Abstract
Context
Acute ST-segment elevation myocardial infarction (STEMI) is a leading cause of morbidity and mortality. In experimental models of MI, erythropoietin reduces infarct size and improves left ventricular (LV) function.
Objective
To evaluate the safety and efficacy of a single intravenous bolus of epoetin alfa in patients with STEMI.
Design, Setting, and Patients
Prospective, randomized, double-blind, placebo-controlled trial with a dose-escalation safety phase and a single-dose (60,000 units of epoetin alfa) efficacy phase involving 222 patients with STEMI who underwent successful percutaneous coronary intervention (PCI) as a primary or rescue reperfusion strategy.
Intervention
Participants were randomly assigned to treatment with intravenous epoetin alfa or matching saline placebo administered within 4 hours of reperfusion.
Main Outcome Measure
Infarct size, expressed as a percentage of LV mass, assessed by cardiac magnetic resonance (CMR) imaging 2–6 days after study medication administration.
Results
In the efficacy cohort (n=138), infarct size did not differ between groups at either 2–6 days (15.8±10.3 vs. 15.0±10.0, P=.666) or 12±2 weeks (10.6±8.6 vs. 10.4±7.6, P=.886). Left ventricular ejection fraction also did not differ between groups at either the early (48.2±9.1 vs. 48.9±8.7, P=.671) or late (52.5±9.3 vs. 52.0±8.8, P=.760) timepoints. In pre-specified analyses of patients aged ≥70 years (n=21), mean infarct size within the first week was larger in the epoetin alfa arm than in the placebo group (19.9±9.9 vs.11.7±7.2, P=.026). Patients who received epoetin alfa had a higher incidence of the composite endpoint of death, myocardial infarction, stroke, or stent thrombosis (4.0% vs. 0.0%, P=.042), and a higher incidence of serious adverse events (20.0% vs. 10.3%, P=.052).
Conclusions
In STEMI patients successfully reperfused with primary or rescue PCI, a single intravenous bolus of epoetin alfa did not reduce infarct size and was associated with higher rates of adverse cardiovascular events. Furthermore, it may be associated with increased infarct size in older patients.
Trial Registration
clinicaltrials.gov identifier NCT00378352.
Keywords: Myocardial infarction, MRI, Randomized controlled trial, Erythropoietin, Recombinant
INTRODUCTION
Despite advances in the management of ST-segment elevation myocardial infarction (STEMI), it remains a significant cause of morbidity, mortality, and disability,1,2 particularly among older persons.3 Patients who survive STEMI are at risk for developing infarct expansion (the process of myocardial thinning and infarct zone dilation that begins soon after coronary occlusion)4 and left ventricular (LV) remodeling (the topographical and functional changes in the infarct zone and the non-infarcted myocardium).5 Both are strongly associated with heart failure and death.6 Risk factors for infarct expansion and LV remodeling include infarct size, extent of apoptosis, anterior location of the infarction,7 severe microvascular obstruction,8 and older age.9 Given the global burden of ischemic heart disease and heart failure, therapies that limit infarct size and attenuate or reverse LV remodeling are needed.
Erythropoietin is a 165 amino acid glycoprotein hormone whose production and secretion are regulated by tissue oxygen levels. Beyond its effects on red cell production, erythropoietin exhibits pleiotropic effects in cells and tissues, including stimulation of angiogenesis and protection against apoptosis.10 Further, erythropoietin receptors have been identified on endothelial cells and cardiomyocytes.11 Preclinical studies have shown that erythropoietin plays a cardioprotective role in various experimental models of myocardial ischemia and ischemia-reperfusion.12–17 In these investigations, erythropoietin was associated with significant reductions in infarct size and improvements in LV function that were partly attributed to its anti-apoptotic and angiogenic properties.
To determine whether erythropoietin has similar cardioprotective effects in the clinical setting, we performed the Reduction of Infarct Expansion and Ventricular Remodeling with Erythropoietin After Large Myocardial Infarction (REVEAL) trial to evaluate the safety and effect on infarct size of a single intravenous bolus of recombinant human erythropoietin (epoetin alfa) in patients with STEMI who have undergone successful primary or rescue percutaneous coronary intervention (PCI).
METHODS
REVEAL was a multicenter, phase II, randomized, double-blind, placebo-controlled clinical trial that evaluated the safety and efficacy of a single intravenous bolus of epoetin alfa in STEMI patients.
