Abstract
Objectives
Cigarette smoking-induced airway disease commonly results in an overall increase of non-specific lung markings on chest radiography. This has been described as “dirty chest”. As the morphological substrate of this condition is similar to the anthracosilicosis of coal workers, we hypothesised that it is possible to quantify the radiological changes using the International Labour Organization (ILO) classification of pneumoconiosis. The aims of this study were to evaluate whether there is a correlation between the extent of cigarette smoking and increased lung markings on chest radiography and to correlate the chest radiographic scores with findings on CT studies.
Methods
In a prospective analysis a cohort of 85 smokers was examined. The cigarette consumption was evaluated in pack years (defined as 20 cigarettes per day over 1 year). Film reading was performed by two board-certified radiologists. Chest radiographs were evaluated for the presence of thickening of bronchial walls, the presence of linear or nodular opacities, and emphysema. To correlate the smoking habits with the increase of overall lung markings in chest radiography, the ILO profusion score was converted to numbers ranging from zero to nine. Chest radiographs were rated according to the complete set of standard films of the revised ILO classification.
Results
63/85 (74%) of the smokers showed an increase in overall lung markings on chest radiography; 32 (37%) had an ILO profusion score of <1/1, 29 (34%) had an ILO profusion score of <2/2 and 2 (2%) had an ILO score of ≥2/2. There was a significant positive linear correlation between the increase of overall lung markings on chest radiography and the cigarette consumption quantified as pack years (r=0.68). The majority of the heavy smokers (>40 pack years) showed emphysema; there was no significant difference between the prevalence of emphysema as diagnosed by CT (62%) or chest radiography (71%) (p<0.05).The most common findings in CT were thickening of bronchial walls (64%) and the presence of emphysema (62%) and of intralobular opacities (61%). Ground-glass opacities were seen in only 7% of our patients.
Conclusion
Bronchial wall thickening and intralobular opacities as seen in CT showed a positive linear correlation with the increase of overall lung markings on chest radiography.
Tobacco smoke is the most important and well-known causative factor for the development of chronic bronchitis, bronchial cancer and emphysema [1]. Cigarette smoking also results in a focal accumulation of macrophages within the walls of the respiratory bronchioles and adjacent alveoli described as respiratory bronchiolitis-associated interstitial lung disease (RB-ILD) [2-6]. The common finding of “dirty chest”, an overall increase in non-specific lung markings on chest radiography in such patients, is frequently identified in daily routine [7-10]. Remy-Jardin et al [11] found that the morphological substrate of cigarette smoking-induced changes in chest radiography were parenchymal micronodules and intralobular opacities comparable to the findings in anthracosilicosis of coal workers. Therefore, we hypothesised that it might be possible to quantify the smoking-induced changes of the lung using the International Labour Organization (ILO) classification [12].
The aims of this study were to evaluate whether there was a correlation between the extent of cigarette smoking and increased lung markings in chest radiography and to correlate chest radiographic scores with findings on CT studies. To the best of our knowledge, an evaluation like this has not been described in the clinical literature so far.
Methods and material
We performed a prospective analysis on a consecutive cohort of smokers who were admitted to the Department of Radiology at Allgemeines Krankenhaus, Hagen, Germany, within a 3-month period. Thoracic CT and chest radiography were simultaneously performed for diagnostic purposes within 1 week. All patients were clinically stable at the time of the study. To avoid misinterpretation of parenchymal changes due to acute infectious processes, patients were excluded from this evaluation if they had any evidence of pneumonia. Other exclusion criteria were knowledge of interstitial lung disease such as sarcoidosis, extrinsic allergic alveolitis, lymphangiosis carcinomatosa or silicosis. The study was approved by the local ethics committee and all patients gave informed consent.
During the observation period a total of 85 patients were enrolled. The demographic data and clinical features are shown in Table 1.
Table 1. Demographic data, indication and smoking habit of the 85 enrolled patients.
| Age (years), mean | 65.5 | |||
| Age (years), range | 42–82 | |||
| Gender | ||||
| Male, n (%) | 56 (66) | |||
| Female, n (%) | 29 (34) | |||
| Indication, n (%) | Suspected malignancy: 18 (21) | Pulmonary embolism: 9 (11) | Staging: 40 (47) | Others: 18 (21) |
| Cigarette consumption, n | <20 py: 33 | 20–<40 py: 30 | 40–<60 py: 13 | ≥60 py: 9 |
py, pack years.
