Abstract
Exposure to ionising radiation results in mutagenesis and cell death, and the clinical manifestations depend on the dose and the involved body area. Reducing carcinogenesis in patients treated with radiotherapy, exposed to diagnostic radiation or who are in certain professional groups is mandatory. The prevention or treatment of early and late radiotherapy effects would improve quality of life and increase cancer curability by intensifying therapies. Experimental and clinical data have given rise to new concepts and a large pool of chemical and molecular agents that could be effective in the protection and treatment of radiation damage. To date, amifostine is the only drug recommended as an effective radioprotectant. This review identifies five distinct types of radiation damage (I, cellular depletion; II, reactive gene activation; III, tissue disorganisation; IV, stochastic effects; V, bystander effects) and classifies the radioprotective agents into five relevant categories (A, protectants against all types of radiation effects; B, death pathway modulators; C, blockers of inflammation, chemotaxis and autocrine/paracrine pathways; D, antimutagenic keepers of genomic integrity; E, agents that block bystander effects). The necessity of establishing and funding central committees that guide systematic clinical research into evaluating the novel agents revealed in the era of molecular medicine is stressed.
All life forms on our planet are exposed to cosmic radiation, albeit at a level low enough to allow genetic stability. The Earth's atmosphere forms a transparent shield, allowing in the right amount of radiation to reach soil and water, and supporting life. Experiments in shielded conditions have shown that low-dose ionising radiation is necessary to maintain optimum physiological function [1,2].
Exposure to higher doses of ionising radiation (above some cGy), however, results in an increased rate of genetic mutations and cell death. A total body acute exposure of mammals to doses of 5–12 Gy results in death due to bone marrow, gastrointestinal, lung or brain damage. Such high exposures occur, however, only after accidental exposure or nuclear weapon usage, or as a medical intervention in the context of bone marrow transplantation for patients with malignancy. Partial exposure of the human body to much higher doses, equivalent to a single acute dose of 16–20 Gy, occurs during radiotherapy of cancer patients. Although in these cases death from radiation tissue damage is rare, late radiation sequelae may severely compromise the quality of life of cancer survivors; therefore, radioprotectants are important in protecting cancer patients during radiotherapy and in preventing remote, sometimes life-threatening, sequelae.
Radioprotectants are also important in minimising the accumulation of genetic mutations in patients receiving radiotherapy or individuals exposed to non-lethal, but higher than normal, levels of radiation. Levels of exposure to diagnostic radiation during CT scan range from 0.01 to 0.2 Gy, and genetic damage and mutations can be identified in the lymphocytes of individuals undergoing CT [3]. The frequency of deoxyribonucleic acid (DNA) mutation is dose dependent [4,5] and appears to increase linearly for doses between 0.1 and 5 Gy [6]. The risk of second carcinomas following radiotherapy and/or chemotherapy is not negligible. The lung cancer incidence following breast radiotherapy is as high as 2% [7], and 18% of deaths following successful treatment for Hodgkin's lymphoma are due to secondary carcinomas [8], although chemotherapy is certainly a component of such oncogenesis. It is therefore important to find ways to reduce the cancer risk following radiotherapy, given that cancer survivors account for >3.5% of the total population of developed countries [9]. Whether effective radioprotectants with acceptable side-effect profiles could further decrease the risk of radiation carcinogenesis in young patients undergoing radiological tests or in professional groups receiving a higher than average radiation dose is an issue of current interest. The field of research on radioprotectants is also of great importance in space science [10], given that manned missions are expected to increase in the near future.
Radiation tissue damage
Tissues are well-organised structures composed of cells of epithelial and connective tissue origin. The epithelial and haemopoietic bone marrow cell components are continuously regenerating compartments: the doubling time of regenerating clonogenic cells in the crypts of the small intestine is less than 1 day, and the whole intestinal tract epithelial compartment renews entirely within a few days [11,12]. Conversely, fibroblasts and the endothelial vascular cells that compose the underlying stroma are slowly proliferating cells; consequently, the stroma is a slow-renewing compartment. The endothelial cell turnover in conduit blood vessels in vivo is approximately 1 to several years, with only 0.1% of cells actively proliferating in quiescent vessels [13,14]. Similarly, only 0.3% of submucosal fibroblasts of the intestine are found in the proliferative phase [15]. Under stressful conditions, however, the proliferation rate of these cells can increase dramatically. Neurons, on the other hand, never proliferate.
Owing to this distinct cellular compartmentalisation, we propose that exposure of tissues to ionising radiation has five main effects:
Type I early radiation effects/cellular depletion, which involve cellular death and cellular depletion of a tissue followed by a proliferative response of stem cells. At doses used in clinical radiotherapy (<80 Gy), this damage is more evident in the epithelial (or haemopoietic) compartment. This is the early-responding cellular compartment, the depopulation of which results in so-called “acute” or “early” radiation toxicity. Doses higher than those used in radiotherapy may result in complete extinction of such cells. Experimental and clinical data suggest that for this compartment—which has a high α/β ratio—the damage induced depends mainly on the total physical dose and less on the dose per fraction (in cases of fractionated radiotherapy) [16].
Type II early radiation effects/reactive gene activation, which involves cellular and tissue dysfunction followed by increased vascular permeability, tissue oedema, production of growth factor and cytokines on behalf of fibroblasts and endothelial cells and chemo-attraction of macrophages and other white cells, leading to radiation inflammation. This is an entirely different type of early radiation damage because it is a consequence of reactive activation of genes involved in inflammation, vascular permeability, angiogenesis, apoptosis inhibition and others [17,18]. This Type II early response of the stromal compartment can persist for weeks or months until it settles. Experimental and clinical data suggest that for this compartment—which has a low α/β ratio—the damage induced strongly depends on the dose per fraction (in cases of fractionated radiotherapy) [16].
Type III late radiation effects/tissue disorganisation. If the radiation damage to fibroblasts and vessels (or other cells of connective origin) is high enough, Type II early effects can progress to permanent tissue disorganisation and dysfunction, with proliferation of fibroblasts and vessels, leading to the known clinical signs of oedema, fibrosis and telangiectasia. Severe damage of the vascular component may lead to necrosis. This is a result of an unbreakable vicious cycle of growth factor and cytokine production by fibroblasts, endothelium and immune cells, leading to organ dysfunction that manifests 6 months or even 2–3 years (or more) after irradiation [18].
Type IV radiation effects/stochastic effects, in which mutations of the exposed somatic cell genome may accumulate in surviving cells and be passed to offspring. This may occur in blood cells, leading to haematological malignancies, or in all epithelial or connective tissue cells, leading to solid tumours [9]. Moreover, although not supported by strong epidemiological or experimental data, germ cell mutations can be inherited in males and manifested after a number of generations.
Type V radiation effects/bystander effects. Although we have been aware of this effect for many decades [19], it has only recently become a target of intensive research to clarify its molecular mechanisms [20,21]. This effect refers to the radiation damage induced in cells within an organ or the whole body that have not been directly exposed to radiation. This is a result of transmission of molecular signals through gap junctions between cells (local level), or via secreted molecules in the organ environment or in the blood, that reach and damage cells in distant organs (long-range abscopal level). Bystander (non-targeted) and directly irradiated cells undergo a similar type of DNA damage, which includes single- and double-strand breaks, induction of micronuclei, sister chromatid exchange and γ-Η2ΑΧ foci, in addition to loss of DNA methylation, resulting in increased genomic instability [22,23]. In this way, Type V effects may significantly contribute to the early and late effects of radiation and, importantly, to the Type V stochastic effects.
Mechanistic classification of radioprotectants
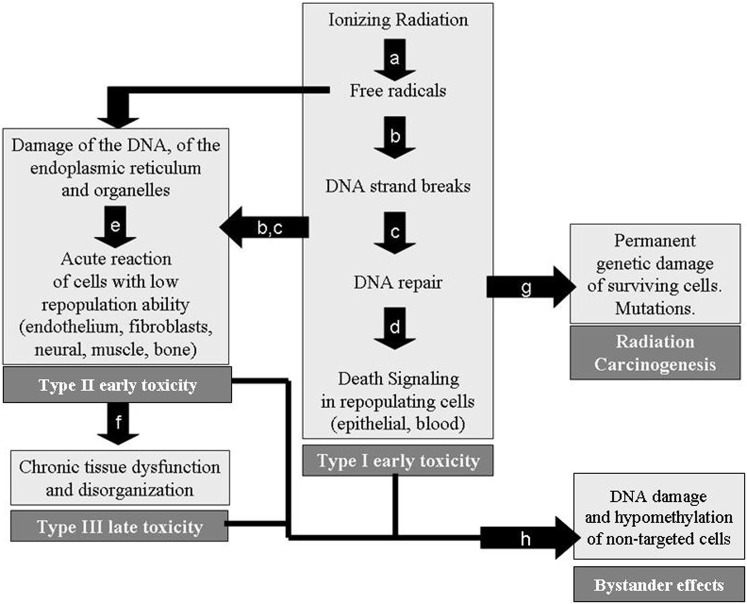
From cell exposure to radiation to the appearance of genetic mutation, cell death or tissue disorganisation, we can identify discrete steps where radioprotective policies can intervene to reverse the destructive process (Figure 1). Exposure of cells to ionising radiation leads to free radical formation that damages both DNA and the cytoplasmic organelles and endoplasmic reticulum. High linear energy transfer (LET) radiation can directly damage DNA strands without affecting the activity of free radicals. A repair process follows, resulting in either the survival of the cell or the triggering of death pathways such as apoptosis. Inadequate survival of cells with mutations leads to carcinogenesis. Damage to late-responding cellular compartments leads to early dysfunction and, eventually, a chronic phase resulting in organ destruction. Each of these steps can be blocked via pharmacological or molecular interference. Classification of the radioprotectants according to their position in the above described process is shown in Table 1.
Figure 1.
We can interfere in radiation damage by: (a) blocking the formation of free radicals; (b) blocking the free radicals, once formed, with free radical scavengers; (c) enhancing the deoxyribonucleic acid (DNA) repair process; (d) inhibiting death signalling pathways; (e) preventing the acute release of cytokines and growth factors by fibroblasts and endothelium during radiotherapy; (f) breaking the vicious cycle that continuously stimulates fibroblast and endothelium dysfunction; (g) preventing the survival of somatic mutated cells that repopulate the tissue with initiated cells; (h) preventing the DNA damage and hypomethylation of bystander cells.
Table 1. Mechanistic classification of radioprotectants according to their biological activity.
| A. Protectants against all types of radiation effects |
| 1. Blockers of oxygen consumption |
| 2. Free radical scavengers (endogenous and exogenous) |
| 3. DNA repair boosters |
| B. Protectants against Type I early radiation damage |
| 1. Death pathway modulators |
| 2. Growth factors |
| C. Protectants against Type II early and Type III late radiation effects |
| 1. Blockers of radiation inflammation and chemotaxis |
| 2. Blockers of autocrine/paracrine pathways |
| D. Protectants against Type IV stochastic effects |
| 1. Antimutagenic keepers of genomic integrity |
| E. Protectants against Type V bystander effects |
DNA, deoxyribonucleic acid.
Type A1 radioprotectants: blockers of oxygen consumption
The first step in radiation interaction with the cell is the formation of free radicals as a result of ionisation of water and other molecules. In 1953, Gray et al [24] showed that the biological effect of radiation is reduced under anoxic conditions. Oxygen-free radicals created by the ionising irradiation interaction with intracellular oxygen assault the DNA strands [25]. Dysfunction of mitochondria, the endoplasmic reticulum and other organelles induced by free radicals may also contribute to mutagenesis and death [26,27]. Reduction of oxygen tension in the body extremities to protect tissues by using tourniquet techniques has been proposed in the past for normal tissue protection [28]. Since the early 1960s, reports have proposed that compounds such as hydroxytryptamine [29] that reduce oxygen consumption could have a role as radioprotectants. Amifostine, the only radioprotectant currently approved for clinical use, has such a role [30], although its major cytoprotective pathway is its activity as a free radical scavenger. The oxygen tension and saturation of haemoglobin is increased in the venous blood 30 min following injection of the agent; glucose levels drop, showing a shift of normal tissue metabolism to anaerobic pathways [31].
Another approach to reduce the cellular oxygen consumption is amplifying the activity of the hypoxia-inducible factors-1 alpha and 2 alpha (HIF-1α and HIF-2α, respectively). These are key transcription factors regulating the expression of a variety of genes involved in glycolysis and angiogenesis [32]. These proteins are constantly degraded by the proteasome pathway; therefore, under normal oxygen tension, their concentration remains low. Under hypoxic conditions, however, degradation is inhibited and HIFαs are accumulated in the cytoplasm. Following heterodimerisation, HIFαs bind the DNA to the hypoxia response elements (HREs) of a large number of target genes involved in angiogenesis [e.g. vascular endothelial growth factor (VEGF), erythropoiesis and apoptosis/survival regulating proteins (e.g. BNIP3)], switching on their transcription. The lactate dehydrogenase A gene (LDHA) is under the direct control of HIFs [33], and overexpression of LDHA leads to suppression of oxidative phosphorylation and anaerobic metabolism. Stabilisation of HIFs in normal tissue would reduce oxygen consumption and eventually decrease radiation damage by oxygen species. In fact, this may be a key pathway in the Warburg effect noted in tumours, whereby cancer cells prefer anaerobic glycolysis instead of mitochondrial oxidative pathways, regardless of the presence of oxygen [34]. Stabilisation of HIFs in normal tissue would reduce oxygen consumption and eventually lead to decreased radiation damage by oxygen species.
Cobalt chloride (CoCl2), the iron chelator desferrioxamine, and the organomercurial compound mersalyl are important inducers of HIFs, which they achieve by mimicking hypoxia [35-37], probably by reducing the ubiquitination of HIF [38]. Clioquinol, a copper (Cu) (II)/zinc (Zn) (II) chelator, also inhibits the degradation of HIF-1α and leads to expression of downstream genes in normoxic cells [39]. Isoflurane, a volatile anaesthetic drug, can also upregulate HIF-1α and enhance HIF-1-responsive genes [40]. Okadaic acid and vanadate also appear to promote HIF-1α expression via Akt activation [41,42].
The activity of prolyl-hydroxylase (PHD), the direct sensor of hypoxia, is decreased under hypoxic conditions. Failure of hydroxylation of HIF results in reduced ubiquitination and HIF accumulation. Inhibitors of PHDs have been developed. Tilorone is a low molecular weight antiviral and a potent activator of the HIF pathway in neuronal cell lines, enhancing the expression of downstream target genes [43]. Baicalein suppresses ubiquitination of HIF-1α by inhibiting the HIF-specific hydroxylases and increasing the target gene transcription in tissue culture cells [44]. A novel PHD inhibitor, FG-4497, which stabilises HIF-1α and drives the expression of downstream genes, has been shown to protect the intestinal mucosa against chemical insult [45].