Study Design
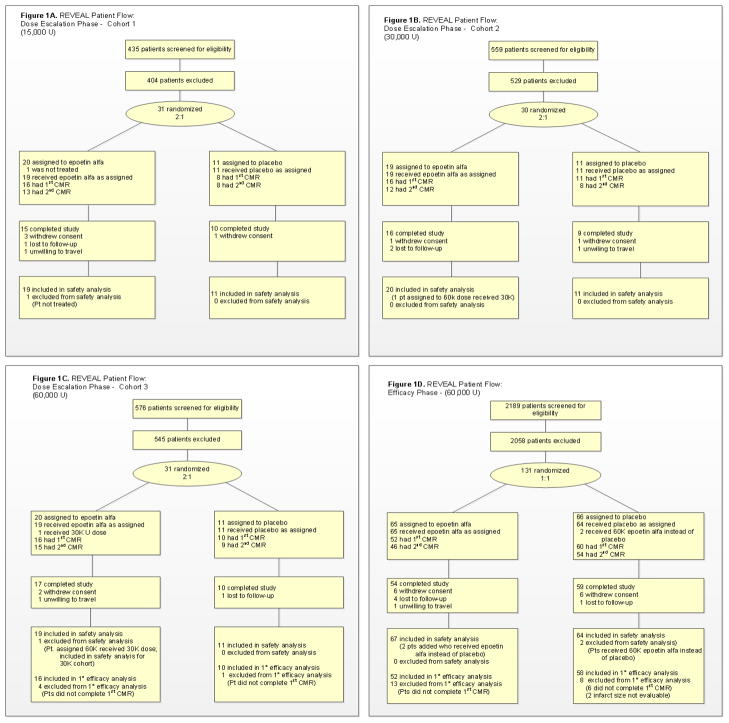
The design and rationale of REVEAL have been previously published.18 Briefly, the study consisted of a dose-escalation phase and an efficacy phase (Figure 1). During the dose-escalation phase, three doses of epoetin alfa were sequentially evaluated: 15,000 (15k), 30,000 (30k), and 60,000 (60k) units (U). These doses were chosen with guidance from the FDA to balance safety concerns arising from preclinical studies that used relatively high doses of erythropoietin. Patients were randomly allocated in a 2:1 ratio to each dose of epoetin alfa or placebo, respectively. Dose escalation was guided by an independent data and safety monitoring board (DSMB), who reviewed clinical and safety data at two timepoints for each dose cohort.18 By study design, the highest of the three doses of epoetin alfa deemed acceptable by the DSMB was used in the efficacy phase, in which participants were randomly allocated in a 1:1 ratio to epoetin alfa or placebo.
Figure 1.
REVEAL study flow.
Eligibility Criteria
Study eligibility criteria are listed in Supplementary Table 1. Briefly, patients were eligible if they had acute STEMI with TIMI flow grade 0–1 in a major epicardial artery or large branch vessel during index angiography and underwent successful primary or rescue PCI within 8 hours of onset of ischemic symptoms. To minimize confounding of infarct-size measurement, patients with a history of LV dysfunction (ejection fraction ≤50%), MI, coronary artery bypass grafting (CABG), or revascularization in the territory of the culprit artery were excluded.
Randomization
Eligible patients who provided written informed consent were randomly assigned to epoetin alfa or matching placebo according to the allocation ratio for each dosing cohort. Randomization was performed with a fixed-block randomization scheme using a Web-based application (WebEZ) fully integrated with drug supply management information. Randomization was stratified according to age (<70 or ≥70 years) and infarct location (left anterior descending [LAD] or non-LAD coronary artery).
Study Medication
Study medication (epoetin alfa or matching saline placebo) was administered within 4 hours of successful primary or rescue PCI, defined as time of restoration of ≥TIMI 2 flow in the IRA. During the initial phase of REVEAL, epoetin alfa and matching placebo were provided free of charge by Centocor Ortho Biotech Clinical Affairs (Bridgewater, NJ). However, during the efficacy phase, the company elected to cease providing study medication, and the study sponsor (the National Institute on Aging [NIA]) purchased active study medication from Centocor Ortho Biotech and contracted with Florida Biologix (Alachua, FL) to manufacture matching placebo. All other treatments, including PCI techniques, were at the discretion of the treating physicians, who were encouraged to adhere to guideline recommendations for the management of STEMI patients.19
Cardiac Magnetic Resonance
Patients underwent one cardiac magnetic resonance (CMR) scan 2–6 days after study medication administration and another at 12±2 weeks. Each CMR examination consisted of (1) a cine-CMR for assessment of LV volumes (end-systolic volume [LVESV] and end-diastolic volume [LVEDV]), LV mass (LVM), and LV function quantified by ejection fraction (LVEF); and (2) a delayed contrast-enhanced CMR for assessment of infarct size. LVESV, LVEDV, and LVM were indexed to body surface area to yield LVESVi, LVEDVi and LVMi, respectively. De-identified CMR studies were analyzed at a central core laboratory (Duke Cardiovascular Magnetic Resonance Center, Durham, NC) by investigators masked to treatment assignment and clinical outcomes. Details of the imaging protocol have been published.18
Primary and Secondary Endpoints
The primary efficacy endpoint of the study was infarct size in the territory of the IRA, expressed as percentage of LV mass and measured by CMR 2–6 days (48–144 hours) after study medication administration. Secondary endpoints included infarct size, LV remodeling parameters (LVESVi, LVEDVi, and LVMi) and LV function (LVEF) at 12±2 weeks. We also examined LVESVi, LVEDVi, LVMi, and LVEF measured at 2–6 days.
Safety endpoints included vital signs, hemoglobin, reticulocyte count, markers of cardiac injury, and clinical events including death, recurrent MI, unplanned PCI, arterial thrombotic events (stent thrombosis), venous thrombotic events (deep venous thrombus; pulmonary embolus), heart failure, and neurologic events (stroke; transient ischemic attack).
Statistical Analysis
The primary comparison was infarct size measured by CMR at 2–6 days after study medication administration between participants who received the highest dose of epoetin alfa deemed safe by the DSMB and those who received placebo. The sample size for the first three patient cohorts in the dose-escalation phase was fixed (n=30/cohort), as agreed upon by study investigators and the FDA. Because the distribution of infarct size data is consistently non-normal, the efficacy phase sample size was determined with an empirical approach that used available infarct size data (mean±SD 19.5±10.6) from studies carefully matched to the conditions of the present investigation. Power values were estimated by simulations, which suggested that a sample size of 55 subjects/group in the efficacy phase, together with 30 subjects from the corresponding dose-escalation phase, would provide >80% power to detect a difference of ≥20% in infarct size between treatment groups. Thus, REVEAL continued to accrue patients until 110 participants who received study medication in the efficacy phase had completed their first CMR scan.
Efficacy data were analyzed on a modified intent-to-treat basis, which excluded one subject who was randomized but did not receive study medication. The efficacy cohort comprised all patients from the efficacy phase plus all patients from the 60k-cohort of the dose-escalation phase who received study medication and underwent CMR 2–6 days later.