Data acquisition
Postero-anterior chest radiographs were obtained in full inspiration on a standard system (Multix R; Siemens, Erlangen, Germany) with the following parameters: 120 kV, 2.5 mAs and antiscatter grid with a 180-cm focus–detector distance. Storage-phosphor images were obtained (Agfa CR 85 X; Agfa, Mortsel, Belgium) using 35×43 cm imaging plates (model CR MD4.0; Agfa). The post-processing algorithms provided by the manufacturer permitted images closely resembling film–screen images. Hard copies were printed via a laser printer (LR 3300; Agfa) on laser film.
Multidetector CT was carried out using a Toshiba Aquilion 64 machine (Toshiba Medical Systems, Tokyo, Japan). Images were obtained in full inspiration using a 64×0.75 mm slice collimation with a tube voltage of 120 kV. The tube current (milliampere) was adjusted relative to patient attenuation by means of the Sure Exposure® modus (Toshiba Medical Systems). The reconstruction slice thickness was 3–5 mm and three reconstruction planes (axial, frontal and sagittal) were automatically obtained. The images were reconstructed in a high spatial resolution algorithm and displayed with a wide window setting at a width of 2000 HU and a level of −500 HU. During CT, 100–120 ml of a 300/400 mg l−1 I contrast medium (Imeron®; Altana, Koblenz, Germany) was administered intravenously at a rate of 2–3 ml s−1 with a power injector followed by a 30 ml normal saline “chaser”.
Assessment of chest radiograph and CT
Film reading was performed by two board-certified radiologists. Thickening of the bronchial walls (visible as tram tracks or ring shadows) and the presence of linear or nodular opacities were noted. The presence of emphysema was classified semiquantitatively as moderate or severe. In order to quantify the overall increase of lung markings, we rated the chest radiographs according to the complete set of standard films of the revised ILO classification [12]. The profusion score was converted to numerical numbers from zero to nine according to increasing density of small opacities as follows: 0/0=0, 0/1=1, 1/0=2, 1/1=3 etc.
The CT scans were reviewed by two chest radiologists. CT scans were analysed for the presence of bronchial abnormalities, decreased lung attenuation, increased lung opacification and nodules. The presence of directly visible small airways with an internal diameter of <2 mm or a distance of <1 cm to the pleura was noted as a sign of thickening of small airways. The thickening of bronchial walls as a sign of chronic bronchitis was classified by the two readers as moderate or severe compared with the representative high resolution CT (HRCT) images of airway wall thickness as published by Awadh et al [13]. Vascular attenuation (thinning of pulmonary vessels and reduction in their number) and distortion (increased branching angle or straightening) were considered as signs of emphysema. Centrilobular emphysema was defined as round areas of low attenuation ≤1 cm in diameter within a homogeneous background of normal lung parenchyma. Panlobular emphysema was characterised as large and extensive areas of uniform low attenuation.
Increased lung opacification was described as ground-glass attenuation, consolidation or reticular opacities. Ground-glass opacification was defined by areas of hazy parenchymal opacities that did not obscure the underlying vasculature, whereas an opacity obscuring the pulmonary vessels was defined as a region of consolidation. Reticular opacities were divided into inter- or intralobular septal thickening. Thickened interlobular septa were defined as linear opacities in the lung periphery measuring 1–2 cm in length and extending to the pleural surface. The interlobular septal thickening outlined lobules 1–2.5 cm in diameter and appeared polygonal in shape. Reticular patterns which did not fit the characteristics of interlobular septal thickening were defined as intralobular septal thickening. Abnormalities considered under this heading were pathologically heterogeneous and included thickening of the alveolar wall and abnormalities of the respiratory bronchus. These intralobular lines may be fine and difficult to see or coarse and irregular [14].
Micronodules were defined as rounded opacities with a diameter of <5 mm and were classified as sharp or ill defined, centrilobular or subpleural. Ill-defined centrilobular opacities as well as intralobular lines were added to the term “intralobular opacities”.