Type A2 radioprotectants: free radical scavengers
Generation of free radicals in the nuclear environment leads to a direct reaction with DNA chains and causes strand breakage. Use of free radical scavengers at this stage would reduce the number of free radicals that damage DNA or organelles. All cells contain a certain level of endogenous scavengers, the expression of which can be induced under stressful conditions. Zn, Cu or the mitochondrial manganese (Mn) superoxide dismutase (SOD) are important enzymes converting oxygen radicals to hydrogen peroxide [46]. Glutathione is also an important endogenous scavenger bearing a thiol group [47]. Several compounds can induce the expression of endogenous scavengers, such as N-acetyl-cysteine [48]. Amifostine is one of the most potent inducers of MnSOD; levels are maintained high for several days, a phenomenon that may explain the delayed radioprotective activity of amifostine [49]. The expression of endogenous SOD can also be enhanced using adenoviral vectors containing SOD genes [50].
The dephosphorylated thiolic form of amifostine (WR-1065) is a potent exogenous free radical scavenger with established clinical position in the protection against platinum toxicities and the prevention of radiation xerostomia [51]. In the recent European Society for Medical Oncology recommendations, the potential value of amifostine in the protection of radiation proctitis and oesophagitis is highlighted [52]. Experimental studies have shown important protection of cells against hypoxanthine–guanine phosphoribosyl transferase (HPRT) gene mutations after exposure to radiation [53]. Amifostine can be administered as a simple subcutaneous injection, has an acceptable tolerance profile and is a selective protectant of normal tissues in various malignancies, as shown in a large number of randomised studies [54]. More recently, an oral nanoparticle formulation of WR-1065 has been tested in pre-clinical models [55].
Fullerenes are crystal forms of carbon molecules that are neither graphite nor diamond. They consist of a spherical, ellipsoid or cylindrical arrangement of dozens of carbon atoms. The addition of a hydroxy moiety on each carbon of a 60-fullerene gives 60-fullerenol, a water-soluble molecule with potent free radical scavenger activity. In a study involving rats, the administration of a high dose of this compound resulted in radioprotection against total body irradiation (TBI) of 8 Gy, similar to that provided by amifostine [56]. However, the tolerance of the drug and its selectivity remains unknown. Cerium oxide (CeO2) nanoparticles have also been shown to protect the gastrointestinal epithelium and lung tissue against radiation damage in mice by acting as free radical scavengers and increasing the endogenous production of SOD [57,58].
Nitroxides have also been tested as free radical scavengers. The lead compound of oxidised forms of nitroxides, tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl), has shown radioprotective efficacy in experimental models [59]. The unique quality of the drug to have paramagnetic properties allows the study of the accumulation and clearance using MRI, which may be useful in optimising the administration time before radiotherapy to prevent tumour protection [60].
Type A3 radioprotectants: DNA repair boosters
Immediately following the formation of DNA strand breaks, a complex machinery involving a large number of enzymes is activated to enable rapid and accurate repair of the damage, so that the cell can survive with unaltered genomes. The recent application of fluorescent immunohistochemical techniques with antibodies recognising the phosphorylated form of (phosphorylated histone 2AX) that appears immediately after the formation of a double-strand break enables us to easily investigate the rate of repair and how putative radioprotectants affect the process [61].
Oxidation of the WR-1065 thiolic product of amifostine in cells leads to a disulphidic form, WR-33278, with DNA repair activity. An effect of the molecule on the polyamine-induced compaction and aggregation of DNA has been reported [62] as decreasing DNA strand break accumulation. WR-33278 has structural similarities to the polyamines putrescine, spermidine and spermine [63], and is transported at the same velocity by the polyamine transport system. Polyamines are integral components of the cell chromatin structure and are involved in DNA repair mechanisms [64-66]. Inhibitors decreasing their intracellular concentration result in enhanced radiation cell killing and mutagenic effect. In supercoiled plasmid DNA experiments, WR-33278 has protected DNA even following neutron exposure, by scavenging of hydroxyl radicals and by reducing the accessibility of radiolytic attack sites via the induction of packaging of DNA in liquid crystalline condensates [67]. Enhancement of the topoisomerase I-mediated unwinding of supercoiled DNA is a process mediated by WR-33278 [68]. Both WR-1065 and WR-33278 molecules have the ability to remove the platinum adducts from DNA [69].
Resveratrol (3,4-trihydroxy-trans-stilbene) is a polyethanol extract from red grapes that is available as a dietary supplement; several studies have shown that it increases life expectancy in experimental animals and prevents carcinogenesis. The addition of resveratrol during radiotherapy of mice resulted in a reduction of chromosomal aberrations [70]. A possible mechanism by which resveratrol acts as a radioprotectant is the stimulation of expression of sirtuins (silent information regulator 2), a class of proteins that exhibit histone deacetylase or mono-ribosyltransferase activity accelerating DNA repair [71].
Direct molecular interference in DNA repair machinery by targeting multiple enzymes involved in the process may also prove critical in accelerating the restoration of DNA damage. Oxoguanine DNA glycosylase (OGG1) initiates base excision repair of oxidised purine bases and exhibits 8-OH-G glycosylase activity [72]. Although this enzyme does not interfere with DNA double-strand breaks and cell death, it seems to have an important antimutagenic effect, as shown in cell lines exposed to ultraviolet light [73]. OGG1 seems also to prevent oxidative damage of mitochondrial DNA [74]. Butin, a 7,3′,4′-trihydroxydihydroflavone, protects cells against hydrogen peroxide-induced damage of DNA by enhancing the transcriptional activity of OGG1 and the expression of phosphorylated Akt, a regulator of OGG1, which makes the compound a candidate antimutagenic agent [75].
A key enzyme of base excision repair is the human apurinic/apyrimidinic endonuclease (HAP1/APE1); the mitochondria-targeted APE1 exhibits robust DNA repair activity. Overexpression vectors of mitochondria-targeted truncated APE1 and full-length APE1 significantly protect normal cells from oxidative stress [76]. Poly-(adenosine diphosphate-ribose) polymerase (PARP-1) is a nuclear enzyme that is possibly involved in DNA base excision repair. PARP-1 is involved in suppressing imprecise repair of endogenous DNA damage, leading to deletion mutation during ageing [77]. PARP-1 amplifies a signal for rapid recruitment of repair factors, enabling efficient restoration of genome integrity [78]. Experiments in PARP-1–/– mice have revealed the importance of the enzyme in the survival of intestinal epithelial stem cells following irradiation [79]. A role of the enzyme in the repair of DNA double-strand breaks following irradiation has also been postulated [80]. Boosting the activity of such enzymes by gene therapy techniques could have an important role in the prevention of radiation mutagenesis or toxicity.
Type B1 radioprotectants: inhibitors of death signalling pathways
The DNA damage and, presumably, the endoplasmic reticulum and mitochondria damage induced by radiation-free radicals immediately trigger the pro-apoptotic cell response [81]. The p53 gene, as a guardian of genomic integrity, is rapidly upregulated, producing the wild type p53 protein, which is considered as the first step of apoptosis. The gap1phase cell-cycle arrest that follows (first death check point) provides the necessary time for the DNA repair process to occur. If the cell considers the repair to be effective then proliferation follows; if not, the cell progresses to the second death check point (G2 arrest), followed by the release of mitochondrial caspases and death. The B-cell lymphoma 2 (bcl-2) family of proteins, being the guardians of this latter check point, can regulate the cell decision towards survival [82]. The anti-apoptotic activity of Bcl-2 is prominent in cancer cells [83].
Blockage of the p53 function will break the apoptotic pathway and prevent death, ignoring sometimes important DNA damage. Sodium orthovanadate (Na3VO4) and Pifithrin-α (imino-tetrahydrobenzothiazol-tolylethanone hydrobromide) are potent p53 inhibitors. The administration of these compounds in mice receiving lethal TBI (8 Gy) significantly enhances survival and protects the gastrointestinal mucosa from radiation damage [84]. Antisense oligonuleotides blocking the p53 upregulated modulator of apoptosis (PUMA) protein, a p53 downstream protein which is important for the apoptotic process, also protect mice against 15 Gy of TBI and allow regeneration of the intestinal progenitor cells, preventing the gastrointestinal syndrome [85]. Such p53 blocking approaches seem important for the prevention of Type I early radiation toxicities due to restriction of cellular and stem cell depletion of epithelial tissues. However, one should keep in mind that by blocking apoptosis a higher percentage of cells with mutations will survive, because the p53 guardian gene will not be allowed to eliminate such cells. In this way, Type I toxicities may be prevented, but Type IV stochastic effects would increase the enhancement of radiation carcinogenesis.
An important group of proteins controlling apoptosis at the post-p53 level is the Bcl-2 family of proteins. Bcl-2 and Bcl-extra large (Bcl-xl) are anti-apoptotic, while bcl-2 associated protein X (Bax) and bcl-2 homologous antagonist/killer protein are apoptosis-inducing proteins. Pre-treatment of mice with small-molecule inhibitors of glycogen synthase kinase 3β (GSK-3β), SB216763 and SB415286, shows a significant reduction of Bax expression and Bcl-2 upregulation in intestinal crypt cells after radiation with 4–8 Gy compared with radiation alone, and reduces the apoptotic rate of these cells and protects mice against gastrointestinal radiation syndrome [86]. Viral proteins such as the Human Papilloma Virus 16E5 protein seem to have anti-apoptotic effects by stimulating the ubiquitin–proteasome-mediated degradation of the Bax protein [87], and may become models for the development of anti-apoptotic radioprotectants. The angiotensin receptor blockers pioglitazone and TAK-491 also have anti-apoptotic functions; they increase Akt and extracellular signal-regulated kinase (ERK) activity and inhibit Bax activation [88].
Nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) is an important transcription factor regulating the expression of a variety of genes including Bcl-2 and Bcl-xl, as well as the expression of the inhibitors of apoptosis proteins (IAPs) [89,90]. NF-κB is usually found as a complex with an NF-κB inhibitor, keeping NF-κB inactive in the cytoplasm. Activation of specific receptors such as the Toll-like receptors leads to activation of the NF-kappa-B essential modulator/inhibitor of nuclear factor kappa-B kinase-interacting proteins that induce degradation of the NF-κB inhibitor protein, releasing the NF-κB protein that shifts into the nucleus [91]. Flagellin is a bacterial protein extracted from Salmonella enterica that binds to the TLRs. CBLB502 is an engineered derivative containing the TLR-5-activating domains of flagellin [92]. Administration of CBLB502 before 13 Gy of TBI in mice results in preservation of the normal crypt cell proliferation rate and significantly protects against death, showing a more potent effect than that of amifostine [92]. CBLB502 also protects monkeys exposed to 6.5 Gy TBI. On the other hand, direct inhibitors of NF-κB activity, including ethyl pyruvate and the synthetic triterpenoid CDDO-TFEA (RTA401), not only protect against but also mitigate radiation toxicity when given 1–2 h post-exposure to zebrafish embryos [93]. Inhibitor of kappa B (IκB) kinase inhibitors also improve the overall survival of lethally irradiated zebrafish embryos. These may imply a complex role of NF-κB in radiation sensitivity.
An important pathway controlling death and survival of cells is the autophagic machinery. Although manipulation of autophagy with specific inducers and blockers alters the radiosensitivity of cancer cells [94], there are no data on the role of these in the prevention or treatment of normal tissue radiation damage [95]. Preliminary (unpublished) data from our group suggest that radiation leads to aberrant autophagosome formation and accumulation, implying an important role of autophagy as a stress response pathway to radiation. Studies investigating the phenomenon and assessing the eventual impact on radioprotection are ongoing.
Type B2 radioprotectants: growth factors
Depopulation of the early responding component of tissues (epithelial and blood cells) induces accelerated proliferation of the relevant stem cells, in order to balance the cellular loss. The pick of this Type I early radiation toxicity appears 15–20 days after exposure to radiation. Specific growth factors acting directly on the stem cell population of epithelial or haematological tissues enhance the rate of repopulation and toxicity healing.
Haematopoietic growth factors such as erythropoietin (EPO) and granulocyte (G) or granulocyte–macrophage (GM) colony-stimulating factor (CSF) have an established position in the treatment of anaemia and neutropenia following chemotherapy or radiochemotherapy [96]. GM-CSF may also have a role in the healing of acute radiation mucositis [97]. Second-generation thrombopoietin (TPO) mimetic agents such as romiplostim and eltrombopag are important agents activating the TPO receptor [98] and have been approved for the treatment of immune thrombocytopenic purpura. The keratinocyte growth factor palifermin has been approved for the treatment of severe oral mucositis that occurs after high-dose chemotherapy and radiotherapy followed by stem cell rescue [99].
Becaplermin, a recombinant platelet-derived growth factor B-chain homodimer, has shown important activity in wound healing and seems also to have an important role in osteogenesis [100]. Intracoronary infusion of recombinant human (rh) VEGF, a potent angiogenic factor, induces revascularisation and improves angina in patients with stable exertional angina [101]. Repeated local application of the rhVEGF telbermin in chronic diabetic foot ulcer has been applied in Phase I trials [102]. An eventual role of such topical gel formulations [103] to accelerate healing of severe radiation mucositis is postulated as VEGF overexpression, and has been shown to occur during the healing of oesophageal acute radiation damage [97].
A peptide derived from the receptor-binding domain of fibroblast growth factor (FGF)-2, FGF-P is a potent mitogen that promotes stem cell renewal, progenitor cell differentiation and epithelial proliferation. 5-day administration of this peptide in mice immediately after sub-TBI (to avoid death by myelosuppression) shows enhanced proliferation of crypt cells of the small intestine, preservation of the gastrointestinal function and prevention of weight loss, suggesting an eventual role of this approach in preventing acute gastrointestinal radiation syndrome [104]. An FGF-1:FGF-2 chimeric growth factor with universal FGF receptor specificity significantly protects the small intestine crypts when given intraperitoneally to BALB/c mice prior to whole-body irradiation [105]. Velafermin, the recombinant FGF-20, reduces lethality of TBI in mice by increasing expression of nuclear factor-erythroid 2 p45-related factor 2 and of MnSOD, and by activating the ERK and Akt signal transduction pathways [106].