Baseline differences between the epoetin alfa and placebo groups were evaluated using the Fisher mid-P or Fisher exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. The primary outcome variable, infarct size expressed as percentage of LV mass 2–6 days after study medication administration, was compared between groups using a log-rank test,20 both without and with adjustment for age group (<70 vs. ≥70 years), IRA location (LAD vs. non-LAD), and enrollment phase. Between-groups comparisons for all other cardiac variables assessed by CMR were performed with ANOVA for unadjusted analyses and with ANCOVA for adjusted analyses. The adjusted analyses controlled for age group, IRA location, and enrollment phase. Adjustment for enrollment phase was performed to control for implicit differences that may exist between patients recruited in the dose-escalation phase and patients recruited in the efficacy phase. For cardiac variables measured at 12±2 weeks, the adjusted models also controlled for values of the same variables obtained on earlier CMR (2–6 days after study medication administration).
Primary and secondary endpoints were further analyzed within the prespecified subgroups of patient age (<70 vs. ≥70 years) and IRA location (LAD vs. non-LAD).
Safety data were analyzed on an as-treated basis. The safety cohort comprised all patients who received study medication. Composite safety endpoints were added to the analysis plan at the suggestion of the DSMB and were compared using the Fisher mid-P test.
One prespecified interim test of efficacy was performed, using the Haybittle-Peto rule.21 Due to rounding, the interim analysis did not result in a penalty for the final alpha level.
A two-sided alpha level of 0.05 was considered statistically significant. P values were not adjusted for multiple comparisons. All analyses were performed in SAS version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Between October of 2006 and November of 2009, 222 participants at 22 U.S. sites were randomized and received study medication. Of these, 189 had a CMR scan performed within 2–6 days after study drug administration: 24 in the 15k cohort; 27 in the 30k cohort; and 138 in the 60k efficacy cohort. Most participants in the efficacy cohort (n=124; 89.9%) underwent follow-up CMR at 12±2 weeks.
The respective baseline clinical characteristics of the total and efficacy cohorts, stratified according to treatment assignment, are summarized in Table 1. The active medication and placebo groups were well matched, although the former had a significantly lower prevalence of diabetes mellitus, less use of rescue PCI, and a longer time from randomization until study drug administration.
Table 1.
Baseline Characteristics of Study Patients
| Total Cohort (n=222)
|
Efficacy Cohort (n=138)
|
|||
|---|---|---|---|---|
| Epoetin alfa | Placebo | Epoetin alfa | Placebo | |
| Demographics | ||||
|
| ||||
| n (%)a | 123 (55.4) | 99 (44.6) | 68 (49.3) | 70 (50.7) |
|
| ||||
| Age, y | 56.8 (12.4) | 58.8 (12.5) | 55.6 (12.6) | 57.4 (11.9) |
|
| ||||
| Female sex, n (%) | 23 (18.7) | 21 (21.2) | 7 (10.3) | 14 (20.0) |
|
| ||||
| White race, n (%) | 104 (84.6) | 81 (81.8) | 59 (86.8) | 61 (87.1) |
|
| ||||
| Weight, kg | 89.9 (18.6)b | 87.4 (16.0) | 89.3 (16.8) | 87.1 (15.3) |
|
| ||||
| Height, cm | 174.4 (9.2)b | 173.2 (9.7) | 174.6 (8.3) | 173.7 (9.5) |
|
| ||||
| Comorbid conditions | ||||
|
| ||||
| Diabetes, n (%) | 14 (11.4) | 25 (25.3)c | 6 (8.8) | 17 (24.3)c |
|
| ||||
| Hypertension, n (%) | 58 (47.2) | 53 (53.5) | 28 (41.2) | 34 (48.6) |
|
| ||||
| Hyperlipidemia, n (%) | 50 (40.7) | 43 (44.8) | 24 (35.3) | 30 (43.5) |
|
| ||||
| Current smoker | 47 (38.8)d | 42 (42.4) | 27 (40.3)e | 32 (45.7) |
|
| ||||
| Baseline medications, n (%) | ||||
|
| ||||
| Aspirin | 110 (90.2) | 93 (93.9) | 62 (91.2) | 64 (91.4) |
|
| ||||
| Clopidogrel | 118 (96.7) | 94 (95.9) | 66 (97.1) | 66 (94.3) |
|
| ||||
| GP IIb/IIIa inhibitor | 88 (72.7) | 71 (71.7) | 46 (67.6) | 45 (64.3) |
|
| ||||
| Heparinf | 108 (87.9) | 86 (86.9) | 60 (88.2) | 60 (85.7) |
|
| ||||
| Thrombolytic therapy | 9 (7.4) | 8 (8.1) | 3 (4.4) | 6 (8.6) |
|
| ||||
| Patient subgroups | ||||
|
| ||||
| Age category | ||||
|
| ||||
| <70 y, n (%) | 103 (83.7) | 81 (81.8) | 57 (83.8) | 59 (84.3) |
|
| ||||
| ≥70 y, n (%) | 20 (16.3) | 18 (18.2) | 11 (16.2) | 11 (15.7) |
|
| ||||
| Infarct-related artery | ||||
|
| ||||
| Non-anterior, n (%) | 89 (72.4) | 72 (72.7) | 48 (70.6) | 51 (72.9) |
|
| ||||
| Anterior, n (%) | 34 (27.6) | 27 (27.3) | 20 (29.4) | 19 (27.1) |
|
| ||||
| Clinical characteristics | ||||
|
| ||||
| Type of PCI | ||||
|
| ||||
| Primary, n (%) | 110 (89.4) | 82 (82.8)c | 64 (94.1) | 55 (78.6)c |
|
| ||||
| Rescue, n (%) | 13 (10.6) | 17 (17.2) | 4 (5.9) | 15 (21.4) |
|
| ||||
| Killip class I, n (%)g | 115 (100) | 91 (94.8) c | 62 (100) | 64 (95.5) |
|
| ||||
| Heart rate, BPM | 77.4 (16.1) | 76.5 (13.6) | 77.0 (14.5) | 75.6 (13.2) |
|
| ||||
| Systolic BP, mm HG | 129.5 (17.9) | 126.5(18.8) | 132.8 (18.1) | 126.7 (19.3) |
|
| ||||
| Diastolic BP, mm HG | 78.4 (12.7) | 76.0 (13.7) | 79.9 (12.2) | 76.8 (13.4) |
|
| ||||
| Time from symptom onset to restoration of TIMI 2–3 flow, min | 207.5 (106.8) | 208.3 (105.7) | 210.9 (98.3) | 201.9 (111.2) |
|
| ||||
| Time from TIMI flow restoration to study drug administration, min | 176.3 (77.3) | 183.0 (74.1) | 172.4 (75.0) | 175.5 (75.3) |
|
| ||||
| Time from randomization to study drug administration, min | 47.5 (48.0)h | 39.9 (32.9)c | 47.9 (32.6)i | 39.6 (34.6)c |
Continuous variables are given as mean (SD). Abbreviations: PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
Values given according to treatment assignment.