Clinical features
We evaluated the patients' medical reports with regard to the indication for chest radiograph, CT and underlying illnesses. The patients were interviewed regarding their cigarette consumption evaluated in pack years (defined as 20 cigarettes per day over 1 year), occupational dust exposure or knowledge of chronic lung disease.
Statistic evaluation
We correlated the smoking habits with the increase of overall lung markings in chest radiographs, classified according to the ILO profusion score, by means of a linear regression model (Excel 2007; Microsoft Corp., Redmond, WA). Pearson's χ2 tests were performed to test significant correlations between the extent of cigarette consumption and different findings (e.g. bronchial wall thickening, increase of linear pattern, emphysema) in both chest radiography and CT. Statistical significance was set at p<0.05. Post-hoc sample size analysis was performed using the G*Power3.1 Program (Faul, Erdfelder, Lang and Buchner, Düsseldorf, Germany [15]). 88 patients were needed to reach an acceptable confidence level of 95% and statistical power of at least 80%.
Results
Chest radiography
Thickening of bronchial walls was seen in 63 out of 85 (74%) patients without any significant differences regarding the extent of cigarette consumption (p<0.05). An increase of linear structures was found in 22/85 (26%) patients. Here we found a significant difference between smokers with ≥20 pack years and smokers with <20 pack years (χ2=5.4, p<0.01). In total, 60/85 (71%) of the included patients showed emphysema, which was classified as moderate in 39 of 60 (65%) patients and severe in 21 of 60 (35%) patients. Severe emphysema was more often observed in heavy smokers with ≥60 pack years (6 of 9 patients, 67%); there was a significant difference between this group and moderate smokers with <20 pack years (χ2=9.7, p<0.05) as well as moderate smokers with 20–<40 pack years (χ2=7.0, p<0.05).
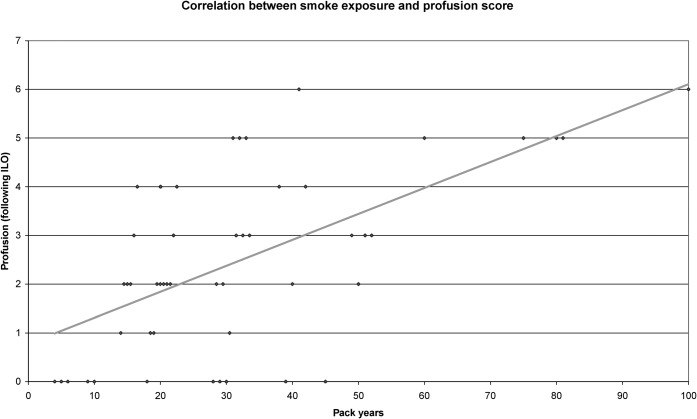
63 of 85 (74%) patients showed an increase in overall lung markings in chest radiography. Only 22 (26%) of our patients demonstrated a normal appearance of lung parenchyma. An increase of overall lung markings according to the ILO profusion score <1/1 was diagnosed in 32 (37%) patients; the majority of these patients were smokers with <20 pack years (n=16), but smokers with <40 pack years (n=12) also showed this finding. Only 4 of 22 smokers with ≥40 pack years showed an ILO score of <1/1. 31 of 85 (36%) patients showed findings indicating an ILO score of ≥1/1 (Figure 1). In this group, we found only 3 patients with <20 pack years (4%) but 28 patients with ≥20 pack years (33%). The highest profusion score seen in our evaluation was ILO 2/2 (n=2). 8 (89%) of the heavy smokers with ≥60 pack years showed ILO profusion scores of ≥2/1. Details pertaining to findings in chest radiography are presented in Table 2. There was a significant positive linear correlation between the reticulonodular opacity classified according to the ILO profusion score and the tobacco exposition quantified as pack years (r=0.68, p<0.0001; Figure 2).
Figure 1.