Type C1 radioprotectants: blockers of radiation inflammation and chemotaxis
Following irradiation, the slowly proliferating cell compartments of tissues suffer a reactive upregulation and downregulation of genes, changing the functional profile of the cells [17]: an event that results in early Type II radiation toxicity. Agents targeting this effect may be extremely useful in reducing acute vascular dysfunction and leukocyte chemotaxis and infiltration during radiation pneumonitis and colitis, as well as in preventing acute breast, laryngeal/pharyngeal or even brain oedema. Type C1 radioprotectants could block the appearance of Type II toxicities when given during or immediately after the end of a radiotherapy course. These are also likely to act prophylactically against the subsequent development of Type III toxicities by preventing the establishment of autocrine/paracrine pathways. Moreover, they may have a role in the treatment of Type III toxicities by interfering in immunological pathways together with Type C2 radioprotectants.
Blockers of the leukocyte chemotaxis may be useful in the avoidance of the early inflammatory response that leads to vasodilation and oedema. Anti-inflammatory interleukins (ILs) such as IL-10 suppress tumour necrosis factor α (TNFα) and inhibit the expression of endothelial adhesion molecules [e.g. intercellular adhesion molecules (ICAMs)], preventing leukocyte extravasation [107,108]. Endogenous IL-10 limits Ang II-mediated oxidative stress and vascular dysfunction [109]. Inhibitors of the IL-6 pro-inflammatory cytokine, which is considered to be a major cause of lymphocytic alveolitis, may be important blockers of this phase [110]. In studies of radiation brain injury, neutralising antibodies against either IL-1β or TNFα prevented the expression of ICAM-1, and thus of leukocyte migration [111].
Recombinant IL-10 (Tenovil™; Schering Corporation, Kenilworth, NJ) has passed the clinical phase of experimentation in patients with Crohn's disease, who have shown an excellent tolerance profile for 12 week subcutaneous administration [112]. Dekavil, a recently synthesised immunocytokine produced by the fusion of IL-10 with the fibronectin-8 antibody recognising fibronectin and tenascin-C, is being tested as an inhibitor of collagen-induced arthritis [113]. Given the fact that fibronectin and tenascin-C are upregulated by radiation [114], blocking approaches would be of interest in the prevention and treatment of radiation tissue fibrosis via multiple immunomodulatory and fibroblast targeting pathways. Airway-directed gene transfer of IL-10 using viruses also seems to be an interesting approach. The recombinant Sendai virus seems to be an efficient IL-10 gene transfer approach to the airway epithelium [115], with eventual applications in radiation pneumonitis. Noradrenaline elicits anti-inflammatory actions in the central nervous system (CNS), inducing IL-10 production and signalling in the CNS, which may protect against neurodegeneration. The noradrenaline reuptake inhibitor reboxetine combined with the α2-adrenoceptor antagonist idazoxan induces a profound increase in IL-10 in the brain [116], and could be tested as a neuroprotector during radiotherapy or in the treatment of radiation CNS syndrome. Compounds such as Y-40138 [117], the peroxisome proliferator-activated receptor PPARα ligand WY14643 [118] and the anti-tumour alkyl-lysophospholipid analogue edelfosine [119] are potent inducers of IL-10.
Infliximab, an anti-TNFα agent, reduces the infiltration of the synovium by TNFα-producing inflammatory cells [120]. Antibodies against the IL-6 receptor such as tocilizumab have a potent anti-inflammatory effect by blocking leukocyte adhesion to the endothelium [121,122]. The novel calcineurin/nuclear factor of activated T cells blocker A-285222 reduces IL-6 production by arteries [123]. Baicalein, a component of Scutellaria radix, blocks IκBα degradation followed by downregulation of IL-6 [124]. Anakinra, an IL-1 receptor antagonist, is also an effective agent that inhibits inflammatory pathways and exhibits potent antirheumatoid activity [125].
Irradiation of mice lung results in a sharp increase of lung tissue messenger (m) ribonucleic acid (RNA) for ICAM-1/cluster of differentiation 54 (CD54), vascular cell adhesion molecule and P-selectin and E-selectin in the pulmonary endothelium some hours after irradiation, which is sustained at a high level for several days and returns to normal within 1 week [126]. Transendothelial migration into the alveoli and interstitium is thereby facilitated. Anti-CD54 blocking antibodies lead to attenuation of inflammatory cell infiltration in the irradiated lung and ICAM-1 knockout mice show no lung inflammatory response to radiation [127]. ICAM-1 is induced in the mouse CNS following irradiation, suggesting a role in leukocyte migration and brain vascular inflammation [128]. Pravastatin, a new hydroxymethylglutaryl coenzyme A reductase inhibitor, exerts persistent anti-inflammatory and antithrombotic effects on irradiated endothelial cells [129] by inhibiting the overproduction of monocyte chemoattractant protein 1, IL-6 and IL-8 and the enhanced expression of ICAM-1. It seems to be an important antifibrotic agent in radiation-induced enteropathy [130], because it inhibits Type I collagen and fibronectin accumulation, modulating the secretory phenotype of mesenchymal cells. It also seems to prevent or reduce radiation-induced skin damage [131].
Type C2 radioprotectants: blockers of autocrine/paracrine pathways
Protracted gene activation and stromal cell functional deregulation leads to the establishment of autocrine/paracrine loops, stimulating fibroblast and endothelial proliferation and leading to structural disorganisation and organ failure (Type III radiation toxicity). Identification of the key molecules involved in this cycle and of specific inhibitors would allow the breakage of the loop, interruption of damage progression and, possibly, reversal of the process. Type C2 radioprotectants could be useful during the immediate post-radiotherapy period to prevent the establishment of late radiation sequelae or could be used in the treatment of the already established Type III toxicities.
Lung hypoxia is a common event, with maximum levels reached within 6 months following irradiation, as shown in experimental studies applying pimonidazol localisation appraisal [132]. Blockage of the prolyl- and asparaginyl hydroxylase activity and subsequent accumulation of hypoxia-inducible factors 1 and 2 is, therefore, expected to dominate Type III radiation toxicities. A variety of genes, including VEGF, EPO and lactate dehydrogenase-A (which is involved in angiogenesis, glycolysis and apoptosis regulation), are under the direct transcriptional control of HIFs [32]. Because VEGF induces VEGF receptors in fibroblasts, myofibroblasts and endothelial cells [133,134], blockage of the VEGF-related pathway by VEGF tyrosine, kinase inhibitors (vatalanib, pazopanib and cediranib) may also lead to cessation of the autocrine or paracrine loops acting on fibroblasts and endothelial cells. Blockage of VEGF attenuates pulmonary fibrosis induced by bleomycin [135]. The TNP-470 anti-angiogenic compound suppresses the proliferation of myofibroblasts in experimental models by reducing the expression of VEGF [136]. Specific HIF inhibitors have been developed, such as PX-478 and YC-1 [137,138], which, apart from finding a position in the treatment of cancer, may prove to be useful tools in the prevention and treatment of late radiation sequelae.
Transforming growth factor beta (TGFβ) is a key cytokine in the fibrotic process [139] because it induces phenotypic modulation of human lung fibroblasts to myofibroblasts and stimulates collagen protein synthesis [140,141]. TGFβ gene expression increases rapidly within 14 days after irradiation [142]. Following thoracic irradiation, elevated plasma TGFβ-1 levels are noted in patients [143]. Rats treated with an adenoviral-mediated soluble TGFβ Type II receptor showed markedly reduced fibroproliferative changes following irradiation [144]. SMAD inhibitors such as naringenin downregulate expression and phosphorylation of SMAD protein in fibroblasts, blocking TGFβ signalling [145]. A small molecular weight molecule, halofuginone, inhibits the TGFβ signalling pathway by increasing the inhibitory SMAD-7 expression and significantly lessens radiation-induced fibrosis [146]. Relaxin, a potent antifibrotic agent and TGFβ inhibitor, also seems to exploit the SMAD pathway [147]. Specific inhibitors of the activin receptor-like kinase activity of the TGFβ-1 receptor, such as SB-525334, exhibit antifibrotic activity in lungs [148,149]. The SB203580 and WP631 SMAD inhibitors abrogate excessive proliferation of human lung fibroblasts induced by γ-rays [150]. Pirfenidone, a novel drug approved for the treatment of idiopathic pulmonary fibrosis, reduces fibroblast proliferation and collagen production [151,152]. Pentoxifylline also exerts antifibrotic activity by downregulating TGFβ [153].
Platelet-derived growth factor (PDGF) isoforms have an important role in stimulating the proliferation and migration of myofibroblasts during fibrosis. PDGF action is directed towards PDGFα and -β receptors with tyrosine kinase activity, present on the surface of stimulated fibroblasts [154]. Radiation induces expression of PDGF and phosphorylation of PDGF receptors that persists for a long period (26 weeks) even in the late phase of radiation-induced fibrosis [83]. PDGF receptor tyrosine kinase inhibitors reduce radiation-induced fibroblast and endothelial cell activation [155]. Administration of imatinib or SU9518 (an agent that blocks PDGF receptors) results in prolongation of survival of mice receiving 20 Gy to the lungs, and reduces the radiomorphological signs of lung fibrosis. Imatinib also inhibits bleomycin-induced lung fibrosis [156]. In a recent study, administration of imatinib (100 mg kg–1) daily after 18 Gy whole lung irradiation of mice until euthanasia significantly reduced lung fibrosis [157,158].
TNFα is produced by activated macrophages and released in large amounts during the fibrotic process [159]. It stimulates fibroblast proliferation, secretion of extracellular matrix proteins and secretion of pro-inflammatory cytokines such as IL-1 and IL-6. Thoracic irradiation of mice results in lung infiltration by TNFα, producing macrophages. This reaction settles within 4 months, suggesting that TNFα probably has a role in the early phase of radiation pneumonitis [160]. Blockage of TNFα activity with recombinant TNFα receptor administration results in resolution of lung fibrotic lesions [161]. TNF receptor 1 inhibition using antisense oligonucleotides preserves lung function following local fractionated irradiation of lung mice [162]. Inhibitors of TNFα such as infliximab, a chimeric monoclonal immunoglobulin G1 (IgG1) antibody against the tumour necrosis factor used in Crohn's disease and rheumatoid arthritis, downregulate both basic FGF (bFGF) and VEGF in the serum of patients [163]. Administration of infliximab in a patient with lung fibrosis and pulmonary hypertension associated with advanced systemic sclerosis has resulted in stabilisation of lung function tests and pulmonary arterial pressures that progressively worsened after cessation of therapy [164]. Anti-TNFα treatment protects normal brain vasculature against radiation [165].
The combination of pentoxifylline and vitamin E in the prevention and treatment of fibrotic lesions has been tested in clinical trials. Pentoxifylline inhibits TNFα [166] and leukotriene synthesis, and reduces inflammation. Prolonged treatment with this combination appears to reduce radiation skin fibrosis [167]. Other clinical trials on breast, lung and pelvic tissue fibrosis have provided inconclusive results [168-171].
Hepatocyte growth factor (HGF) is a tyrosine kinase, a product of the c-Met gene, with angiogenic properties. HGF appears to be important in reparative lung response following injury [172]. Activation of NF-κB by HGF has been reported and abrogation of NF-κB activity by IκBα repressors have resulted in loss of HGF/scatter factor-mediated protection of renal cells against doxorubicin [173]. HGF promotes the proliferation of ECV304 cells and inhibits radiation-induced apoptosis of endothelial cells [174]. Intramyocardial injection of adenoviral vectors transferring the HGF gene protect against experimental radiation-induced heart disease [175]. Continuous infusion of HGF in mice attenuates bleomycin-induced lung damage; furthermore, administration of HGF after establishment of bleomycin fibrosis reverses the fibrotic process and accelerates the proliferation of alveolar epithelial cells [176]. HGF plasmids, when injected into the liver and transferred by electroporation, significantly protect rats against radiation-induced liver damage [177]. Retinoic acid, an active metabolite of vitamin A that acts on specific receptors on cells, prevents fibrosis by counteracting the activity of TGFβ and stimulating HGF promoter activity and HGF receptor phosphorylation [178].
bFGF activates the ERK mitogen-activated protein kinase pathway and induces activator protein-1 binding, a nuclear factor that regulates the expression of a variety of genes involved in fibrosis [179]. bFGF promotes fibroblast growth and differentiation, and is produced by endothelial cells within hours following radiation exposure [180,181]. Serum concentrations of bFGF (together with TNFα and IL-6) are consistently higher in patients receiving lung irradiation [182]. Blockage of bFGF may be useful in the treatment of radiation fibrosis only after the completion of radiotherapy, because blockage of this pathway during irradiation may worsen Type I radiation toxicity.
There is some limited evidence for the protective effect of angiotensin-converting enzyme inhibitors, especially captopril and an angiotensin II Type 1 receptor blocker, on radiation-induced pulmonary injury [183]. Finally, cyclo-oxygenase selective inhibitors may also have a role in preventing radiation pneumopathy [184].
Type D radioprotectants: keepers of genomic integrity
Patients undergoing radiotherapy are exposed to inhomogeneous low-dose whole-body radiation (ranging from 0.004% to 1% of the dose fraction [185,186]) as a consequence of the scattering of the radiation outside the portals used; in addition, varying volumes of normal tissues receive high doses ranging from 10% to 85% of the total fraction delivered to the tumour. The estimated overall incidence of secondary radiotherapy-induced carcinoma is 1–2% [187], which is low compared with the benefit of cure rates, but significant considering that 3.5% of the general population are cancer survivors [9]. Diagnostic radiology, like CT scans, also delivers a dose in the range of 2 cGy to large body areas [188] and the increasing application of CT scanning in medicine highlights worries regarding potential increased radiation carcinogenesis in the general population [189]. Type D radioprotectants are therefore important in decreasing the overall cancer incidence induced by medical applications. Such agents may also be useful in protecting, for example, radiologists, nuclear industry workers [190], aircraft crew [191] and astronauts [10] against occupational exposure.
Experimental studies show that Type I radioprotectants such as amifostine are important in decreasing the mutation load, given that incorrectly repaired DNA double-strand breaks are considered to be the major cause of radiation mutagenesis [192]. However, no study has yet to assess their efficacy in protecting against secondary carcinomas in a series of patients undergoing radiotherapy and, given the 1–2% excess rate expected within 10–20 years, it is unlikely that such data will ever be available. Administration of 100 mg kg–1 of amifostine in mice has been shown to reduce cyclophosphamide-induced mutagenesis at the HPRT by fivefold [193]. Mutagenesis of splenic T lymphocytes at the HPRT locus following whole-body irradiation of neutrons (single doses, 50–150 cGy) and 60Co photons (single doses, 250–750 cGy) also declined when amifostine administration (400 mg kg–1) preceded irradiation, by factors of 1.4 and 2.4, respectively [194]. Amifostine has also been shown to reduce the frequencies of radiation-induced altered hepatocyte foci in Sprague Dawley® (Charles River, Wilmington, MA) rats [195].
