Abstract
Objectives
The purpose of this prospective study was to elucidate the efficacy of using contrast-enhanced ultrasound to characterise focal hepatic lesions appearing non-hypervascular on contrast-enhanced CT in chronic liver diseases.
Methods
The study population included 22 patients with cirrhosis or chronic hepatitis, who between them had 27 focal hepatic lesions smaller than 20 mm (mean 13.9±3.4) that appeared non-hypervascular on contrast-enhanced CT. Contrast-enhanced ultrasound with perflubutane microbubble agent (Sonazoid, 0.0075 ml kg–1) was performed prior to ultrasound-guided needle biopsy, and intensity analysis was done for hepatic lesions in the early phase (−60 s) and late phase (600 s post injection).
Results
All seven early-phase hyperenhanced lesions were hepatocellular carcinoma (HCC). 20 lesions iso- or hypoenhanced during the early phase consisted of 11 regenerative nodules (RNs) and 9 HCCs. HCC was more frequent in early-phase hyperenhanced lesions than in iso- or hypoenhanced lesions (p=0.0108). Both late-phase hypoenhanced lesions were HCCs, whereas 25 late-phase isoenhanced lesions consisted of 11 RNs and 14 HCCs. The enhancement patterns of the 11 RNs included isoenhanced appearance in both the early and late phases in 8 lesions, and early-phase hypoenhancement combined with late-phase isoenhancement in the remaining 3. Both of these enhancement patterns (i.e. either iso–iso or hypo–iso) were found in 9 malignant lesions, 9 (75%) of the 12 well-differentiated HCCs.
Conclusion
Hypervascularity on contrast-enhanced ultrasound with Sonazoid strongly suggested HCC regardless of non-hypervascularity on CT, and late-phase hypoenhancement was another possible finding of HCC. However, characterisation of hepatic lesions with other enhancement patterns was difficult using our technique.
The development of hepatocellular carcinoma (HCC) has a profound influence on the prognosis of patients with chronic liver disease (CLD), and there is nearly universal consensus on the importance of adequate HCC surveillance for these patients [1,2]. However, because reliable surveillance of HCC cannot be achieved solely by assessing tumour markers such as α-fetoprotein, it is necessary to utilise currently available imaging modalities effectively and efficiently in patients at risk for developing this neoplasm [3,4]. Differential diagnosis of focal hepatic lesions is a major challenge that must be overcome in order to provide appropriate clinical management of these patients.
Based on diagnostic imaging, a hypervascular appearance of focal hepatic lesions in patients with CLD strongly suggests HCC, and a hypervascular lesion larger than 20 mm can be diagnosed as HCC without performing a biopsy [1,5]. On the other hand, non-hypervascular hepatic lesions include non-malignant lesions such as regenerative nodules (RNs) and both low- and high-grade dysplastic nodules; some well-differentiated HCCs also appear as non-hypervascular lesions prior to tumour vascularisation during the multistep process of carcinogenesis [5-9]. Characterisation of non-hypervascular hepatic lesions may prove challenging in patients with CLD. For example, the invasive nature of a needle biopsy is a drawback in cirrhotic patients with impaired coagulation, so diagnostic imaging tools merit serious consideration in this clinical situation.
Technical improvements in ultrasound have been outstanding in the past two decades, with the resulting advantages of real-time observation, simple technique and non-invasiveness [10]. Moreover, the use of a microbubble contrast agent allows detailed observation of tumour haemodynamics, which can prove helpful in the detection and characterisation of focal hepatic lesions [11,12]. Stable and sufficient contrast enhancement, including improved signal-to-noise ratio, is achieved in the liver using harmonic imaging in combination with a second-generation contrast agent [13].
Sonazoid (GE Healthcare, Little Chalfont, UK) is a novel perflubutane microbubble contrast agent whose clinical efficacy has been demonstrated for the diagnosis of focal hepatic lesions and diffuse liver diseases [14-16]. The microbubbles of this agent are captured in the liver parenchyma during the agent's circulation in the blood; therefore, contrast-enhanced sonography can generate both haemodynamic-phase and accumulated-phase images [17,18]. These dual-phase images may offer improved diagnostic performance for non-hypervascular hepatic lesions. The purpose of the current study was to examine the clinical significance of using contrast-enhanced ultrasound with Sonazoid to characterise focal hepatic lesions that show a non-hypervascular appearance on contrast-enhanced CT in patients with CLD.
Patients and methods
Patients
This prospective study was approved by the ethics committee of our department, and was performed from July 2007 to December 2009; all patients gave their written informed consent before participating in the study. The following inclusion criteria were used to screen potential participants (patients had to meet all criteria in order to participate in the study): (1) presence of both CLD and focal hepatic lesions detected by ultrasound when performed as surveillance for HCC; (2) hepatic lesions showing a non-hypervascular appearance on contrast-enhanced CT with dynamic imaging performed for the characterisation of hepatic lesions; and (3) scheduled percutaneous ultrasound-guided biopsy following contrast-enhanced ultrasound examination. Patients with any of the following were excluded from participation in the study: (1) target hepatic lesion ≥20 mm; (2) presence of another hepatic lesion with hypervascular appearance or lesions >20 mm coincidentally found on contrast-enhanced ultrasound; (3) history of treatment for HCC; (4) fatty liver, portal vein thrombosis or portal vein tumour thrombosis found by ultrasound/CT; (5) pathological results of hepatic lesions indicating malignancies other than HCC; (6) severely impaired coagulation (platelet count <50 000 μ l–1 or prothrombin time <40%); or (7) egg allergy, which is a contraindication to the use of Sonazoid.