Total n=122.
P<.05 for between-group comparison.
Total n=121.
Total n=67.
Includes both unfractionated and low-molecular-weight heparin.
Total cohort: epoetin alfa, total n=115; placebo, total n=96. Efficacy cohort: epoetin alfa, total n=62; placebo, total n=67.
Total n=122.
Total n=67.
Efficacy Analyses
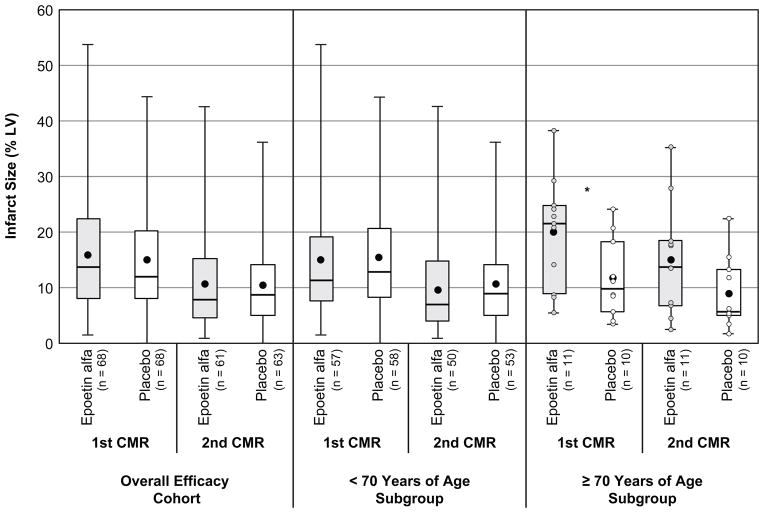
Cardiac variables assessed by CMR in the efficacy cohort are shown in Table 2. The primary study endpoint (infarct size at 2–6 days after study medication administration) did not differ between the epoetin alfa and placebo groups in either the unadjusted (Figure 2A) or adjusted analysis, which controlled for age group, IRA location, and enrollment phase.
Table 2.
Infarct Size, Infarct Mass, LV Ejection Fraction, LV Volumes, and LV Mass by Treatment Group and Timepoint—Efficacy Cohort
| First CMR: 2–6 days (N=136)
|
Second CMR: 12 ± 2 weeks (N=124)
|
|||||
|---|---|---|---|---|---|---|
| Epoetin alfa (n=68) | Placebo (n=70) | P | Epoetin alfa (n=61) | Placebo (n=63) | P | |
| Infarct size, % LV | 15.8 (10.3) | 15.0 (10.0) | .666 | 10.6 (8.6) | 10.4 (7.6) | .886 |
|
| ||||||
| Infarct mass, g | 24.6 (18.9) | 21.1 (14.5) | .221 | 14.9 (13.3) | 12.7 (9.2) | .291 |
|
| ||||||
| LVEF, % | 48.2 (9.1) | 48.9 (8.7) | .671 | 52.5 (9.3) | 52.0 (8.8) | .760 |
|
| ||||||
| LVESVi, mL/m2 | 34.7 (14.7) | 32.6 (10.6) | .343 | 34.1 (14.0) | 32.0 (11.7) | .364 |
|
| ||||||
| LVEDVi, mL/m2 | 65.6 (18.2) | 63.4 (15.4) | .437 | 70.0 (17.1) | 66.6 (19.1) | .303 |
|
| ||||||
| LVMi, g/m2 | 74.2 (15.2) | 69.2 (13.0) | .040 | 67.3 (14.7) | 61.8 (14.1) | .036 |
All values given as mean (SD). P values are from unadjusted analysis. Abbreviations: LV, left ventricle. LVEF, left ventricular ejection fraction. LVESVi, left ventricular end-systolic volume, indexed to body surface area (m2); LVEDVi, left ventricular end-diastolic volume, indexed to body surface area (m2); LVMi, left ventricular mass, indexed to body surface area.
Figure 2.
Comparison of infarct size, expressed as percentage of LV mass, between epoetin alfa and placebo groups in the overall efficacy cohort (A), in patients <70 years of age (B), and in patients ≥70 years of age (C). *P=.026. Boxes extend to 25th and 75th percentiles. Whiskers extend to minimum and maximum values. Mean values are represented by a dot within the box; medians are represented by a horizontal line within the box.