Free radical scavenging is certainly a major antimutagenic pathway, but it seems that amifostine also exploits additional pathways. p53 is a key tumour suppressor gene, the functional loss of which provides resistance to irradiation. Ionising radiation dramatically accelerates lymphoma development in p53 heterozygous mice. Upon irradiation, p53 disruption confers a selective advantage and expansion of p53-deficient clones followed by an increased incidence of lymphoma [196]. Amifostine restores the normal p53 gene function in the presence of specific p53 gene mutations [197,198] and also suppresses the activation of the c-Myc oncogene [199]. This suggests that amifostine could potentially act as an antileukemogenic agent in cases of genetic predisposition. At low intracellular levels, amifostine exhibits antimutagenic activity against nucleoside reverse transcriptase inhibitors used for the treatment of human immunodeficiency virus 1 infection [200]. Amifostine may, therefore, be classified as a Type D radioprotectant, owing to its genetic interference in addition to its scavenger activity.
The exact nature of the mutagenic event induced by radiation remains elusive. Irradiation of peripheral blood mononuclear cells with 3 Gy results in prolonged up- and downregulation of a variety of genes [201]. The upregulated guanine nucleotide binding protein α 15 and the downregulated chemokine (CX3C) receptor 1 are involved in G-protein-coupled receptor protein signalling pathways. The H4 histone family, member G, replication protein A3 and topoisomerase 2α all act as DNA binding molecules in DNA metabolism and are downregulated. V-Maf, a musculoaponeurotic fibrosarcoma (avian) oncogene homologue with transcription factor activity, is also upregulated. The identification of a specific phenotype that marks a normal cell as initiated would allow the development of treatment approaches aiming to cleanse the body from such cells.
p53 gene mutations appear to occur late in the process of radiation carcinogenesis [202], and amplification of the murine double minute (mdm2; p53 inactivating) protein has been also documented in some experimental radiation-induced tumours [203]. Anti-p53 Type II radioprotectants are expected to increase carcinogenesis, while an attempt to restore p53 function once radiotherapy is accomplished seems a reasonable approach to eliminate p53-linked radiation carcinogenesis. The direct activity of amifostine on p53 gene function restoration implies that an antimutagenic effect could be achieved even if the compound is administered late after cessation of radiotherapy. Whether p53 gene therapy (INGN201) [204] or mdm2 inhibitors such as Nutlin-3 or MI-219 [205,206] are useful approaches as a cleansing method following irradiation should be tested experimentally. Whether Bcl-2 inhibition (i.e. with oblimersen sodium [207]) during radiotherapy may also prove significant in promoting the death of mutated cells is also an interesting speculation. Levels of the anti-apoptotic Bcl-xl protein have been shown to increase following irradiation promoting the aggressiveness of cancer cells [208,209].
Non-homologous end joining (NHEJ) of DNA double-stranded breaks, an error-prone repair pathway, is common in mammalian cells compared with the error-free homologous recombination pathway [210]. The D-NHEJ depends on the activities of DNA-dependent protein kinase (DNA-PK) and DNA ligase IV/XRCC4/XLF, while B-NHEJ uses DNA ligase III/XRCC1, PARP-1 and histone H1 [211,212]. Even partial deficiency of DNA-PKcs leads to increased ionising radiation induced mutagenesis (that is directly related to the level of deficiency) and telomere dysfunction [213]. High mobility group adenine–thymine-hook 2 protein (HMGA2) overexpression interferes with NHEJ processes, promoting genomic instability and tumorigenesis [214]. Vanillin derivatives, on the other hand, bearing importent antimutagenic properties, selectively inhibit the activity of DNA-PK so that blockage of the NHEJ is considered to be the cause of its antimutagenic effect, presumably by shifting the balance towards homologous error-prone recombination [215]. Ribonucleoproteins (RNPs) are involved in the packaging, processing and export of pre-RNA molecules in the cytoplasm, and have an important role in gene regulation. A subclass of TNPs, heterogeneous nuclear ribonucleoproteins (hnRNPs), show either mRNA transcript or protein quantity changes following ionising radiation. A role for hnRNPs A1, A18, A2/B1, C1/C2, K and P2 in regulating double-stranded break repair pathways by promoting preferentially homologous recombination or NHEJ repair is implied [216]. This certainly represents a field of research to identify pathways involved in radiation carcinogenesis. The stimulation of homologous error-free recombination repair of double-strand breaks is a field for research for the development of Type D radioprotectants, and hnRNPs may represent a putative target.
Centrosome aberrations rapidly appear following irradiation of normal cells [217]. These are linked to prolonged cell-cycle arrest and are considered as an important mechanism involved in radiation-induced genomic instability, predisposing cells for the subsequent development of tumours. Exposure of human mammary epithelial cells to 2 Gy irradiation leads to an increase of centrosomal aberrations that are reduced when cells are treated for 8 post-irradiation days with TGFβ [218]. This effect of TGFβ is not related to a reduction of induced aberrations but to elimination of cells with established centrosomal aberrations. This observation is interesting because a growth factor, namely TGFβ, appears to be an important agent for the cleansing of cells with genomic instability. This effect was proved to be mediated by p53 activation, highlighting again the potential for eliminating mutated cells via this approach. Because TGFβ is also a factor involved in Type II and III radiation sequelae, it is possible that anti-TGFβ agents aiming to mitigate such radiation toxicities may instead enhance genomic instability, and that TGFβ administration aiming to decrease genomic instability may enhance Type II/III toxicities. However, short-term administration of TGFβ in the form of, for example, avotermin [219] for a short period after the cessation of Type II radiation sequelae may be feasible and could be tested at experimental level. Such an approach may also be significant in reducing the load of mutated cells after exposure to lower, Type II/III toxicity-free radiation doses such as those used in diagnostic radiology or following exposure during space missions.
Telomerases also appear to have an important role in radiation-induced genomic instability. Telomeric repeat binding factor (TRF-2) is a telomere-binding protein with roles in telomere length regulation, and is upregulated in certain tumours. In the absence of telomerase activity, TRF-2 becomes a potent oncogene accelerating epithelial carcinogenesis [220]. There is increasing evidence that many DNA double-strand break repair proteins, including Ku, DNA-PKcs, RAD51D and MRN, BRCA1, hRad9 and PARP1, are involved in telomere maintenance. TRF-2 is not confined to its telomeric environment but it may migrate to the sites of DNA breakage following radiation [221]. X-ray induces phosphorylation of TRF-2 and plays a functional role in DNA double-strand break repair [222]. There is, therefore, increasing evidence that telomere maintenance mechanisms constitute an integral part of DNA damage response machinery. NF-κB, a transcription factor that becomes functionally activated upon radiation exposure, upregulates the telomerase activity by binding to the κB-binding region in the promoter region of the TERT gene [223]. Enhancing NF-κB, therefore, may protect genomic integrity in addition to protecting normal tissues against Type I toxicities.
Type E radioprotectants: protecting bystander cells
The exact molecular events defining the non-targeted effects of radiation remain poorly understood. This is an important field of research, given that understanding the pathways may help in the reduction of radiotherapy sequelae, enhance its antitumour efficacy and, most importantly, reduce the risk of secondary carcinomas.
Gap junctions allow the flow between cells of small molecules (1000–1500 Da) such as calcium ions, nucleotides and peptides, and this route is considered a major pathway of bystander effect manifestation within an organ [224,225]. Targeting gap junctions and their constituent proteins, namely connexins, may prove important in blocking the bystander effects within a partially irradiated organ (e.g. the lung or liver). Gamma-hexachlorocyclohexane (lindane) induces gap junction endocytosis, a process that is activated by the ERK pathway [226]. TGFβ-3 treatment downregulates connexin 43 and induces the ERK pathway [227]. Phorbol ester 12-O-tetradecanoylphorbol-13-acetate and chlorohydroxyfuranones, by-products of drinking water chlorination, also appear to inhibit gap junction [228].
The long-range abscopal effects are postulated to be mediated through large molecules (1000–10 000 kDa) such as lipid peroxide products and cytokines (IL-1, IL-6, TGFβ and TNFα). Such cytokines induce nitric oxide synthase 2 and increased nitric oxide content in target cells. Injection of Cu/Zn-SOD or nitric oxide synthase inhibitors such as L-NAME lead to reduced expression of bystander effects in experimental animals [229]. Macrophages appear to be the source of the long- and short-range bystander signals through cytokine production [230]. Whether abrogation of macrophage activation may have a role in protection against Type D radiation toxicities is an emerging hypothesis. Macrophage migration inhibitory factor (MIF) is a macrophage-produced cytokine that induces TNFα secretion and nitric oxide production, also contributing to the recruitment of leukocytes. Its activity can be blocked with monoclonal antibodies or by targeting MIF tautomerase activity using small molecules such as (S,R)-3-(4-hydroxyphenyl)-4, 5-dihydro-5-isoxazole acetic acid methyl ester [231]. Inhibitors of protein kinase-C, such as 1-(5-isoquinolinesulphonyl)-2-methylpiperazine dihydrochloride, also display important inhibitory activity on macrophage activation [232].
Because hypomethylation of DNA is a bystander effect of radiation [22] that may be important in genomic instability and carcinogenesis, agents that can restore this effect may also be useful Type V radioprotectants. CpG dinucleotides are major sites of DNA methylation in mammals. During somatic cell differentiation, DNA methylation occurs and represses germline-specific genes, a process catalysed by DNA methyltransferases (DNMTs). Demethylation is an important subsequent step in permitting expression of tissue-specific genes and occurs either by inhibition of DNMTs or as an active process involving the DNA repair-related demethylase [233]. The latter process is largely obscure, although it seems to be related to DNA repair machinery, including DNA glycosylases. Growth arrest and DNA-damage-inducible protein GADD45α is a non-enzymatic factor involved in base excision repair that actively promotes DNA demethylation. Cytidine deaminase also appears to be involved, converting 5-methylcytosine into a thymine; this is followed by excision and replacement of methylated nucleotides. DNA demethylation correlates with extensive histone modification and exchange that is facilitated by histone chaperone proteins such as histone cell cycle regulation defective homolog A and nucleosome assembly protein. It is expected that explanation of the process of demethylation will help in the development of strategies to block radiation-induced demethylation in bystander cells.
Conclusion
It is evident that during the past decade a large number of experimental and clinical data providing new concepts have been accumulated, in addition to a large pool of chemical and molecular agents that can be effective in the protection and treatment of early and late radiation damage, as well as in the reduction of carcinogenesis following exposure to therapeutic or diagnostic ionising radiation. Where we stand in the clinical setting is rather disappointing. Amifostine, an important multitype radioprotective agent, is the only agent tested and officially approved for a very narrow spectrum of radiation toxicity; namely, radiation-induced xerostomia, regardless of the large amount of clinical data supporting its activity in the prevention of oesophagitis, pneumonitis and intestinal toxicity. Such indications, however, have been included in the clinical recommendation guidelines of certain organisations [234,235].
Table 2 summarises the types of radiation damage and the corresponding agents expected to have a radioprotective role. It is obvious that many of the agents may be valuable for protection against multiple types of radiation damage, given that the underlying biological pathways overlap to a certain extent. It is also stressed that some agents, although protective against one type of radiation, can aggravate another type of damage; therefore, the time points of their usage may be a critical issue for clinical trials. Establishing central committees, recruiting experts in the field and absorbing significant funds from governmental and charitable agencies active in the field of cancer and radiation protection is required to sustain large-scale clinical experimentation with an aim to uncover the value of the plethora of agents with putative radioprotectant activity.
Table 2. Known agents with radioprotective activity.
| Radioprotectant type | Agent | Reference | Radioprotectant type | Agent | Reference | ||
| Type A | Type C | ||||||
| A1 | Hydroxytryptamine | [24] | C1 | Tenovil (rhIL-10) | [107] | ||
| Amifostine | [25] | Dekavil (IL-10/F8 fusion) | [108] | ||||
| Cobalt chloride | [30] | SeV- mediated transfer of IL-10 gene | [110] | ||||
| Deferoxamine | [31] | IL-10 inducers (Y-40128, WY14643. reboxetine, edelfosine) | [111-114] | ||||
| Clioquinol | [34] | Infliximab (anti-TNFα) | [115] | ||||
| Isofluran | [35] | Tolcizumab (anti-IL6 Ab) | [116,117] | ||||
| Okadaic acid | [36] | IL-6 blockers (A-285222, Baicalein) | [118,119] | ||||
| Vanadate | [37] | Anakinra (IL-1 receptor antagonist) | [120] | ||||
| Tilorone | [38] | Anti-CD54 Ab | [122] | ||||
| Baicalein | [39] | Pravastatin | [124-126] | ||||
| FG-4497 | [40] | C2 | VEGF blockers | [130] | |||
| A2 | Superoxide dismutases | [41] | TNP-470 | [131] | |||
| Glutathione | [42] | HIF blockers (PX-478, YC-1) | [132,133] | ||||
| N-acetyl-cystein | [43] | TGFβ blockers (naringenin, halogunginon, relaxin, SB-525334, SB203580, pirferidone, pentoxyfylline) | [140-148] | ||||
| Amifostine (WR-1065) | [25] | PDGFR inhibitors (imatinib, SU9518) | [150,151] | ||||
| Fullerenols | [51] | Infliximab (anti-TNFα Ab) | [158] | ||||
| Cerium oxide | [52] | HGF gene transfer | [170-172] | ||||
| Tempol | [54] | Retinoic acid | [173] | ||||
| A3 | Amifostine (WR-33278) | [57,58] | Anti-bFGF | [177] | |||
| Resveratrol | [65] | ACE inhibitors | [178] | ||||
| Butin | [70] | COX inhibitors | [179] | ||||
| Vectors with repair enzymes | [71-75] | ||||||
| Type B | Type D | Amifostine | [191-194] | ||||
| B1 | Sodium orthovanadate | [79] | INGN201 (p53 gene therapy) | [199] | |||
| Antisense-PUMA | [80] | Mdm2 inhibitors (until-1, MI-219) | [200,201] | ||||
| Inhibitors of GSK-3β | [81] | Oblimersen sodium (anti-bcl-2) | [202] | ||||
| HPV16 E5 viral protein | [82] | Vanillin derivatives | [209] | ||||
| Angiotensin receptor blockers | [83] | Avotermin (TGFβ) | [213] | ||||
| Flagellin analogues | [87] | NF-κB inducers | [217] | ||||
| RTA401 | [88] | Macrophage activation suppressors | [218] | ||||
| Autophagy modulators | [90] | ||||||
| B2 | Haemopoietin growth factors | [91,92] | Type E | ||||
| Keratinocyte growth factor | [94] | Amifostine and Type A agents | |||||
| Becaplermin (rhPDGF-BB) | [95] | Type D agents | |||||
| Telbermin (rhVEGF) | [97,98] | Gap junction inhibitors (lindane, TPA) | [226-228] | ||||
| FGF-P peptide | [99] | NOS-inhibitors (L-NAME) | [229] | ||||
| FGF1:FGF2 chimeric GF | [100] | Macrophage activation inhibitors | [231,232] | ||||
| Velafermin (rhFGF-20) | [101] | Demethylation targeting agents | [233] |
ACE, angiotensin-converting-enzyme; COX, cyclooxygenase; FGF, fibroblast growth factor; GSK-3β, glycogen synthase kinase 3-beta; HGF, hepatocyte growth factor; HIF, hypoxia inducible factor; HPV, human papillomavirus; IL, interleukin; NOS, nitric oxide synthase; PDGFR, platelet derived growth factor receptor; PUMA, p53 upregulated modulator of apoptosis; TGF, transforming growth factor; TNF, tumour necrosis factor; TNP, methionine aminopeptidase-2 (MetAP-2) inhibitor; VEGF, vascular endothelial growth factor.