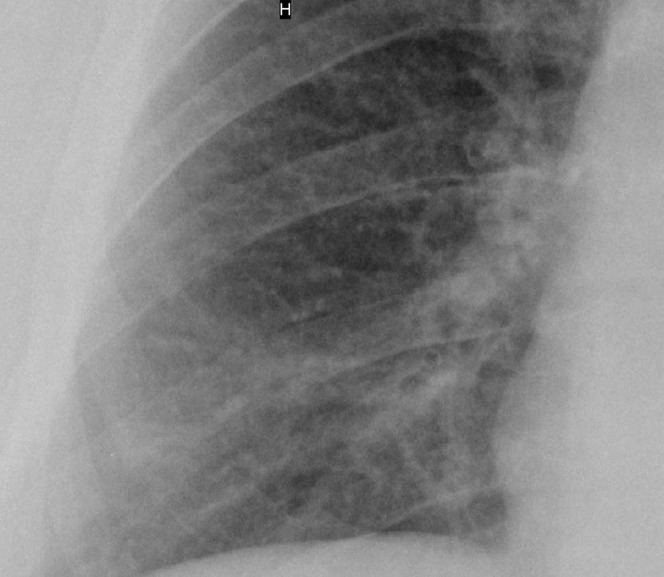
Chest radiograph of a heavy smoker (38 pack years) showing an increase in overall lung markings with numerous small nodules, singular linear opacities and diffuse bronchial wall thickening; the normal lung markings are partially obscured. The finding is comparable to the profusion score of 2/1 according to the International Labour Organization classification.
Table 2. Findings in chest radiography correlated with the cigarette consumption.
| Findings in chest radiography | Cigarette consumption (pack years) |
Total (n=85) | |||||
| <20 (n=33) | 20–<40 (n=30) | 40–<60 (n=13) | ≥60 (n=9) | ||||
| Bronchial wall thickening | 28 (85%) | 19 (64%) | 10 (77%) | 6 (67%) | 63 (74%) | ||
| Linear pattern | 4 (12%) | 11 (37%) | 4 (31%) | 3 (33%) | 22 (26%) | ||
| Overall marking score | 0 | 0/0 | 14 (42%) | 5 (17%) | 3 (23%) | 0 | 22 (26%) |
| 1 | 0/1 | 8 (24%) | 5 (17%) | 0 | 1 (11%) | 14 (16%) | |
| 2 | 1/0 | 8 (24%) | 7 (23%) | 3 (23%) | 0 | 18 (21%) | |
| 3 | 1/1 | 1 (3%) | 5 (17%) | 5 (38%) | 2 (22%) | 13 (15%) | |
| 4 | 1/2 | 2 (6%) | 4 (13%) | 2 (15%) | 2 (22%) | 10 (12%) | |
| 5 | 2/1 | 0 | 3 (10%) | 0 | 3 (33%) | 6 (7%) | |
| 6 | 2/2 | 0 | 1 (3%) | 0 | 1 (11%) | 2 (2%) | |
| Emphysema | Moderate | 13 (40%) | 16 (53%) | 8 (61%) | 2 (22%) | 39 (46%) | |
| Severe | 5 (15%) | 6 (20%) | 4 (13%) | 6 (67%) | 21 (25%) | ||
| Total | 18 (55%) | 22 (73%) | 12 (92%) | 8 (89%) | 60 (71%) | ||
Figure 2.
Graphic analysis of regression showing a significant positive linear correlation between the reticulonodular opacity and tobacco exposition (r=0.68). ILO, International labour Organizition.
CT findings
The most common findings in CT were bronchial wall thickening (64%), emphysema (62%) and the presence of intralobular opacities (61%). Intralobular smooth nodular opacities (Figure 3) were seen in 39% of patients and intralobular septal thickening was seen in 58%. The latter was often accompanied by more prominent interlobular septal lines. Thickening of interlobular septa without intralobular septal lines was only seen in 6%. There was no significant difference between moderate smokers (<20 pack years) and heavy smokers (≥20 pack years) with regard to the findings of bronchial wall thickening. Intralobular opacities were seen in up to 77% (cigarette consumption 40–<60 pack years). Patients with a cigarette consumption of <20 pack years showed intralobular opacities in 45% of the CT scans. This finding showed a statistically significant difference between the groups with <20 pack years and ≥20 pack years (χ2=5.6, p<0.01). Ground-glass opacities and subpleural micronodules were seen in only 6 patients (7%) and 8 patients (9%), respectively, without any significant differences regarding the tobacco consumption.