Infarct size at 12±2 weeks did not differ between groups in either unadjusted (Figure 2A) or adjusted analyses. Adjusting for baseline clinical characteristics that differed between groups did not affect these findings. As with infarct size, infarct mass did not differ between groups at either timepoint.
LVEF, LVESVi, and LVEDVi did not differ between groups in the unadjusted analysis at either timepoint (Table 2); however, at 12±2 weeks, the adjusted analyses (which controlled for values of LVESVi and LVEDVi on initial CMR) showed that LVESVi and LVEDVi were respectively 8.1% (P=.036) and 7.2% (P=.014) larger in the epoetin alfa group than in the placebo group. Thus, in post hoc comparisons to the placebo group, the epoetin alfa group had a smaller decrease in LVESVi from first to second CMR scan, but a greater increase in LVEDVi.
LVMi was the only endpoint that differed significantly between groups in the unadjusted analysis. On first CMR scan, the mean LVMi was 6.7% higher in the epoetin alfa group than in the placebo group (unadjusted P=.040, adjusted P=.028). This difference persisted at 12±2 weeks, but was due to the difference in LVMi on the first CMR study, as the change in LVMi from first to second CMR did not differ between groups.
Subgroup Analyses
Neither infarct size at 2–6 days or at 12±2 weeks differed between treatment groups according to IRA location in either the unadjusted or adjusted analysis.
The interaction between treatment assignment and age group trended toward significance (P=.084), suggesting that age group may modify the effect of epoetin alfa on infarct size. Infarct size did not differ between groups among patients <70 years of age (Figure 2B). However, among patients ≥70 years (n=21), infarct size at 2–6 days was 41.2% higher in the epoetin alfa group than in the placebo group (19.9±9.9 vs. 11.7±7.2, unadjusted P=.026, adjusted P=.019) (Figure 2C). These results were unchanged when analyses were further adjusted for presence of diabetes mellitus—the only characteristic that significantly differed between the two groups (prevalence of 9.1% and 54.4% in the epoetin alfa and placebo groups, respectively). Among patients ≥70 years, infarct size at 12±2 weeks remained 40.0% higher in the epoetin alfa group than in the placebo group (15.0±10.0 vs. 9.0±6.5, unadjusted P=.121), mainly due to the difference in infarct size on the first CMR (adjusted P=.066 when controlling for IRA location and enrollment phase; adjusted P=.875 when controlling for those plus the initial CMR value of infarct size).
Safety Analyses
Adverse events were reported in 55.2% of participants who received epoetin alfa and 41.2% of those who received placebo (P=.037). Serious adverse events were reported in 20.0% of subjects who received epoetin alfa, and 10.3% of those who received placebo (P=.052) (Table 3). The composite outcome of death, MI, stroke, or stent thrombosis occurred in five (4.0%) participants who received epoetin alfa but in none of those who received placebo (P=.042). Description of these clinical events can be found in the online supplement.
Table 3.
Clinical Adverse Events, Total Cohort (N = 222)
| Epoetin alfa (n = 125)a | Placebo (n = 97)a | P Value | |
|---|---|---|---|
| Death | 1 (0.8) | 0 | .719 |
| Myocardial infarctionb | 2 (1.6) | 0 | .348 |
| Unstaged PCI | 6 (4.8) | 0 | .022 |
| CABG | 0 | 1 (1.0) | .219 |
| Stroke | 1 (0.8) | 0 | .719 |
| DVT/pulmonary embolism | 0 | 0 | – |
| New/worsening heart failure | 5 (4.0) | 2 (2.1) | .358 |
| Stent thrombosis | 3 (2.4) | 0 | .170 |
| LV thrombus | 3 (2.4) | 2 (2.1) | .828 |
| Composite events | |||
| Any adverse event | 69 (55.2) | 40 (41.2) | .037 |
| Any serious adverse event | 25 (20.0) | 10 (10.3) | .052 |
| Death/MI/stroke/stent thrombosis | 5 (4.0) | 0 | .042 |
| Death/MI/stroke | 4 (3.2) | 0 | .084 |
All values given as n (%).
Number of participants for epoetin alfa and placebo arms for total cohort given according to treatment received.
Does not include index event. Adverse events include events occurring during the period between randomization and discharge or day 7, whichever comes first. Serious adverse events include events occurring during the period between randomization and 12± 2 weeks.
Abbreviations: CABG, coronary artery bypass graft; DVT, deep venous thrombosis; LV, left ventricle; MI, myocardial infarction; PCI, percutaneous coronary intervention.
There were no clinically meaningful differences between groups in hemoglobin levels, reticulocyte counts, or blood pressure at the various timepoints assessed after administration of study medication (data not shown), likely reflecting the fact that only a single infusion of epoetin alfa was administered. Similarly, there were no clinically meaningful differences between groups in the changes in the levels of these variables from baseline or from the preceding timepoint.