References
- 1.Planel H, Soleilhavoup JP, Tixador R, Richoilley G, Conter A, Croute F, et al. Influence on cell proliferation of background radiation or exposure to very low, chronic gamma radiation. Health Phys 1987;52:571–8 [DOI] [PubMed] [Google Scholar]
- 2.Liu SZ. Biological effects of low level exposures to ionizing radiation: theory and practice. Hum Exp Toxicol 2010;29:275–81 [DOI] [PubMed] [Google Scholar]
- 3.Golfier S, Jost G, Pietsch H, Lengsfeld P, Eckardt-Schupp F, Schmid E, et al. Dicentric chromosomes and gamma-H2AX foci formation in lymphocytes of human blood samples exposed to a CT scanner: a direct comparison of dose response relationships. Radiat Prot Dosimetry 2009;134:55–61 [DOI] [PubMed] [Google Scholar]
- 4.Forrester HB, Yeh RF, Dewey WC. A dose response for radiation-induced intrachromosomal DNA rearrangements detected by inverse polymerase chain reaction. Radiat Res 1999;152:232–8 [PubMed] [Google Scholar]
- 5.Hande MP, Azizova TV, Geard CR, Burak LE, Mitchell CR, Khokhryakov VF, et al. Past exposure to densely ionizing radiation leaves a unique permanent signature in the genome. Am J Hum Genet 2003;72:1162–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wedemeyer N, Greve B, Uthe D, Pötter T, Denklau D, Severin E, et al. Frequency of CD59 mutations induced in human-hamster hybrid A(L) cells by low-dose X-irradiation. Mutat Res 2001;473:73–84 [DOI] [PubMed] [Google Scholar]
- 7.Roychoudhuri R, Evans H, Robinson D, Møller H. Radiation-induced malignancies following radiotherapy for breast cancer. Br J Cancer 2004;91:868–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favier O, Heutte N, Stamatoullas-Bastard A, Carde P, Van't Veer MB, Aleman BM, et al. Survival after Hodgkin lymphoma: causes of death and excess mortality in patients treated in 8 consecutive trials. Cancer 2009;115:1680–91 [DOI] [PubMed] [Google Scholar]
- 9.Travis LB, Rabkin CS, Brown LM, Allan JM, Alter BP, Ambrosone CB, et al. Cancer survivorship—genetic susceptibility and second primary cancers: research strategies and recommendations. J Natl Cancer Inst 2006;98:15–25 [DOI] [PubMed] [Google Scholar]
- 10.Testard I, Ricoul M, Hoffschir F, Flury-Herard A, Dutrillaux B, Fedorenko B, et al. Radiation-induced chromosome damage in astronauts' lymphocytes. Int J Radiat Biol 1996;70:403–11 [DOI] [PubMed] [Google Scholar]
- 11.Barker N, van deWetering M, Clevers H. The intestinal stem cell. Genes Dev 2008;22:1856–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. Bioessays 2002;24:91–8 [DOI] [PubMed] [Google Scholar]
- 13.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998;91:3527–61 [PubMed] [Google Scholar]
- 14.Hobson B, Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer 1984;49:405–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sivridis E, Giatromanolaki A, Koukourakis MI. Proliferating fibroblasts at the invading tumour edge of colorectal adenocarcinomas are associated with endogenous markers of hypoxia, acidity, and oxidative stress. J Clin Pathol 2005;58:1033–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowler JF. Review: total doses in fractionated radiotherapy—implications of new radiobiological data. Int J Radiat Biol Relat Stud Phys Chem Med 1984;46:103–20 [DOI] [PubMed] [Google Scholar]
- 17.Boerma M, van derWees CG, Vrieling H, Svensson JP, Wondergem J, van derLaarse A, et al. Microarray analysis of gene expression profiles of cardiac myocytes and fibroblasts after mechanical stress, ionising or ultraviolet radiation. BMC Genomics 2005;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys 2006;66:1281–93 [DOI] [PubMed] [Google Scholar]
- 19.Murphy JB, Liu JH, Sturm E. Studies on X-ray effects. IX. The action of serum from X-rayed animals on lymphoid cells in vitro. J Exp Med 1922;35:373–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baskar R. Emerging role of radiation induced bystander effects: Cell communicatons and carcinogenesis. Genome Integr 2010;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prise KM, O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer 2009;9:351–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilnytsky Y, Koturbash I, Kovalchuk O. Radiation-induced bystander effects in vivo are epigenetically regulated in a tissue specific manner. Environ Mol Mutagen 2009;50:105–13 [DOI] [PubMed] [Google Scholar]
- 23.Hall EJ, Hei TK. Genomic instability and bystander effects induced by high-LET radiation. Oncogene 2003;22:7034–42 [DOI] [PubMed] [Google Scholar]
- 24.Gray LH, Conger AD, Ebert M. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953;26:683. [DOI] [PubMed] [Google Scholar]
- 25.Henderson BW, Miller AC. Effects of scavengers of reactive oxygen and radical species on cell survival following photodynamic treatment in vitro: comparison to ionizing radiation. Radiat Res 1986;108:196–205 [PubMed] [Google Scholar]
- 26.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, et al. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal 2007;9:49–89 [DOI] [PubMed] [Google Scholar]
- 27.Zhang B, Wang Y, Pang X, Su Y, Ai G, Wang T. ER stress induced by ionizing radiation in IEC-6 cells. Int J Radiat Biol 2010;86:429–35 [DOI] [PubMed] [Google Scholar]
- 28.McCartney R, Kerr RC, Cass NM, Van DenBrenk HA. Blood flow in limbs determined with 131 I-HSA after application tourniquets to deliver radiation under anoxic conditions in sarcomata of the extremities. Australas Radiol 1966;10:240–2 [DOI] [PubMed] [Google Scholar]
- 29.Renson J. Radioprotection of the mouse by 5-hydroxytryptamine and some related substances. Arch Int Physiol Biochim 1960;68:531–4 [PubMed] [Google Scholar]
- 30.Glover D, Negendank W, Delivoria-Papadopoulos M, Glick JH. Alterations in oxygen transport following WR-2721. Int J Radiat Oncol Biol Phys 1984;10:1565–8 [DOI] [PubMed] [Google Scholar]
- 31.Koukourakis MI, Giatromanolaki A, Chong W, Simopoulos C, Polychronidis A, Sivridis E, et al. Amifostine induces anaerobic metabolism and hypoxia-inducible factor 1 alpha. Cancer Chemother Pharmacol 2004;53:8–14 [DOI] [PubMed] [Google Scholar]
- 32.Semenza GL, Shimoda LA, Prabhakar NR. Regulation of gene expression by HIF-1. Novartis Found Symp 2006;272:2–8 [PubMed] [Google Scholar]
- 33.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 1996;271:32529–37 [DOI] [PubMed] [Google Scholar]
- 34.Warburg O. The metabolism of tumors. New York, NY: R. R. Smith, Inc., 1931 [Google Scholar]
- 35.Piret JP, Mottet D, Raes M, Michiels C. CoCl2, a chemical inducer of hypoxia-inducible factor-1, and hypoxia reduce apoptotic cell death in hepatoma cell line HepG2. Ann N Y Acad Sci 2002;973:443–7 [DOI] [PubMed] [Google Scholar]
- 36.Jiang Y, Xue ZH, Shen WZ, Du KM, Yan H, Yu Y, et al. Desferrioxamine induces leukemic cell differentiation potentially by hypoxia-inducible factor-1 alpha that augments transcriptional activity of CCAAT/enhancer-binding protein-alpha. Leukemia 2005;19:1239–47 [DOI] [PubMed] [Google Scholar]
- 37.Agani F, Semenza GL. Mersalyl is a novel inducer of vascular endothelial growth factor gene expression and hypoxia-inducible factor 1 activity. Mol Pharmacol 1998;54:749–54 [DOI] [PubMed] [Google Scholar]
- 38.Metzen E, Zhou J, Jelkmann W, Fandrey J, Brüne B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell 2003;14:3470–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi SM, Choi KO, Park YK, Cho H, Yang EG, Park H. Clioquinol, a Cu(II)/Zn(II) chelator, inhibits both ubiquitination and asparagines hydroxylation of hypoxia-inducible factor-1alpha, leading to expression of vascular endothelial growth factor and erythropoietin in normoxic cells. J Biol Chem 2006;281:34056–63 [DOI] [PubMed] [Google Scholar]
- 40.Li QF, Wang XR, Yang YW, Su DS. Up-regulation of hypoxia inducible factor 1alpha by isoflurane in Hep3B cells. Anesthesiology 2006;105:1211–19 [DOI] [PubMed] [Google Scholar]
- 41.Kim YS, Ahn KH, Kim SY, Jeong JW. Okadaic acid promotes angiogenesis via activation of hypoxia-inducible factor-1. Cancer Lett 2009;276:102–8 [DOI] [PubMed] [Google Scholar]
- 42.Gao N, Ding M, Zheng JZ, Zhang Z, Leonard SS, Liu KJ, et al. Vanadate-induced expression of hypoxia-inducible factor 1 alpha and vascular endothelial growth factor through phosphatidylinositol 3-kinase/Akt pathway and reactive oxygen species. J Biol Chem 2002;277:31963–71 [DOI] [PubMed] [Google Scholar]
- 43.Ratan RR, Siddiq A, Aminova L, Langley B, McConoughey S, Karpisheva K, et al. Small molecule activation of adaptive gene expression: tilorone or its analogs are novel potent activators of hypoxia inducible factor-1 that provide prophylaxis against stroke and spinal cord injury. Ann N Y Acad Sci 2008;1147:383–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho H, Lee HY, Ahn DR, Kim SY, Kim S, Lee KB, et al. Baicalein induces functional hypoxia-inducible factor-1alpha and angiogenesis. Mol Pharmacol 2008;74:70–81 [DOI] [PubMed] [Google Scholar]
- 45.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 2008;134:145–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutteridge JM, Halliwell B. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann N Y Acad Sci 2000;899:136–47 [DOI] [PubMed] [Google Scholar]
- 47.Bump EA, Brown JM. Role of glutathione in the radiation response of mammalian cells in vitro and in vivo. Pharmacol Ther 1990;47:117–36 [DOI] [PubMed] [Google Scholar]
- 48.Murley JS, Kataoka Y, Cao D, Li JJ, Oberley LW, Grdina DJ. Delayed radioprotection by NFkappaB-mediated induction of Sod2 (MnSOD) in SA-NH tumor cells after exposure to clinically used thiol-containing drugs. Radiat Res 2004;162:536–46 [DOI] [PubMed] [Google Scholar]
- 49.Murley JS, Kataoka Y, Baker KL, Diamond AM, Morgan WF, Grdina DJ. Manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by the free thiol form of amifostine and tumor necrosis factor alpha. Radiat Res 2007;167:465–74 [DOI] [PubMed] [Google Scholar]
- 50.Duffy AM, O'Brien T, McMahon JM. Generation of antioxidant adenovirus gene transfer vectors encoding CuZnSOD, MnSOD, and catalase. Methods Mol Biol 2010;594:381–93 [DOI] [PubMed] [Google Scholar]
- 51.Koukourakis MI. Amifostine in clinical oncology: current use and future applications. Anticancer Drugs 2002;13:181–209 [DOI] [PubMed] [Google Scholar]
- 52.Peterson DE, Bensadoun RJ, Roila F; ESMO Guidelines Working Group Management of oral and gastrointestinal mucositis: ESMO clinical recommendations. Ann Oncol 2009;20:174–7 [DOI] [PubMed] [Google Scholar]
- 53.Grdina DJ, Shigematsu N, Dale P, Newton GL, Aguilera JA, Fahey RC. Thiol and disulfide metabolites of the radiation protector and potential chemopreventive agent WR-2721 are linked to both its anti-cytotoxic and anti-mutagenic mechanisms of action. Carcinogenesis 1995;16:767–74 [DOI] [PubMed] [Google Scholar]
- 54.Koukourakis MI. Amifostine: is there evidence of tumor protection? Semin Oncol 2003;30:18–30 [DOI] [PubMed] [Google Scholar]
- 55.Pamujula S, Kishore V, Rider B, Agrawal KC, Mandal TK. Radioprotection in mice following oral administration of WR-1065/PLGA nanoparticles. Int J Radiat Biol 2008;84:900–8 [DOI] [PubMed] [Google Scholar]
- 56.Trajković S, Dobrić S, Jaćević V, Dragojević-Simić V, Milovanović Z, Dordević A. Tissue-protective effects of fullerenol C60(OH)24 and amifostine in irradiated rats. Colloids Surf B Biointerfaces 2007;58:39–43 [DOI] [PubMed] [Google Scholar]
- 57.Colon J, Hsieh N, Ferguson A, Kupelian P, Seal S, Jenkins DW, et al. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine 2010;6:738–44 [DOI] [PubMed] [Google Scholar]
- 58.Colon J, Herrera L, Smith J, Patil S, Komanski C, Kupelian P, et al. Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine 2009;5:225–31 [DOI] [PubMed] [Google Scholar]
- 59.Hahn SM, Tochner Z, Krishna CM, Glass J, Wilson L, Samuni A, et al. Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res 1992;52:1750–3 [PubMed] [Google Scholar]
- 60.Cotrim AP, Hyodo F, Matsumoto K, Sowers AL, Cook JA, Baum BJ, et al. Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assessment of Tempol by magnetic resonance imaging. Clin Cancer Res 2007;13:4928–33 [DOI] [PubMed] [Google Scholar]
- 61.Mah LJ, El-Osta A, Karagiannis TC. gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia 2010;24:679–86 [DOI] [PubMed] [Google Scholar]
- 62.Newton GL, Aguilera JA, Ward JF, Fahey RC. Effect of polyamine-induced compaction and aggregation of DNA on the formation of radiation-induced strand breaks: quantitative models for cellular radiation damage. Radiat Res 1997;148:272–84 [PubMed] [Google Scholar]