Figure 3.
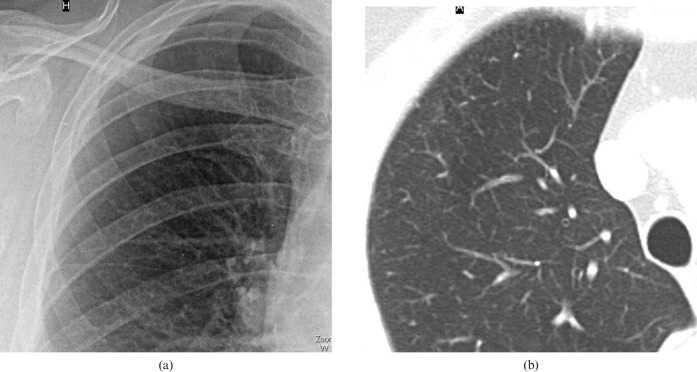
Correlation of chest radiography and CT appearances in a 69-year-old female smoker (22 pack years). The chest radiograph shows (a) a considerable increase of overall lung markings with small nodular opacities; normal lung markings are still visible. The finding is comparable to the profusion score of small opacities 1/0 according to the International Labour Organization. (b) Transverse CT scan targeted to the right lung shows intralobular thickening and numerous intralobular smooth opacities in the periphery of the upper lobe.
The vast majority (20 of 22, 91%) of patients with a cigarette consumption of >40 pack years showed emphysema; there was no difference between the percentage (91%) of emphysema diagnosed by CT or chest radiography in this group. The presence of emphysema differed significantly between the group of smokers with <20 pack years and ≥20 pack years (χ2=12, p<0.005). Details concerning the CT findings are presented in Table 3.
Table 3. Findings in CT correlated with the cigarette consumption.
| CT findings | Smoke burden (pack years) |
Total (n=85) | |||
| <20 (n=33) | 20–<40 (n=30) | 40–<60 (n=13) | ≥60 (n=9) | ||
| Interlobular | 16 (48%)a | 22 (73%) | 11 (84%) | 5 (56%) | 54 (64%) |
| Intralobular | 15 (45%)a | 21 (70%) | 10 (77%) | 6 (67%) | 52 (61%) |
| Micronodules | 2 (6%) | 2 (7%) | 1 (8%) | 2 (22%) | 8 (9%) |
| Ground glass | 1 (3%) | 3 (10%) | 1 (8%) | 1 (11%) | 6 (7%) |
| Bronchial wall thickening | 20 (61%) | 16 (53%) | 11 (84%) | 7 (78%) | 54 (64%) |
| Emphysema | 13 (40%)a | 20 (67%) | 12 (92%) | 8 (89%) | 53 (62%) |
aSignificant difference compared with the other groups.
Discussion
Since the decline of the coal mining and steel industries, cigarette smoke remains the most serious environmental hazard for the lung in western countries [5]. It has been demonstrated that respiratory illness is associated with the degree of tobacco smoke exposure in particular [5,11]. The pathological entity of smoke-induced RB-ILD is also called “smokers' bronchiolitis” as it is seen almost exclusively in current or former smokers. While numerous publications have investigated the CT appearance of emphysematous changes of the lung parenchyma [16,17] or alterations of the bronchial walls [18-21], less attention has been directed to the abnormalities of chest radiographs caused by chronic bronchitis or RB-ILD.
Our study demonstrates that cigarette smoking generally results in an increase of overall lung markings on chest radiography. This fact is perhaps not surprising and was assumed in the literature many years ago [7,8,10]. In accordance with other investigators, we verified that only a small number of smokers show normal lung parenchyma. Abnormal chest radiographs have been reported in up to three-quarters of all patients suffering from RB-ILD [3,5,22,23]. Heyneman et al [3] reported that the most common finding of RB-ILD on chest radiography is the thickening of the walls of the central or peripheral bronchi, which is seen in about 75% of the patients. Moon et al [22] found a reticulonodular pattern in the chest radiographs of 5 out of 10 patients and ground-glass opacities in 2 out of 10 patients with histologically proven RB-ILD. In our study, only 8 (15%) of the 52 smokers with a cigarette consumption of >20 pack years showed a lower profusion score of reticular pattern—ILO <0/1. It should also be noted that this might disprove the existence of a considerable natural resistance to tobacco smoke.