DISCUSSION
In the REVEAL Trial, a single bolus of intravenous epoetin alfa in STEMI patients who underwent successful primary or rescue PCI failed to demonstrate a benefit, was associated with increased infarct size in older patients, and was associated with more cardiovascular adverse events, including stent thrombosis. Clinical interest in the use of erythropoietin as a cardioprotective agent emanated from a growing body of experimental evidence showing that erythropoietin’s non-erythropoietic effects include anti-inflammatory, anti-apoptotic, and angiogenic properties.10,22 In preclinical models, erythropoietin promotes neovascularization and induces mobilization of endothelial progenitor cells from bone marrow.23 In animal models of ischemic myocardial injury, erythropoietin was shown to reduce apoptotic cells,12,17 diminish cardiomyocyte loss,16 improve functional recovery,14 decrease infarct size16,17 and attenuate infarct expansion.17
Previous clinical studies evaluating the effects of erythropoietin on infarct size have yielded conflicting results. In four of these studies, infarct size was indirectly estimated from serial measurements of enzymatic markers of cardiac injury. In the HEBE III trial, a single dose of erythropoietin (60,000 U) within 3 hours of primary PCI in STEMI patients reduced enzymatic infarct size by 6.7% (P=.06) but did not improve LVEF at 6 weeks.26 In a small pilot study, a single dose of erythropoietin (33,000 U) reduced enzymatic infarct size by 30%.27 However, in two other trials (one in non-STEMI patients and one in STEMI patients treated with fibrinolysis), a single dose of erythropoietin (40,000 U) did not reduce enzymatic infarct size.24,25
In the pilot study mentioned above,27 erythropoietin did not affect infarct size at 6 months as assessed by contrast-enhanced CMR, a powerful, validated tool that allows accurate and reproducible measurement of infarct size.28,29 Using CMR, the REVIVAL-3 study found that three daily doses of erythropoietin (33,000 U each) did not reduce infarct size at 5 days or 6 months30; 6-month LVEF also remained unimproved.
Taken together with the REVEAL trial, these results indicate that no clinical study to date has shown any beneficial effect of erythropoietin on CMR-measured infarct size. Furthermore, the results in the subgroup of participants ≥70 years in REVEAL suggest that erythropoietin may adversely affect infarct size in this high-risk population. Although this concerning finding should be interpreted with caution due to the small number of older patients enrolled in REVEAL and the lack of multiplicity adjustment in the analyses, it suggests the need for added vigilance before enrolling older patients in any future trial evaluating erythropoietin in the setting of MI.
In the small (N=30) pilot study that reported a 30% reduction in enzymatic infarct size with erythropoietin, the erythropoietin group had a greater decrease in LVESV than the placebo group, and a similar increase in LVEDV.27 Our larger study failed to replicate these observations. Instead, the epoetin alfa arm in REVEAL showed a smaller decrease in LVESVi and a larger increase in LVEDVi than the placebo group, suggesting a greater reliance on the Frank-Starling mechanism to augment stroke volume (data not shown). Thus, administration of erythropoietin may be associated with adverse LV remodeling at 3 months.
In REVEAL, we also found a significantly increased risk of death, recurrent MI, stroke, or stent thrombosis with erythropoietin, suggesting an increased thrombotic risk with erythropoietin in STEMI patients. This is consistent with other studies involving erythropoietin in non-cardiac populations.31–33 Chronic administration of erythropoietin can lead to increases in vasoconstriction, blood pressure, blood viscosity,34 and thrombotic risk.35 Some previous studies in patients with MI did not detect an increased risk of adverse events with erythropoietin,25,27,36 and the HEBE III trial even reported a lower risk of major adverse cardiovascular outcomes in patients receiving erythropoietin.24 On the other hand, the REVIVAL-3 observed an increased risk of death, recurrent MI, stroke, or target vessel revascularization in patients who received erythropoietin.30
Reconciling Animal and Clinical Studies
It is conceivable that the effects of erythropoietin differ across species. For example, although erythropoietin reduced infarct size in a rodent model of coronary occlusion,17 it failed to do so in a porcine model.37 Moreover, the average infarct size in REVEAL (approximately 15%–16%) was smaller than infarct sizes reported in animal studies. However, the average infarct size in the REVIVAL-3 study was approximately 27%–28%29 and erythropoietin still was not effective in reducing infarct size. In addition, the doses of erythropoietin used in animal studies (3000–5000 U/kg) were generally higher than those used in clinical studies. However, the cardioprotective effects of erythropoietin have been demonstrated at doses as low as 150 U/kg in rodents.38
Importantly, animal studies indicate that there is a therapeutic window of time beyond which the tissue-protective effects of erythropoietin are attenuated; further, the duration of this window is directly related to erythropoietin dose.31 For example, in a rodent model of coronary ligation, the benefits of erythropoietin at 3000 U/kg can still be observed when administration is delayed up to 12 (but not 24) hours after ischemic injury; whereas at 150 U/kg, these benefits are observed only when erythropoietin is administered within 4 (but not 8) hours of ischemic injury. Further, in a rodent model of ischemia (2 hours)-reperfusion (wherein infarct size is expected to be smaller than that achieved with permanent coronary ligation), an erythropoietin dose of 3000 U/kg was effective in reducing infarct size if administered at time of reperfusion, but not if infused 2 hours later (Talan M, unpublished data). In REVEAL, erythropoietin was infused, on average, >6 hours after symptom onset. Thus, we cannot exclude the possibility that administration of erythropoietin in our study occurred beyond the therapeutic window. We did not observe an association between infarct size and time from symptom onset to time of erythropoietin administration (data not shown). In addition, the REVIVAL-3 trial30 did not observe an effect on infarct size although erythropoietin was administered at time of PCI. Whether earlier administration of erythropoietin might reveal cardioprotective properties in humans remains to be determined.
In summary, a single bolus of 60,000 U of epoetin alfa in STEMI patients within 4 hours following successful PCI does not reduce infarct size and may increase infarct size among patients aged ≥70 years. Epoetin alfa was associated with unfavorable remodeling and higher risk of adverse clinical events. Whether very early administration of erythropoietin after symptom onset might reveal cardioprotective properties in humans remains to be determined.