- 63.Mitchell JL, Judd GG, Diveley Jr RR, Choe CY, Leyser A. Involvement of the polyamine transport system in cellular uptake of the radioprotectants WR-1065 and WR-33278. Carcinogenesis 1995;16:3063–8 [DOI] [PubMed] [Google Scholar]
- 64.Prager A, Terry NH, Murray D. Influence of intracellular thiol and polyamine levels on radioprotection by aminothiols. Int J Radiat Biol 1993;64:71–81 [DOI] [PubMed] [Google Scholar]
- 65.Snyder RD, Schroeder KK. Radiosensitivity of polyamine-depleted HeLa cells and modulation by the aminothiol WR-1065. Radiat Res 1994;137:67–75 [PubMed] [Google Scholar]
- 66.Gerner EW, Tome ME, Fry SE, Bowden GT. Inhibition of ionizing radiation recovery processes in polyamine-depleted Chinese hamster cells. Cancer Res 1988;48:4881–5 [PubMed] [Google Scholar]
- 67.Savoye C, Swenberg C, Hugot S, Sy D, Sabattier R, Charlier M, et al. Thiol WR-1065 and disulphide WR-33278, two metabolites of the drug ethyol (WR-2721), protect DNA against fast neutron-induced strand breakage. Int J Radiat Biol 1997;71:193–202 [DOI] [PubMed] [Google Scholar]
- 68.Holwitt EA, Koda E, Swenberg CE. Enhancement of topoisomerase I-mediated unwinding of supercoiled DNA by the radioprotector WR-33278. Radiat Res 1990;124:107–19 [PubMed] [Google Scholar]
- 69.Treskes M, Nijtmans LG, Fichtinger-Schepman AM, van derVijgh WJ. Effects of the modulating agent WR2721 and its main metabolites on the formation and stability of cisplatin-DNA adducts in vitro in comparison to the effects of thiosulphate and diethyldithiocarbamate. Biochem Pharmacol 1992;43:1013–19 [DOI] [PubMed] [Google Scholar]
- 70.Carsten RE, Bachand AM, Bailey SM, Ullrich RL. Resveratrol reduces radiation-induced chromosome aberration frequencies in mouse bone marrow cells. Radiat Res 2008;169:633–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003;425:191–6 [DOI] [PubMed] [Google Scholar]
- 72.Nishimura S. Mammalian Ogg1/Mmh gene plays a major role in repair of the 8-hydroxyguanine lesion in DNA. 1. Prog Nucleic Acid Res Mol Biol 2001;68:107–23 [DOI] [PubMed] [Google Scholar]
- 73.Dahle J, Brunborg G, Svendsrud DH, Stokke T, Kvam E. Overexpression of human OGG1 in mammalian cells decreases ultraviolet A induced mutagenesis. Cancer Lett 2008;267:18–25 [DOI] [PubMed] [Google Scholar]
- 74.Dobson AW, Grishko V, LeDoux SP, Kelley MR, Wilson GL, Gillespie MN. Enhanced mtDNA repair capacity protects pulmonary artery endothelial cells from oxidant-mediated death. Am J Physiol Lung Cell Mol Physiol 2002;283:205–10 [DOI] [PubMed] [Google Scholar]
- 75.Kang KA, Lee JH, Chae S, Zhang R, Piao MJ, Kim HS, et al. Butin decreases oxidative stress-induced 8-hydroxy-2′-deoxyguanosine levels via activation of oxoguanine glycosylase 1. Chem Biol Interact 2009;181:338–42 [DOI] [PubMed] [Google Scholar]
- 76.Li MX, Wang D, Zhong ZY, Xiang DB, Li ZP, Xie JY, et al. Targeting truncated APE1 in mitochondria enhances cell survival after oxidative stress. Free Radic Biol Med 2008;45:592–601 [DOI] [PubMed] [Google Scholar]
- 77.Shibata A, Maeda D, Ogino H, Tsutsumi M, Nohmi T, Nakagama H, et al. Role of Parp-1 in suppressing spontaneous deletion mutation in the liver and brain of mice at adolescence and advanced age. Mutat Res 2009;664:20–7 [DOI] [PubMed] [Google Scholar]
- 78.Mortusewicz O, Amé JC, Schreiber V, Leonhardt H. Feedback-regulated poly(ADP-ribosyl)ation by PARP-1 is required for rapid response to DNA damage in living cells. Nucleic Acids Res 2007;35:7665–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ishizuka S, Martin K, Booth C, Potten CS, de Murcia G, Bürkle A, et al. Poly(ADP-ribose) polymerase-1 is a survival factor for radiation-exposed intestinal epithelial stem cells in vivo. Nucleic Acids Res 2003;31:6198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mitchell J, Smith GCM, Curtin NJ. Poly(ADP-ribose) polymerase-1 and DNA-dependent protein kinase have equivalent roles in double strand break repair following ionizing radiation. Int J Radiat Oncol Biol Phys 2009;75:1520–7 [DOI] [PubMed] [Google Scholar]
- 81.Fei P, El-Deiry WS. P53 and radiation responses. Oncogene 2003;22:5774–83 [DOI] [PubMed] [Google Scholar]
- 82.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell 2010;37:299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel MP, Masood A, Patel PS, Chanan-Khan AA. Targeting the Bcl-2. Curr Opin Oncol 2009;21:516–23 [DOI] [PubMed] [Google Scholar]
- 84.Morita A, Yamamoto S, Wang B, Tanaka K, Suzuki N, Aoki S, et al. Sodium orthovanadate inhibits p53-mediated apoptosis. Cancer Res 2010;70:257–65 [DOI] [PubMed] [Google Scholar]
- 85.Qiu W, Carson-Walter EB, Liu H, Epperly M, Greenberger JS, Zambetti GP, et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell 2008;2:576–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thotala DK, Geng L, Dickey AK, Hallahan DE, Yazlovitskaya EM. A new class of molecular targeted radioprotectors: GSK-3beta inhibitors. Int J Radiat Oncol Biol Phys 2010;76:557–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oh JM, Kim SH, Cho EA, Song YS, Kim WH, Juhnn YS. Human papillomavirus type 16 E5 protein inhibits hydrogen-peroxide-induced apoptosis by stimulating ubiquitin-proteasome-mediated degradation of Bax in human cervical cancer cells. Carcinogenesis 2010;31:402–10 [DOI] [PubMed] [Google Scholar]
- 88.Ye Y, Keyes KT, Zhang CF, Perez-Polo JR, Lin Y, Birnbaum Y. Additive effect of TAK-491, a new angiotensin receptor blocker, and pioglitazone, in reducing myocardial infarct size. Cardiovasc Drugs Ther 2010;24:107–20 [DOI] [PubMed] [Google Scholar]
- 89.Khoshnan A, Tindell C, Laux I, Bae D, Bennett B, Nel AE. The NF-kappa B cascade is important in Bcl-xL expression and for the anti-apoptotic effects of the CD28 receptor in primary human CD4+ lymphocytes. J Immunol 2000;165:1743–54 [DOI] [PubMed] [Google Scholar]
- 90.Erl W, Hansson GK, de Martin R, Draude G, Weber KS, Weber C. Nuclear factor-kappa B regulates induction of apoptosis and inhibitor of apoptosis protein-1 expression in vascular smooth muscle cells. Circ Res 1999;84:668–77 [DOI] [PubMed] [Google Scholar]
- 91.Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 2008;320:226–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 2008;320:226–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Daroczi B, Kari G, Ren Q, Dicker AP, Rodeck U. Nuclear factor kappaB inhibitors alleviate and the proteasome inhibitor PS-341 exacerbates radiation toxicity in zebrafish embryos. Mol Cancer Ther 2009;8:2625–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen N, Karantza-Wadsworth V. Role and regulation of autophagy in cancer. Biochim Biophys Acta 2009;1793:1516–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zois CE, Koukourakis MI. Radiation-induced autophagy in normal and cancer cells: towards novel cytoprotection and radio-sensitization policies? Autophagy 2009;5:442–50 [DOI] [PubMed] [Google Scholar]
- 96.Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol 2009;27:127–45 [DOI] [PubMed] [Google Scholar]
- 97.Koukourakis MI, Flordellis CS, Giatromanolaki A, Koukouraki S, Kapsoritakis A, Potamianos S, et al. Oral administration of recombinant human granulocyte macrophage colony-stimulating factor in the management of radiotherapy-induced esophagitis. Clin Cancer Res 1999;5:3970–6 [PubMed] [Google Scholar]
- 98.Kuter DJ. New thrombopoietic growth factors. Clin Lymphoma Myeloma 2009;9:S347–56 [DOI] [PubMed] [Google Scholar]
- 99.Balfour JA, Noble S. Becaplermin. BioDrugs 1999;11:359–64 [DOI] [PubMed] [Google Scholar]
- 100.Moore DC, Ehrlich MG, McAllister SC, Machan JT, Hart CE, Voigt C, et al. Recombinant human platelet-derived growth factor-BB augmentation of new-bone formation in a rat model of distraction osteogenesis. J Bone Joint Surg Am 2009;91:1973–84 [DOI] [PubMed] [Google Scholar]
- 101.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation 2003;107:1359–65 [DOI] [PubMed] [Google Scholar]
- 102.Hanft JR, Pollak RA, Barbul A, van Gils C, Kwon PS, Gray SM, et al. Phase I trial on the safety of topical rhVEGF on chronic neuropathic diabetic foot ulcers. J Wound Care 2008;17:30–2 [DOI] [PubMed] [Google Scholar]
- 103.Ji JA, Liu J, Shire SJ, Kamerzell TJ, Hong S, Billeci K, et al. Characteristics of rhVEGF release from topical hydrogel formulations. Pharm Res 2010;27:644–54 [DOI] [PubMed] [Google Scholar]
- 104.Zhang L, Sun W, Wang J, Zhang M, Yang S, Tian Y, et al. Mitigation effect of an FGF-2 peptide on acute gastrointestinal syndrome after high-dose ionizing radiation. Int J Radiat Oncol Biol Phys 2010;77:261–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Motomura K, Hagiwara A, Komi-Kuramochi A, Hanyu Y, Honda E, Suzuki M, et al. An FGF1:FGF2 chimeric growth factor exhibits universal FGF receptor specificity, enhanced stability and augmented activity useful for epithelial proliferation and radioprotection. Biochim Biophys Acta 2008;1780:1432–40 [DOI] [PubMed] [Google Scholar]
- 106.Maclachlan T, Narayanan B, Gerlach VL, Smithson G, Gerwien RW, Folkerts O, et al. Human fibroblast growth factor 20 (FGF-20; CG53135-05): a novel cytoprotectant with radioprotective potential. Int J Radiat Biol 2005;81:567–79 [DOI] [PubMed] [Google Scholar]
- 107.Song S, Ling-Hu H, Roebuck KA, Rabbi MF, Donnelly RP, Finnegan A. Interleukin-10 inhibits interferon-gamma-induced intercellular adhesion molecule-1 gene transcription in human monocytes. Blood 1997;89:4461–9 [PubMed] [Google Scholar]
- 108.Lindner H, Holler E, Gerbitz A, Johnson JP, Bornkamm GW, Eissner G. Influence of bacterial endotoxin on radiation-induced activation of human endothelial cells in vitro and in vivo: Interleukin-10 protects against transendothelial migration. Transplantation 1997;64:11370–3 [DOI] [PubMed] [Google Scholar]
- 109.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension 2009;54:619–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen Y, Rubin P, Williams J, Hernady E, Smudzin T, Okunieff P. Circulating IL-6 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys 2001;49:641–8 [DOI] [PubMed] [Google Scholar]
- 111.Kyrkanides S, Olschowka JA, Williams JP, Hansen JT, O'Banion MK. TNF alpha and IL-1beta mediate intercellular adhesion molecule-1 induction via microglia-astrocyte interaction in CNS radiation injury. J Neuroimmunol 1999;95:95–106 [DOI] [PubMed] [Google Scholar]
- 112.Colombel JF, Rutgeerts P, Malchow H, Jacyna M, Nielsen OH, Rask-Madsen J, et al. Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn's disease. Gut 2001;49:42–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwager K, Kaspar M, Bootz F, Marcolongo R, Paresce E, Neri D, et al. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res Ther 2009;11:R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Geffrotin C, Tricaud Y, Crechet F, Castelli M, Lefaix JL, Vaiman M. Unlike tenascin-X, tenascin-C is highly up-regulated in pig cutaneous and underlying muscle tissue developing fibrosis after necrosis induced by very high-dose gamma radiation. Radiat Res 1998;149:472–81 [PubMed] [Google Scholar]
- 115.Shoji F, Yonemitsu Y, Okano S, Yoshino I, Nakagawa K, Nakashima Y, et al. Airway-directed gene transfer of interleukin-10 using recombinant Sendai virus effectively prevents post-transplant fibrous airway obliteration in mice. Gene Ther 2003;10:213–18 [DOI] [PubMed] [Google Scholar]
- 116.McNamee EN, Ryan KM, Griffin EW, González-Reyes RE, Ryan KJ, Harkin A, et al. Noradrenaline acting at central beta-adrenoceptors induces interleukin-10 and suppressor of cytokine signaling-3 expression in rat brain: Implications for neurodegeneration. Brain Behav Immun 2010;24:660–71 [DOI] [PubMed] [Google Scholar]
- 117.Tsujimoto T, Kawaratani H, Kitazawa T, Yoshiji H, Fujimoto M, Uemura M, et al. Immunotherapy for nonalcoholic steatohepatitis using the multiple cytokine production modulator Y-40138. World J Gastroenterol 2009;15:5533–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yanagisawa J, Shiraishi T, Iwasaki A, Maekawa S, Higuchi T, Hiratuka M, et al. PPARalpha ligand WY14643 reduced acute rejection after rat lung transplantation with the upregulation of IL-4, IL-10 and TGFbeta mRNA expression. J Heart Lung Transplant 2009;28:1172–9 [DOI] [PubMed] [Google Scholar]
- 119.Mollinedo F, Gajate C, Morales AI, del Canto-Jañez E, Justies N, Collía F, et al. Novel anti-inflammatory action of edelfosine lacking toxicity with protective effect in experimental colitis. J Pharmacol Exp Ther 2009;329:439–49 [DOI] [PubMed] [Google Scholar]
