado A, Rezai A. Cognitive effects of DBS in the ventral striatum in patients with severe major depression and obsessive-compulsive disorder. Biol Psychiatry. 2008;63:232S. [Google Scholar]
- 31.Christmas D, Matthews K, Eljamel MS. Neurosurgery for mental disorder. Br J Psychiatry. 2004;185:173–174. doi: 10.1192/bjp.185.2.173-a. author reply 174. [DOI] [PubMed] [Google Scholar]
- 32.Halpern C, Hurtig H, Jaggi J, Grossman M, Won M, Baltuch G. Deep brain stimulation in neurologic disorders. Parkinsonism Relat Disord. 2007;13:1–16. doi: 10.1016/j.parkreldis.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, et al. Vagus nerve stimulation for treatment-resistant depression:A randomized, controlled acute phase trial. Biol Psychiatry. 2005;58:347–354. doi: 10.1016/j.biopsych.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 34.Rauch SL, Dougherty DD, Malone D, Rezai A, Friehs G, Fischman AJ, et al. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive-compulsive disorder. J Neurosurg. 2006;104:558–565. doi: 10.3171/jns.2006.104.4.558. [DOI] [PubMed] [Google Scholar]
- 35.Van Laere K, Nuttin B, Gabriels L, Dupont P, Rasmussen S, Greenberg BD, Cosyns P. Metabolic imaging of anterior capsular stimulation in refractory obsessive-compulsive disorder: A key role for the subgenual anterior cingulate and ventral striatum. J Nucl Med. 2006;47:740–747. [PubMed] [Google Scholar]
- 36.Haber SN, McFarland NR. The concept of the ventral striatum in nonhuman primates. Ann N Y Acad Sci. 1999;877:33–48. doi: 10.1111/j.1749-6632.1999.tb09259.x. [DOI] [PubMed] [Google Scholar]
- 37.Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamicpituitary-adrenal axis. Brain Res Brain Res Rev. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 39.Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]