In addition to this result, a crucial finding of our study is the affirmation of a linear correlation between the extent of cigarette consumption and an increase of overall lung markings on chest radiography, a fact which, as far as we know, until now was not proven.
While previous literature argues that neither chest radiographs nor HRCT have much to offer in the diagnosis and management of patients suffering from chronic obstructive pulmonary disease (COPD) [8,13,16], more recent work on CT demonstrated that it can help differentiate the COPD phenotype (emphysema-predominant, airway-predominant) which is crucial for determining the appropriate management strategy [20]. Remy-Jardin et al [24] demonstrated in a group of 98 asymptomatic smokers with normal findings at pulmonary function tests that parenchymal abnormalities such as subpleural and parenchymal micronodules, mild emphysema and ground-glass attenuation can be detected by means of HRCT. A follow-up study of 57 smokers [25] provided morphological information on longitudinal changes in CT findings caused by smoking. Emphysematous changes were seen in 40% and ground-glass attenuation was seen in 42%; this finding showed significant higher frequency after an average follow-up of 5.5 years. Ill-defined micronodules were observed in 35%. Holt et al [26] found that the CT findings of RB-ILD are variable (atelectasis, ground-glass opacity, emphysema, and linear and reticular abnormalities).
More recently, Park et al [27] analysed chest radiographs and CT scans of 21 smokers and ex-smokers and noted major radiographic findings of bronchial wall thickening (76%) and ground-glass opacity (57%). Major CT findings were central (90%) and peripheral (86%) bronchial wall thickening, centrilobular nodules (71%) and ground-glass opacities (67%). Our sample showed a comparable frequency of centrilobular opacities (61%) but a much lower frequency of ground-glass opacification (7%). This might be explained by the fact that our images were displayed at a rather high window level (−500 HU) and a wide window width (2000 HU).
The CT measurement of airway wall dimensions was estimated to play a front-line role in the study of COPD because airway remodelling represents the major reason for airflow limitation [17-19,21]. In our study, bronchial wall thickening was diagnosed approximately as often in chest radiography (58%) as in CT (64%) and—similar to other investigations [27]—as a major finding. With special regard to this finding, we substantially agree with Müller-Leise et al [28], who evaluated parenchymal lung changes in a group of 82 smokers and found that subpleural dystelectasis and centrilobular and panlobular emphysema are dependent on cigarette consumption, but that ground-glass pattern, centrilobular micronodules and bronchial wall thickening are independent of tobacco burden. This finding is somewhat different to the observation that the extent of centrilobular nodules found on CT correlates with the degree of macrophage accumulation and chronic inflammation in respiratory bronchioles [3,29]. In addition, we did not find any correlation between bronchial wall thickening and the extent of cigarette consumption in our study.
Considering our results, it seems to be somewhat surprising that the overall prevalence of emphysema was high and that the vast majority of the heavy smokers (cigarette consumption of ≥40 pack years) showed emphysema on both chest radiography and CT (20/22, 90%). Recent studies have already demonstrated the diagnostic impact of both modalities in the early diagnosis of emphysema [30,31]. In this context, Spaggiari et al [31] demonstrated that HRCT was more sensitive and specific than functional tests for the evaluation of initial emphysema. Our results show that chest radiography is comparable to CT in predicting emphysema. This observation is in agreement with Miniati et al [32,33] and might be caused by a relatively small number of early emphysema in our sample.
Finally, the question of which morphological substrates result in the proven increase of overall lung markings remains. The CT findings of intralobular opacities and emphysema showed significant differences regarding tobacco consumption. As no other parameter showed a comparable high correlation, we infer that intralobular opacities—perhaps caused by small airway disease—are the major factor for an increase of overall lung markings in chest radiography.
There were several limitations to our study. First, the sample size was small, preventing a generalisation of our results. Second, the study design did not compare the findings of smokers with an age-matched non-smoking cohort; this would have been suitable because increased lung markings on chest radiography are a non-specific finding and are also seen in other conditions.