- 120.Wijbrandts CA, Dijkgraaf MG, Kraan MC, Vinkenoog M, Smeets TJ, Dinant H, et al. The clinical response to infliximab in rheumatoid arthritis is in part dependent on pretreatment tumour necrosis factor alpha expression in the synovium. Ann Rheum Dis 2008;67:1139–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nishimoto N, Kishimoto T, Yoshizaki K. Anti-interleukin 6 receptor antibody treatment in rheumatic disease. Ann Rheum Dis 2000;59:i21–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Suzuki M, Hashizume M, Yoshida H, Mihara M. Anti-inflammatory mechanism of tocilizumab, a humanized anti-IL-6R antibody: effect on the expression of chemokine and adhesion molecule. Rheumatol Int 2010;30:309–15 [DOI] [PubMed] [Google Scholar]
- 123.Nilsson LM, Sun ZW, Nilsson J, Nordström I, Chen YW, Molkentin JD, et al. Novel blocker of NFAT activation inhibits IL-6 production in human myometrial arteries and reduces vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol 2007;292:1167–78 [DOI] [PubMed] [Google Scholar]
- 124.Liu S, Ma Z, Cai H, Li Q, Rong W, Kawano M. Inhibitory effect of baicalein on IL-6-mediated signaling cascades in human myeloma cells. Eur J Haematol 2010;84:137–44 [DOI] [PubMed] [Google Scholar]
- 125.Mertens M, Singh JA. Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol 2009;36:1118–25 [DOI] [PubMed] [Google Scholar]
- 126.Tsujino K, Kodama A, Kanaoka N, Maruta T, Kono M. Expression of pulmonary mRNA encoding ICAM-1, VCAM-1, and P-selectin following thoracic irradiation in mice. Radiat Med 1999;17:283–7 [PubMed] [Google Scholar]
- 127.Hallahan DE, Virudachalam S. Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation. Proc Natl Acad Sci U S A 1997;94:6432–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Olschowka JA, Kyrkanides S, Harvey BK, O'Banion MK, Williams JP, Rubin P, et al. ICAM-1 induction in the mouse CNS following irradiation. Brain Behav Immun 1997;11:273–85 [DOI] [PubMed] [Google Scholar]
- 129.Gaugler MH, Vereycken-Holler V, Squiban C, Vandamme M, Vozenin-Brotons MC, Benderitter M. Pravastatin limits endothelial activation after irradiation and decreases the resulting inflammatory and thrombotic responses. Radiat Res 2005;163:479–87 [DOI] [PubMed] [Google Scholar]
- 130.Haydont V, Bourgier C, Pocard M, Lusinchi A, Aigueperse J, Mathé D, et al. Pravastatin inhibits the Rho/CCN2/extracellular matrix cascade in human fibrosis explants and improves radiation-induced intestinal fibrosis in rats. Clin Cancer Res 2007;13:5331–40 [DOI] [PubMed] [Google Scholar]
- 131.Holler V, Buard V, Gaugler MH, Guipaud O, Baudelin C, Sache A, et al. Pravastatin limits radiation-induced vascular dysfunction in the skin. J Invest Dermatol 2009;129:1280–91 [DOI] [PubMed] [Google Scholar]
- 132.Vujaskovic Z, Anscher MS, Feng QF, Rabbani ZN, Amin K, Samulski TS, et al. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys 2001;50:851–5 [DOI] [PubMed] [Google Scholar]
- 133.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, et al. Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut 2003;52:1347–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chintalgattu V, Nair DM, Katwa LC. Cardiac myofibroblasts: a novel source of vascular endothelial growth factor (VEGF) and its receptors Flt-1 and KDR. J Mol Cell Cardiol 2003;35:277–86 [DOI] [PubMed] [Google Scholar]
- 135.Hamada N, Kuwano K, Yamada M, Hagimoto N, Hiasa K, Egashira K, et al. Anti-vascular endothelial growth factor gene therapy attenuates lung injury and fibrosis in mice. J Immunol 2005;175:1224–31 [DOI] [PubMed] [Google Scholar]
- 136.Yoshio Y, Miyazaki M, Abe K, Nishino T, Furusu A, Mizuta Y, et al. TNP-470, an angiogenesis inhibitor, suppresses the progression of peritoneal fibrosis in mouse experimental model. Kidney Int 2004;66:1677–85 [DOI] [PubMed] [Google Scholar]
- 137.Schwartz DL, Powis G, Thitai-Kumar A, He Y, Bankson J, Williams R, et al. The selective hypoxia inducible factor-1 inhibitor PX-478 provides in vivo radiosensitization through tumor stromal effects. Mol Cancer Ther 2009;8:947–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li SH, Shin DH, Chun YS, Lee MK, Kim MS, Park JW. A novel mode of action of YC-1 in HIF inhibition: stimulation of FIH-dependent p300 dissociation from HIF-1{alpha}. Mol Cancer Ther 2008;7:3729–38 [DOI] [PubMed] [Google Scholar]
- 139.Piguet P-F. Cytokines involved in pulmonary fibrosis. Int Rev Exp Pathol 1993;34:173–81 [DOI] [PubMed] [Google Scholar]
- 140.Hashimoto S, Gon Y, Takeshita I, Matsumoto K, Maruoka S, Horie T. Transforming growth factor-beta1 induces phenotypic modulation of human lung fibroblasts to myofibroblast through a c-Jun-NH2-terminal kinase-dependent pathway. Am J Respir Crit Care Med 2001;163:152–7 [DOI] [PubMed] [Google Scholar]
- 141.Fine A, Goldstein RH. The effect of transforming growth factor-beta on cell proliferation and collagen formation by lung fibroblasts. J Biol Chem 1987;262:3897–902 [PubMed] [Google Scholar]
- 142.Finkelstein JN, Johnson CJ, Baggs R, Rubin P. Early alterations in extracellular matrix and transforming growth factor β gene expression in mouse lung indicative of late radiation fibrosis. Int J Radiat Oncol Biol Phys 1994;28:621–31 [DOI] [PubMed] [Google Scholar]
- 143.Anscher MS, Kong FM, Andrews K, Clough R, Marks LB, Bentel G, et al. Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys 1998;41:1029–35 [DOI] [PubMed] [Google Scholar]
- 144.Nishioka A, Ogawa Y, Mima T, Jin YJ, Sonobe H, Kariya S, et al. Histopathologic amelioration of fibroproliferative change in rat irradiated lung using soluble transforming growth factor-beta (TGF-beta) receptor mediated by adenoviral vector. Int J Radiat Oncol Biol Phys 2004;58:1235–41 [DOI] [PubMed] [Google Scholar]
- 145.Liu X, Wang W, Hu H, Tang N, Zhang C, Liang W, et al. Smad3 specific inhibitor, naringenin, decreases the expression of extracellular matrix induced by TGF-beta1 in cultured rat hepatic stellate cells. Pharm Res 2006;23:82–9 [DOI] [PubMed] [Google Scholar]
- 146.Xavier S, Piek E, Fujii M, Javelaud D, Mauviel A, Flanders KC, et al. Amelioration of radiation-induced fibrosis: inhibition of transforming growth factor-beta signaling by halofuginone. J Biol Chem 2004;279:15167–76 [DOI] [PubMed] [Google Scholar]
- 147.Heeg MH, Koziolek MJ, Vasko R, Schaefer L, Sharma K, Muller GA, et al. The antifibrotic effects of relaxin in human renal fibroblasts are mediated in part by inhibition of the Smad2 pathway. Kidney Int 2005;68:96–109 [DOI] [PubMed] [Google Scholar]
- 148.Grygielko ET, Martin WM, Tweed C, Thornton P, Harling J, Brooks DP, et al. Inhibition of gene markers of fibrosis with a novel inhibitor of transforming growth factor-beta type I receptor kinase in puromycin-induced nephritis. J Pharmacol Exp Ther 2005;313:943–51 [DOI] [PubMed] [Google Scholar]
- 149.Bonniaud P, Margetts PJ, Kolb M, Schroeder JA, Kapoun AM, Damm D, et al. Progressive transforming growth factor {beta}1-induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor. Am J Respir Crit Care Med 2005;171:889–98 [DOI] [PubMed] [Google Scholar]
- 150.Li Y, Song LW, Peng RY, Wang DW, Jin MH, Gao YB, et al. Effects of SB203580 and WP631 on Smad signal transduction pathway in lung fibroblasts after irradiation. Ai Zheng 2008;27:698–702 [PubMed] [Google Scholar]
- 151.Nakazato H, Oku H, Yamane S, Tsuruta Y, Suzuki R. A novel anti-fibrotic agent pirfenidone suppresses tumor necrosis factor-alpha at the translational level. Eur J Pharmacol 2002;446:177–85 [DOI] [PubMed] [Google Scholar]
- 152.Card JW, Racz WJ, Brien JF, Margolin SB, Massey TE. Differential effects of pirfenidone on acute pulmonary injury and ensuing fibrosis in the hamster model of amiodarone-induced pulmonary toxicity. Toxicol Sci 2003;75:169–80 [DOI] [PubMed] [Google Scholar]
- 153.Lin SL, Chen RH, Chen YM, Chiang WC, Lai CF, Wu KD, et al. Pentoxifylline attenuates tubulointerstitial fibrosis by blocking Smad3/4-activated transcription and profibrogenic effects of connective tissue growth factor. J Am Soc Nephrol 2005;16:2702–13 [DOI] [PubMed] [Google Scholar]
- 154.Yu J, Moon A, Kim HR. Both platelet-derived growth factor receptor (PDGFR)-alpha and PDGFR-beta promote murine fibroblast cell migration. Biochem Biophys Res Commun 2001;282:697–700 [DOI] [PubMed] [Google Scholar]
- 155.Li M, Ping G, Plathow C, Trinh T, Lipson KE, Hauser K, et al. Small molecule receptor tyrosine kinase inhibitor of platelet-derived growth factor signaling (SU9518) modifies radiation response in fibroblasts and endothelial cells. BMC Cancer 2006;6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest 2004;114:1308–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thomas DM, Fox J, Haston CK. Imatinib therapy reduces radiation-induced pulmonary mast cell influx and delays lung disease in the mouse. Int J Radiat Biol 2010;86:436–44 [DOI] [PubMed] [Google Scholar]
- 158.Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med 2005;201:925–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Piguet P-F. Is “tumor necrosis factor” the major effector of pulmonary fibrosis? Eur Cytokine Netw 1990;1:257–8 [PubMed] [Google Scholar]
- 160.Hong JH, Jung SM, Tsao TC, Wu CJ, Lee CY, Chen FH, et al. Bronchoalveolar lavage and interstitial cells have different roles in radiation-induced lung injury. Int J Radiat Biol 2003;79:159–67 [DOI] [PubMed] [Google Scholar]
- 161.Piguet PF, Collart MA, Grau GE, Kapanci Y, Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med 1989;170:655–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang M, Qian J, Xing X, Kong FM, Zhao L, Chen M, et al. Inhibition of the tumor necrosis factor-alpha pathway is radioprotective for the lung. Clin Cancer Res 2008;14:1868–76 [DOI] [PubMed] [Google Scholar]
- 163.Di Sabatino A, Ciccocioppo R, Benazzato L, Sturniolo GC, Corazza GR. Infliximab downregulates basic fibroblast growth factor and vascular endothelial growth factor in Crohn's disease patients. Aliment Pharmacol Ther 2004;19:1019–24 [DOI] [PubMed] [Google Scholar]
- 164.Bargagli E, Galeazzi M, Bellisai F, Volterrani L, Rottoli P. Infliximab treatment in a patient with systemic sclerosis associated with lung fibrosis and pulmonary hypertension. Respiration 2008;75:346–9 [DOI] [PubMed] [Google Scholar]
- 165.Ansari R, Gaber MW, Wang B, Pattillo CB, Miyamoto C, Kiani MF. Anti-TNFA (TNF-alpha) treatment abrogates radiation-induced changes in vacular density and tissue oxygenation. Radiat Res 2007;167:80–6 [DOI] [PubMed] [Google Scholar]
- 166.Marques LJ, Zheng L, Poulakis N, Guzman J, Costabel U. Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages. Am J Respir Crit Care Med 1999;159:508–11 [DOI] [PubMed] [Google Scholar]
- 167.Delanian S, Porcher R, Rudant J, Lefaix JL. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. J Clin Oncol 2005;23:8570–9 [DOI] [PubMed] [Google Scholar]
- 168.Magnusson M, Höglund P, Johansson K, Jönsson C, Killander F, Malmström P, et al. Pentoxifylline and vitamin E treatment for prevention of radiation-induced side-effects in women with breast cancer: a phase two, double-blind, placebo-controlled randomised clinical trial (Ptx-5). Eur J Cancer 2009;45:2488–95 [DOI] [PubMed] [Google Scholar]
- 169.Gothard L, Cornes P, Earl J, Hall E, MacLaren J, Mortimer P, et al. Double-blind placebo-controlled randomised trial of vitamin E and pentoxifylline in patients with chronic arm lymphoedema and fibrosis after surgery and radiotherapy for breast cancer. Radiother Oncol 2004;73:133–9 [DOI] [PubMed] [Google Scholar]
- 170.Misirlioglu CH, Demirkasimoglu T, Kucukplakci B, Sanri E, Altundag K. Pentoxifylline and alpha-tocopherol in prevention of radiation-induced lung toxicity in patients with lung cancer. Med Oncol 2007;24:308–11 [DOI] [PubMed] [Google Scholar]
- 171.Gothard L, Cornes P, Brooker S, Earl J, Glees J, Hall E, et al. Phase II study of vitamin E and pentoxifylline in patients with late side effects of pelvic radiotherapy. Radiother Oncol 2005;75:334–41 [DOI] [PubMed] [Google Scholar]
- 172.Morimoto , K , Amano H, Sonoda F, Baba M, Senba M, Yoshimine H, et al. Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am J Respir Cell Mol Biol 2001;24:608–15 [DOI] [PubMed] [Google Scholar]
- 173.Fan S, Gao M, Meng Q, Laterra JJ, Symons MH, Coniglio S, et al. Role of NF-kappaB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene 2005;24:1749–66 [DOI] [PubMed] [Google Scholar]
- 174.Hu SY, Duan HF, Li QF, Yang YF, Chen JL, Wang LS, et al. Hepatocyte growth factor protects endothelial cells against gamma ray irradiation-induced damage. Acta Pharmacol Sin 2009;30:1415–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hu S, Chen Y, Li L, Chen J, Wu B, Zhou X, et al. Effects of adenovirus-mediated delivery of the human hepatocyte growth factor gene in experimental radiation-induced heart disease. Int J Radiat Oncol Biol Phys 2009;75:1537–44 [DOI] [PubMed] [Google Scholar]