Supplementary Material
Acknowledgments
The authors wish to thank the members of the REVEAL Data and Safety Monitoring Board: Lawrence J. Appel, MD, (chair), Victor Ferrari, MD, Mark D. Kelemen, MD, Jon R. Resar, MD, and Michael L. Terrin, MD
The authors also thank Jonathan McCall of the Duke Clinical Research Institute for editorial assistance with this manuscript.
Sources of Funding
This study was supported by the Intramural Research Program of the National Institute on Aging (#HHS-N-260-2005-00010-C).
Role of Sponsor and Access to Data
REVEAL was sponsored by the Intramural Research Program of the NIA. The Duke Clinical Research Institute (DCRI) served as the coordinating center under contract from the NIA. Investigators from the NIA and the DCRI collaborated in designing and supervising the conduct of the study and the investigators had full access to study data. Neither Centocor Ortho Biotech nor Florida Biologix provided funding or had input into the design or conduct of REVEAL; in addition, neither had any input into data collection, data analysis, or the writing of the manuscript. Dr. Najjar had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix
Description of clinical events in the composite outcome
One participant, a man aged 90 years enrolled in the lowest-dose (15k) cohort who received active study medication, developed acute renal failure and subsequently died of heart failure approximately 2 weeks after enrollment. One patient who received 15k of epoetin alfa had an ischemic stroke 7 days later. Three patients developed stent thrombosis, all of whom received epoetin alfa. Stent thrombosis was acute (7 hours after administration of study medication) in one participant who received the 60k dose of epoetin alfa, and subacute (5 days and 4 days after administration of study medication, respectively) in two participants who received the 30k and 60k doses of epoetin alfa, respectively. The latter two sustained a recurrent MI as a result of stent thrombosis. Thus, five (4.0%) participants who received active study medication sustained the composite outcome of death, MI, stroke, or stent thrombosis, while no participants who received placebo sustained that outcome (P=.042).
Footnotes
Primary findings from the REVEAL study were presented on November 17th at the “Clinical Science: Special Reports” session of the 2010 American Heart Association annual meeting in Chicago, Illinois.
Financial Disclosures
Dr. Rao reports receiving research funding from Novartis, Cordis Corporation, and Ikaria; Dr. Rao also consults for sanofi-aventis, Bristol-Meyers Squib, AstraZeneca, Daiichi Sankyo-Lilly, and Terumo USA. Dr. Hasselblad receives salary support from grants from Eli Lilly and Medicure administered through Duke University. A complete listing of disclosure information for Dr. Harrington is available at: https://dcri.org/about-us/conflict-of-interest/Harrington-COI_2010.pdf. Dr. Raman reports grant support from NIH grant R01 HL099563 and from Siemens Corporation. Dr. Kim is an inventor on a U.S. patent for Delayed Enhancement MRI, which is owned by Northwestern University. All other authors report no conflicts of interest.
References
- 1.Young JJ, Kereiakes DJ. Pharmacologic reperfusion strategies for the treatment of ST-segment elevation myocardial infarction. Rev Cardiovasc Med. 2003;4(4):216–227. [PubMed] [Google Scholar]
- 2.McGovern PG, Pankow JS, Shahar E, Doliszny KM, Folsom AR, Blackburn H, Luepker RV. Recent trends in acute coronary heart disease--mortality, morbidity, medical care, and risk factors. The Minnesota Heart Survey Investigators. N Engl J Med. 1996;334(14):884–890. doi: 10.1056/NEJM199604043341403. [DOI] [PubMed] [Google Scholar]
- 3.Maggioni AP, Maseri A, Fresco C, Franzosi MG, Mauri F, Santoro E, Tognoni G. Age-related increase in mortality among patients with first myocardial infarctions treated with thrombolysis. The Investigators of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI-2) N Engl J Med. 1993;329(20):1442–1448. doi: 10.1056/NEJM199311113292002. [DOI] [PubMed] [Google Scholar]
- 4.Eaton LW, Weiss JL, Bulkley BH, Garrison JB, Weisfeldt ML. Regional cardiac dilatation after acute myocardial infarction: recognition by two-dimensional echocardiography. N Engl J Med. 1979;300(2):57–62. doi: 10.1056/NEJM197901113000202. [DOI] [PubMed] [Google Scholar]
- 5.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81(4):1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 6.Weiss JL, Marino PN, Shapiro EP. Myocardial infarct expansion: recognition, significance and pathology. Am J Cardiol. 1991;68(14):35D–40D. doi: 10.1016/0002-9149(91)90259-n. [DOI] [PubMed] [Google Scholar]
- 7.Weisman HF, Healy B. Myocardial infarct expansion, infarct extension, and reinfarction: Pathophysiologic concepts. Prog Cardiovasc Dis. 1987;30(2):73–110. doi: 10.1016/0033-0620(87)90004-1. [DOI] [PubMed] [Google Scholar]
- 8.Ezekowitz JA, Armstrong PW, Granger CB, Theroux P, Stebbins A, Kim RJ, Patel MR. Predicting chronic left ventricular dysfunction 90 days after ST-segment elevation myocardial in Acute Myocardial Infarction (APEX-AMI) Study. Am Heart J. 2010;160(2):272–278. doi: 10.1016/j.ahj.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Ma Y, Han W, et al. Age-related differences in postinfarct left ventricular rupture and remodeling. Am J Physio– Heart Circ Physiol. 2008;294(4):H1815–1822. doi: 10.1152/ajpheart.00831.2007. [DOI] [PubMed] [Google Scholar]
- 10.Chong ZZ, Kang JQ, Maiese K. Angiogenesis and plasticity: role of erythropoietin in vascular systems. J Hematother Stem Cell Res. 2002;11(6):863–871. doi: 10.1089/152581602321080529. [DOI] [PubMed] [Google Scholar]
- 11.Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, Noguchi CT. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91(9):3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tramontano AF, Muniyappa R, Black AD, et al. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biophys Res Commun. 2003;308(4):990–994. doi: 10.1016/s0006-291x(03)01503-1. [DOI] [PubMed] [Google Scholar]