Another limitation to our study is that the findings were not proven by a gold standard such as histological examination. However, owing to the study design there was no indication for performing invasive investigations. Above this, one may believe there was an interpretation bias in our study because the CT protocol cannot be regarded as a standard for the examination of interstitial lung diseases. Some subtle abnormalities, as described in the literature, may be visualised only by thin section high resolution slices in defined inspiration conditions. Therefore, we regard our findings as preliminary.
References
- 1.Hartman TE, Tazelaar HD, Swensen SJ, Müller NL. Cigarette smoking: CT and pathologic findings of associated pulmonary diseases. Radiographics 1997;17:377–90 [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society, European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med 2002;165:277–304 [DOI] [PubMed] [Google Scholar]
- 3.Heyneman LE, Ward S, Lynch DA, Remy-Jardin M, Johkoh T, Müller NL. Respiratory bronchiolitis, respiratory bronchiolitis-associated interstitial lung disease, and desquamative interstitial pneumonia: different entities or part of the spectrum of the same disease process? AJR Am J Roentgenol 1999;173:1617–22 [DOI] [PubMed] [Google Scholar]
- 4.Kanne JP, Bilawich AM, Lee CH, Im JG, Müller NL. Smoking-related emphysema and interstitial lung diseases. J Thorac Imaging 2007;22:286–91 [DOI] [PubMed] [Google Scholar]
- 5.Ryu JH, Colby TV, Hartman TE, Vassallo R. Smoking-related interstitial lung diseases: a concise review. Eur Respir J 2001;17:122–32 [DOI] [PubMed] [Google Scholar]
- 6.Wells AU, Nicholson AG, Hansell DM. Challenges in pulmonary fibrosis. 4: smoking-induced diffuse interstitial lung diseases. Thorax 2007;62:904–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates DV. Chronic bronchitis and emphysema. N Engl J Med 1968;278:546–51 [DOI] [PubMed] [Google Scholar]
- 8.Fraser RG, Fraser RS, Renner JW, Bernard C, Fitzgerald PJ. The roentgenologic diagnosis of chronic bronchitis: a reassessment with emphasis on parahilar bronchi seen end-on. Radiology 1976;120:1–9 [DOI] [PubMed] [Google Scholar]
- 9.Gückel C, Hansell DM. Imaging the ‘dirty lung’—has high resolution computed tomography cleared the smoke? Clin Radiol 1998;53:717–22 [DOI] [PubMed] [Google Scholar]
- 10.Reid L, Simon G., III Pathological findings and radiological changes in chronic bronchitis and emphysema. Br J Radiol 1959;32:291–305 [DOI] [PubMed] [Google Scholar]
- 11.Remy-Jardin M, Remy J, Gosselin B, Becette V, Edme JL. Lung parenchymal changes secondary to cigarette smoking: pathologic-CT correlations. Radiology 1993;186:643–51 [DOI] [PubMed] [Google Scholar]
- 12.International Labour Office Guidelines for the use of ILO international classification of radiographs of pneumonconioses. Geneva: ILO; 2000 [Google Scholar]
- 13.Awadh N, Müller NL, Park CS, Abboud RT, FitzGerald JM. Airway wall thickness in patients with near fatal asthma and control groups: assessment with high resolution computed tomographic scanning. Thorax 1998;53:248–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webb WR. Plain film and high resolution computed tomographic assessment of diffuse infiltrative lung disease. Webb WR, Higgins CB, Thoracic imaging: pulmonary and cardiovascular radiology. Philadelphia, PA: Lippincott, Williams and Wilkins; 2005. pp 306–30 [Google Scholar]
- 15.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods 2009;41:1149–60 [DOI] [PubMed] [Google Scholar]
- 16.Webb WR. Radiology of obstructive pulmonary disease. AJR Am J Roentgenol 1997;169:637–47 [DOI] [PubMed] [Google Scholar]
- 17.Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 2000;162:1102–8 [DOI] [PubMed] [Google Scholar]