- 176.Yaekashiwa M, Nakayama S, Ohnuma K, Sakai T, Abe T, Satoh K, et al. Simultaneous or delayed administration of hepatocyte growth factor equally represses the fibrotic changes in murine lung injury induced by bleomycin. A morphologic study. Am J Respir Crit Care Med 1997;156:1937–44 [DOI] [PubMed] [Google Scholar]
- 177.Chi CH, Liu IL, Lo WY, Liaw BS, Wang YS, Chi KH. Hepatocyte growth factor gene therapy prevents radiation-induced liver damage. World J Gastroenterol 2005;11:1496–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wen X, Li Y, Hu K, Dai C, Liu Y. Hepatocyte growth factor receptor signaling mediates the anti-fibrotic action of 9-cis-retinoic acid in glomerular mesangial cells. Am J Pathol 2005;167:947–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Finlay GA, Thannickal VJ, Fanburg BL, Paulson KE. Transforming growth factor-beta 1-induced activation of the ERK pathway/activator protein-1 in human lung fibroblasts requires the autocrine induction of basic fibroblast growth factor. J Biol Chem 2000;275:27650–6 [DOI] [PubMed] [Google Scholar]
- 180.Girinsky T. Effects of ionizing radiation on the blood vessel wall. J Mal Vasc 2000;25:321–4 [PubMed] [Google Scholar]
- 181.Haimovitz-Friedman A, Vlodavsky I, Chaudhuri A, Witte L, Fuks Z. Autocrine effects of fibroblast growth factor in repair of radiation damage in endothelial cells. Cancer Res 1991;51:2552–8 [PubMed] [Google Scholar]
- 182.Gridley DS, Bonnet RB, Bush DA, Franke C, Cheek GA, Slater JD, et al. Time course of serum cytokines in patients receiving proton or combined photon/proton beam radiation for resectable but medically inoperable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;60:759–66 [DOI] [PubMed] [Google Scholar]
- 183.Molteni A, Moulder JE, Cohen EF, Ward WF, Fish BL, Taylor JM, et al. Control of radiation-induced pneumopathy and lung fibrosis by angiotensin-converting enzyme inhibitors and an angiotensin II type 1 receptor blocker. Int J Radiat Biol 2000;76:523–32 [DOI] [PubMed] [Google Scholar]
- 184.Gore E. Celecoxib and radiation therapy in non-small-cell lung cancer. Oncology (Williston Park). 2004;18:10–14 [PubMed] [Google Scholar]
- 185.Mazonakis M, Varveris H, Damilakis J, Theoharopoulos N, Gourtsoyiannis N. Radiation dose to conceptus resulting from tangential breast irradiation. Int J Radiat Oncol Biol Phys 2003;55:386–91 [DOI] [PubMed] [Google Scholar]
- 186.Mazonakis M, Damilakis J, Varveris H, Gourtsoyiannis N. Therapeutic external irradiation in women of reproductive age: risk estimation of hereditary effects. Br J Radiol 2004;77:847–50 [DOI] [PubMed] [Google Scholar]
- 187.Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys 2003;56:83–8 [DOI] [PubMed] [Google Scholar]
- 188.Mettler FA, Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008;248:254–63 [DOI] [PubMed] [Google Scholar]
- 189.Brenner DJ. Should we be concerned about the rapid increase in CT usage? Rev Environ Health 2010;25:63–8 [DOI] [PubMed] [Google Scholar]
- 190.Jacob P, Rühm W, Walsh L, Blettner M, Hammer G, Zeeb H. Is cancer risk of radiation workers larger than expected? Occup Environ Med 2009;66:789–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Beck P. Overview of research on aircraft crew dosimetry during the last solar cycle. Radiat Prot Dosimetry 2009;136:244–50 [DOI] [PubMed] [Google Scholar]
- 192.Little JB. Radiation carcinogenesis. Carcinogenesis 2000;21:397–404 [DOI] [PubMed] [Google Scholar]
- 193.Kataoka Y, Perrin J, Hunter N, Milas L, Grdina DJ. Antimutagenic effects of amifostine: clinical implications. Semin Oncol 1996;23:53–7 [PubMed] [Google Scholar]
- 194.Kataoka Y, Basic I, Perrin J, Grdina DJ. Antimutagenic effects of radioprotector WR-2721 against fission-spectrum neurons and 60Co gamma-rays in mice. Int J Radiat Biol 1992;61:387–92 [DOI] [PubMed] [Google Scholar]
- 195.Grdina DJ, Peraino C, Carnes BA, Hill CK. Protective effect of S-2-(3-aminopropylamino)ethylphosphorothioic acid against induction of altered hepatocyte foci in rats treated once with gamma-radiation within one day after birth. Cancer Res 1985;45:5379–81 [PubMed] [Google Scholar]
- 196.Marusyk A, Porter CC, Zaberezhnyy V, DeGregori J. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol 2010;8:e1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Maurici D, Monti P, Campomenosi P, North S, Frebourg T, Fronza G, et al. Amifostine (WR2721) restores transcriptional activity of specific p53 mutant proteins in a yeast functional assay. Oncogene 2001;20:3533–40 [DOI] [PubMed] [Google Scholar]
- 198.Grochova D, Vankova J, Damborsky J, Ravcukova B, Smarda J, Vojtesek B, et al. Analysis of transactivation capability and conformation of p53 temperature-dependent mutants and their reactivation by amifostine in yeast. Oncogene 2008;27:1243–52 [DOI] [PubMed] [Google Scholar]
- 199.Liu SC, Murley JS, Woloschak G, Grdina DJ. Repression of c-myc gene expression by the thiol and disulfide forms of the cytoprotector amifostine. Carcinogenesis 1997;18:2457–9 [DOI] [PubMed] [Google Scholar]
- 200.Walker DM, Kajon AE, Torres SM, Carter MM, McCash CL, Swenberg JA, et al. WR1065 mitigates AZT-ddI-induced mutagenesis and inhibits viral replication. Environ Mol Mutagen 2009;50:460–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fält S, Holmberg K, Lambert B, Wennborg A. Long-term global gene expression patterns in irradiated human lymphocytes. Carcinogenesis 2003;24:1837–45 [DOI] [PubMed] [Google Scholar]
- 202.Syljuåsen RG, Krolewski B, Little JB. Molecular events in radiation transformation. Radiat Res 2001;155:215–21 [DOI] [PubMed] [Google Scholar]
- 203.Tachiiri S, Katagiri T, Tsunoda T, Oya N, Hiraoka M, Nakamura Y. Analysis of gene-expression profiles after gamma irradiation of normal human fibroblasts. Int J Radiat Oncol Biol Phys 2006;64:272–9 [DOI] [PubMed] [Google Scholar]
- 204.Senzer N, Nemunaitis J. A review of contusugene ladenovec (Advexin) p53 therapy. Curr Opin Mol Ther 2009;11:54–61 [PubMed] [Google Scholar]
- 205.Impicciatore G, Sancilio S, Miscia S, Di Pietro R. Nutlins and ionizing radiation in cancer therapy. Curr Pharm Des 2010;16:1427–2 [DOI] [PubMed] [Google Scholar]
- 206.Mohammad RM, Wu J, Azmi AS, Aboukameel A, Sosin A, Wu S, et al. An MDM2 antagonist (MI-319) restores p53 functions and increases the life span of orally treated follicular lymphoma bearing animals. Mol Cancer 2010;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Klasa RJ, Gillum AM, Klem RE, Frankel SR. Oblimersen Bcl-2 antisense: facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev 2002;12:193–213 [DOI] [PubMed] [Google Scholar]
- 208.Ho JN, Kang GY, Lee SS, Kim J, Bae IH, Hwang SG, et al. Bcl-X(L) and STAT3 mediate malignant actions of gamma-irradiation in lung cancer cells. Cancer Sci 2010;101:1417–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhan Q, Alamo I, Yu K, Boise LH, Cherney B, Tosato G, et al. The apoptosis-associated gamma-ray response of BCL-X(L) depends on normal p53 function. Oncogene 1996;13:2287–93 [PubMed] [Google Scholar]
- 210.Mahaney BL, Meek K, Lees-Miller SP. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J 2009;417:639–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rosidi B, Wang M, Wu W, Sharma A, Wang H, Iliakis G. Histone H1 functions as a stimulatory factor in backup pathways of NHEJ. Nucleic Acids Res 2008;36:1610–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mitchell J, Smith GC, Curtin NJ. Poly(ADP-ribose) polymerase-1 and DNA-dependent protein kinase have equivalent roles in double strand break repair following ionizing radiation. Int J Radiat Oncol Biol Phys 2009;75:1520–7 [DOI] [PubMed] [Google Scholar]
- 213.Zhang Y, Zhou J, Cao X, Zhang Q, Lim CU, Ullrich RL, et al. Partial deficiency of DNA-PKcs increases ionizing radiation-induced mutagenesis and telomere instability in human cells. Cancer Lett 2007;250:63–73 [DOI] [PubMed] [Google Scholar]
- 214.Li AY, Boo LM, Wang SY, Lin HH, Wang CC, Yen Y, et al. Suppression of nonhomologous end joining repair by overexpression of HMGA2. Cancer Res 2009;69:5699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Durant S, Karran P. Vanillins—a novel family of DNA-PK inhibitors. Nucleic Acids Res 2003;31:5501–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Haley B, Paunesku T, Protić M, Woloschak GE. Response of heterogeneous ribonuclear proteins (hnRNP) to ionising radiation and their involvement in DNA damage repair. Int J Radiat Biol 2009;85:643–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sato N, Mizumoto K, Nakamura M, Ueno H, Minamishima YA, Farber JL, et al. A possible role for centrosome overduplication in radiation-induced cell death. Oncogene 2000;19:5281–90 [DOI] [PubMed] [Google Scholar]
- 218.Maxwell CA, Fleisch MC, Costes SV, Erickson AC, Boissière A, Gupta R, et al. Targeted and nontargeted effects of ionizing radiation that impact genomic instability. Cancer Res 2008;68:8304–11 [DOI] [PubMed] [Google Scholar]
- 219.Ferguson MW, Duncan J, Bond J, Bush J, Durani P, So K, et al. Prophylactic administration of avotermin for improvement of skin scarring: three double-blind, placebo-controlled, phase I/II studies. Lancet 2009;373:1264–74 [DOI] [PubMed] [Google Scholar]
- 220.Blanco R, Muñoz P, Flores JM, Klatt P, Blasco MA. Telomerase abrogation dramatically accelerates TRF2-induced epithelial carcinogenesis. Genes Dev 2007;21:206–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Slijepcevic P. The role of DNA damage response proteins at telomeres—an “integrative” model. DNA Repair (Amst) 2006;5:1299–306 [DOI] [PubMed] [Google Scholar]
- 222.Huda N, Tanaka H, Mendonca MS, Gilley D. DNA damage-induced phosphorylation of TRF2 is required for the fast pathway of DNA double-strand break repair. Mol Cell Biol 2009;29:3597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Natarajan M, Mohan S, Konopinski R, Otto RA, Herman TS. Induced telomerase activity in primary aortic endothelial cells by low-LET gamma-radiation is mediated through NF-kappaB activation. Br J Radiol 2008;81:711–20 [DOI] [PubMed] [Google Scholar]
- 224.Hervé JC, Derangeon M, Bahbouhi B, Mesnil M, Sarrouilhe D. The connexin turnover, an important modulating factor of the level of cell-to-cell junctional communication: comparison with other integral membrane proteins. J Membr Biol 2007;217:21–33 [DOI] [PubMed] [Google Scholar]
- 225.Hervé JC, Dhein S. Peptides targeting gap junctional structures. Curr Pharm Des 2010;16:3056–70 [DOI] [PubMed] [Google Scholar]
- 226.Segretain D, Fiorini C, Decrouy X, Defamie N, Prat JR, Pointis G. A proposed role for ZO-1 in targeting connexin 43 gap junctions to the endocytic pathway. Biochimie 2004;86:241–4 [DOI] [PubMed] [Google Scholar]
- 227.Jin EJ, Lee SY, Jung JC, Bang OS, Kang SS. TGF-beta3 inhibits chondrogenesis of cultured chick leg bud mesenchymal cells via downregulation of connexin 43 and integrin beta4. J Cell Physiol 2008;214:345–53 [DOI] [PubMed] [Google Scholar]
- 228.Hakulinen P, Mäki-Paakkanen J, Naarala J, Kronberg L, Komulainen H. Potent inhibition of gap junctional intercellular communication by 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone (MX) in BALB/c 3T3 cells. Toxicol Lett 2004;151:439–49 [DOI] [PubMed] [Google Scholar]
- 229.Khan MA, Van Dyk J, Yeung IW, Hill RP. Partial volume rat lung irradiation; assessment of early DNA damage in different lung regions and effect of radical scavengers. Radiother Oncol 2003;66:95–102 [DOI] [PubMed] [Google Scholar]
- 230.Lorimore SA, Chrystal JA, Robinson JI, Coates PJ, Wright EG. Chromosomal instability in unirradiated hemaopoietic cells induced by macrophages exposed in vivo to ionizing radiation. Cancer Res 2008;68:8122–6 [DOI] [PubMed] [Google Scholar]
- 231.Al-Abed Y, Dabideen D, Aljabari B, Valster A, Messmer D, Ochani M, et al. ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. J Biol Chem 2005;280:36541–4 [DOI] [PubMed] [Google Scholar]
- 232.Radzioch D, Varesio L. Protein kinase C inhibitors block the activation of macrophages by IFN- beta but not by IFN-gamma. J Immunol 1988;l4:1259–63 [PubMed] [Google Scholar]
- 233.Ma DK, Guo JU, Ming GL, Song H. DNA excision repair proteins and Gadd45 as molecular players for active DNA demethylation. Cell Cycle 2009;8:1526–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Peterson DE, Bensadoun RJ, Roila F; ESMO Guidelines Working Group Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann Oncol 2010;21:v261–5 [DOI] [PubMed] [Google Scholar]
- 235.Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 2007;109:820–31 [DOI] [PubMed] [Google Scholar]