- 13.Wright GL, Hanlon P, Amin K, Steenbergen C, Murphy E, Arcasoy MO. Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. FASEB J. 2004;18(9):1031–1033. doi: 10.1096/fj.03-1289fje. [DOI] [PubMed] [Google Scholar]
- 14.Cai Z, Manalo DJ, Wei G, et al. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation. 2003;108(1):79–85. doi: 10.1161/01.CIR.0000078635.89229.8A. [DOI] [PubMed] [Google Scholar]
- 15.Parsa CJ, Matsumoto A, Kim J, et al. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112(7):999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvillo L, Latini R, Kajstura J, et al. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci U S A. 2003;100(8):4802–4806. doi: 10.1073/pnas.0630444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon C, Krawczyk M, Ahn D, Ahmet I, Paik D, Lakatta EG, Talan MI. Erythropoietin reduces myocardial infarction and left ventricular functional decline after coronary artery ligation in rats. Proc Natl Acad Sci U S A. 2003;100(20):11612–11617. doi: 10.1073/pnas.1930406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melloni C, Rao SV, Povsic TJ, et al. Design and rationale of the Reduction of Infarct Expansion and Ventricular Remodeling with Erythropoietin After Large Myocardial Infarction (REVEAL) trial. Am Heart J. 2010;160(5):795–803. doi: 10.1016/j.ahj.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51(2):210–247. doi: 10.1016/j.jacc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Dudley RA, Harrell FE, Jr, Smith LR, et al. Comparison of analytic models for estimating the effect of clinical factors on the cost of coronary artery bypass graft surgery. J Clin Epidemiol. 1993;46:261–271. doi: 10.1016/0895-4356(93)90074-b. [DOI] [PubMed] [Google Scholar]
- 21.Jennison C, Trunbull BW. Group Sequential Methods with Applications to Clinical Trials. Boca Raton, FL: Chapman & Hall; 2000. [Google Scholar]
- 22.Lipšic E, Schoemaker RG, van der Meer P, Voors AA, van Veldhuisen DJ, van Gilst WH. Protective effects of erythropoietin in cardiac ischemia: from bench to bedside. J Am Coll Cardiol. 2006;48(11):2161–2167. doi: 10.1016/j.jacc.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 23.Bahlmann FH, De Groot K, Spandau JM, et al. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103(3):921–926. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- 24.Voors AA, Belonje AM, Zijlstra F, et al. A single dose of erythropoietin in ST-elevation myocardial infarction. Eur Heart J. 2010 doi: 10.1093/eurheartj/ehq304. [e-pub ahead of print] DOI: 10.1093. [DOI] [PubMed] [Google Scholar]
- 25.Ferrario M, Arbustini E, Massa M, et al. High-dose erythropoietin in patients with acute myocardial infarction: A pilot, randomised, placebo-controlled study. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.10.028. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Liem A, van de Woestijne AP, Bruijns E, et al. Effect of EPO administration on myocardial infarct size in patients with non-STE acute coronary syndromes; results from a pilot study. Int J Cardiol. 2009;131(2):285–287. doi: 10.1016/j.ijcard.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 27.Binbrek AS, Rao NS, Al Khaja N, Assaqqaf J, Sobel BE. Erythropoietin to augment myocardial salvage induced by coronary thrombolysis in patients with ST segment elevation acute myocardial infarction. Am J Cardiol. 2009;104(8):1035–1040. doi: 10.1016/j.amjcard.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 28.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 29.Kim RJ, Albert TS, Wible JH, et al. Performance of delayed-enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: an international, multicenter, double-blinded, randomized trial. Circulation. 2008;117(5):629–637. doi: 10.1161/CIRCULATIONAHA.107.723262. [DOI] [PubMed] [Google Scholar]
- 30.Ott I, Schulz S, Mehilli J, et al. Erythropoietin in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: A randomized, double-blind trial. Circ Cardiovasc Interv. 2010 doi: 10.1161/circinterventions.109.904425. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Ehrenreich H, Weissenborn K, Prange H, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40(12):e647–656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 32.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355(20):2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 33.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299(8):914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 34.Smith KJ, Bleyer AJ, Little WC, Sane DC. The cardiovascular effects of erythropoietin. Cardiovasc Res. 2003;59(3):538–548. doi: 10.1016/s0008-6363(03)00468-1. [DOI] [PubMed] [Google Scholar]
- 35.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339(9):584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 36.Lipšic E, van der Meer P, Voors AA, et al. A single bolus of a long-acting erythropoietin analogue darbepoetin alfa in patients with acute myocardial infarction: a randomized feasibility and safety study. Cardiovasc Drugs Ther. 2006;20(2):135–141. doi: 10.1007/s10557-006-7680-5. [DOI] [PubMed] [Google Scholar]
- 37.Kristensen J, Maeng M, Rehling M, Berg JS, Mortensen UM, Nielsen SS, Nielsen TT. Lack of acute cardioprotective effect from preischaemic erythropoietin administration in a porcine coronary occlusion model. Clin Physiol Funct Imaging. 2005;25(1–2):305–310. doi: 10.1111/j.1475-097X.2005.00626.x. [DOI] [PubMed] [Google Scholar]
- 38.Moon C, Krawczyk M, Paik D, Lakatta EG, Talan MI. Cardioprotection by recombinant human erythropoietin following acute experimental myocardial infarction: dose response and therapeutic window. Cardiovasc Drugs Ther. 2005;19(4):243–250. doi: 10.1007/s10557-005-3189-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.