- 18.Coxson HO. Quantitative computed tomography assessment of airway wall dimensions: current status and potential applications for phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;15:940–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiMango EA, Lubetsky H, Austin JH. Assessment of bronchial wall thickening on posteroanterior chest radiographs in acute asthma. J Asthma 2002;39:255–61 [DOI] [PubMed] [Google Scholar]
- 20.Matsuoka S, Yamashiro T, Washko GR, Kurihara Y, Nakajima Y, Hatabu H. Quantitative CT assessment of chronic obstructive pulmonary disease. Radiographics 2010;30:55–66 [DOI] [PubMed] [Google Scholar]
- 21.Orlandi I, Moroni C, Camiciottoli G, Bartolucci M, Pistolesi M, Villari N, et al. Chronic obstructive pulmonary disease: thin-section CT measurement of airway wall thickness and lung attenuation. Radiology 2005;234:604–10 [DOI] [PubMed] [Google Scholar]
- 22.Moon J, du Bois RM, Colby TV, Hansell DM, Nicholson AG. Clinical significance of respiratory bronchiolitis on open lung biopsy and its relationship to smoking related interstitial lung disease. Thorax 1999;54:1009–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yousem SA, Colby TV, Gaensler EA. Respiratory bronchiolitis-associated interstitial lung disease and its relationship to desquamative interstitial pneumonia. Mayo Clin Proc 1989;64:1373–80 [DOI] [PubMed] [Google Scholar]
- 24.Remy-Jardin M, Remy J, Boulenguez C, Sobaszek A, Edme JL, Furon D. Morphologic effects of cigarette smoking on airways and pulmonary parenchyma in healthy adult volunteers: CT evaluation and correlation with pulmonary function tests. Radiology 1993;186:107–15 [DOI] [PubMed] [Google Scholar]
- 25.Remy-Jardin M, Edme JL, Boulenguez C, Remy J, Mastora I, Sobaszek A. Longitudinal follow-up study of smoker's lung with thin-section CT in correlation with pulmonary function tests. Radiology 2002;222:261–70 [DOI] [PubMed] [Google Scholar]
- 26.Holt RM, Schmidt RA, Godwin JD, Raghu G. High resolution CT in respiratory bronchiolitis-associated interstitial lung disease. J Comput Assist Tomogr 1993;17:46–50 [DOI] [PubMed] [Google Scholar]
- 27.Park JS, Brown KK, Tuder RM, Hale VA, King TE, Jr, Lynch DA. Respiratory bronchiolitis-associated interstitial lung disease: radiologic features with clinical and pathologic correlation. J Comput Assist Tomogr 2002;26:13–20 [DOI] [PubMed] [Google Scholar]
- 28.Müller-Leisse C, Otto A, Berger F, Schmitz E, Günther RW. The recording of parenchymal lung changes in smokers by high-resolution computed tomography. Röfo 1997;166:108–14 [DOI] [PubMed] [Google Scholar]
- 29.Gruden JF, Webb WR. CT findings in a proved case of respiratory bronchiolitis. AJR Am J Roentgenol 1993;161:44–6 [DOI] [PubMed] [Google Scholar]
- 30.Sashidhar K, Gulati M, Gupta D, Monga S, Suri S. Emphysema in heavy smokers with normal chest radiography. Detection and quantification by HCRT. Acta Radiol 2002;43:60–5 [DOI] [PubMed] [Google Scholar]
- 31.Spaggiari E, Zompatori M, Verduri A, Chetta A, Bnà C, Ormitti F, et al. Early smoking-induced lung lesions in asymptomatic subjects. Correlations between high resolution dynamic CT and pulmonary function testing. Radiol Med 2005;109:27–39 [PubMed] [Google Scholar]
- 32.Miniati M, Monti S, Stolk J, Mirarchi G, Falaschi F, Rabinovich R, et al. Value of chest radiography in phenotyping chronic obstructive pulmonary disease. Eur Respir J 2008;31:509–15 [DOI] [PubMed] [Google Scholar]
- 33.Miniati M, Filippi E, Falaschi F, Carrozzi L, Milne EN, Sostman HD, et al. Radiologic evaluation of emphysema in patients with chronic obstructive pulmonary disease. Chest radiography versus high resolution computed tomography. Am J Respir Crit Care Med 1995;151:1359–67 [DOI] [PubMed] [Google Scholar]