Ultrasound examination
Ultrasound examination was performed using the Aplio XG (Toshiba, Tokyo, Japan) with a 3.75 MHz convex probe, after a fast of more than 4 h. The contrast agent Sonazoid (perflubutane microbubbles, a median diameter of 2–3 μm, 0.0075 ml kg–1) was manually injected by HI, followed by 3.0 ml of normal saline. Contrast harmonic sonograms were taken using a mechanical index level ranging from 0.2 to 0.3, during 2 phases: the early phase (dynamic phase, from agent injection to 1 min) and the late phase (accumulated phase, 10 min after injection), in accordance with our previous report [19]. The agent was injected once for each lesion. Thus, in the patients with multiple lesions, the additional injection was conducted after the disappearance of the previous effect.
Qualitative and quantitative assessment of contrast enhancement
First, contrast-enhanced findings of the hepatic lesion were evaluated subjectively by the ultrasound operator (MT), a hepatologist with 7 years' experience in ultrasound examination at the time that the initial case was enrolled. After this evaluation, the intensity in both the hepatic lesion and adjacent liver parenchyma was analysed using ImageLab software (Toshiba, Tokyo, Japan) on digitally recorded ultrasound images by the same operator. The time–intensity curve of the hepatic lesion was obtained by manually superimposing the circular user-defined region of interest (ROI), the size of which corresponded to the size of the hepatic lesion. Then, the intensity difference between tumour and surrounding non-tumour liver parenchyma was measured using two ROIs at the two phases: the intratumour maximum enhancement time point in the early phase (determined by the time–intensity curve of the hepatic lesion), and in the late phase. Next, the distribution of contrast enhancement of the non-tumour liver parenchyma was measured, because heterogeneous enhancement of adjacent liver parenchyma might influence the assessment of contrast enhancement in the hepatic lesions. We positioned three adjacent ROIs at the same depth in the non-tumour liver parenchyma and measured the maximum difference in intensity between these ROIs during the early and late phases. Contrast-enhanced findings for hepatic lesions were defined based on the comparison of the intensity difference in the non-tumour parenchyma versus that between tumour and non-tumour parenchyma. More specifically, when the tumour-non-tumour intensity difference was above the range of the intensity difference in non-tumour parenchyma, the hepatic lesions were assessed as having a hyperenhanced or hypoenhanced appearance; an isoenhanced appearance was defined as falling between these extremes.
Contrast-enhanced CT
Contrast-enhanced CT with dynamic imaging was performed using the Aquilion One system at the 64-row multidetector setting (Toshiba) along with injection of 100 ml of contrast medium (Iopamiron 350; Nihon Schering, Osaka, Japan) at 3 ml s–1 into the antecubital vein by means of a mechanical injection system (Mark V ProVis; Medrad, Warrendale, PA). Images were taken with a 30 s delay between contrast medium administration and start of imaging for the hepatic artery-dominant phase, 80 s delay for the portal vein-dominant phase and 180 s delay for the equilibrium phase. Contrast-enhanced CT findings were evaluated by MY, a hepatologist with 28 years' experience at the time of enrolment of the initial case, and who was blinded to the patient information.
Statistical analysis
All data were expressed as mean±standard deviation (SD) or as percentages. Statistical significance was examined using the χ2 test, and p-values of <0.05 were considered to be significant. Statistical analysis was performed using the SPSS software package (version 13.0J; IBM Corporation, Armonk, NY).
Results
During the study period, a total of 186 patients with CLD underwent both contrast-enhanced ultrasound and contrast-enhanced CT examination for the characterisation of their 314 hepatic lesions found by surveillance ultrasound, and 101 nodules underwent ultrasound-guided needle biopsy in a total of 67 patients. Among these patients, 28 met the study criteria, although 6 of these patients were eventually excluded, because of either an inadequate biopsy specimen (n=5) or a pathological finding of adenocarcinoma (n=1). Therefore, the final study population comprised 22 patients, who had 27 hepatic lesions overall.
There were 12 men and 10 women, with a mean age ±SD of 63.4±12.5 years (range 29–65) and body mass index of 24±3.4 (range 19–33.4). Liver diseases were diagnosed, based on biochemistry and imaging results, as cirrhosis in 17 patients and chronic hepatitis in 5; 15 patients tested positive for hepatitis C virus antibody, and 4 tested positive for hepatitis B virus surface antigen; alcohol abuse was noted in 3 patients. The number of hepatic lesions examined by both contrast-enhanced ultrasound and liver biopsy was 1 lesion each in 17 patients and 2 lesions each in 5 patients, and the maximum diameter measured on the sonogram ranged from 7.5 to 19 mm (mean 13.9±3.4). The serum α-fetoprotein level ranged from 1.8 to 97.7 ng ml–1 (mean 25.3±30.1), and was normal (<20) in 12 patients. Pathological findings for all hepatic lesions were evaluated from the specimens obtained by percutaneous ultrasound-guided needle biopsy using a Sonopsy C1 needle (21 guage; Hakko, Tokyo, Japan). The time period between contrast-enhanced ultrasound and contrast-enhanced CT ranged from 1 to 27 days (mean 6.8±6.5), and that between ultrasound/CT and needle biopsy ranged from 1 to 20 days (mean 4.0±4.5).
Contrast enhancement of focal hepatic lesions on ultrasound and CT images
All hepatic lesions appeared isoechoic on contrast harmonic image before the beginning of contrast enhancement effect. The maximum intensity difference in the non-tumour liver parenchyma was 2.0 dB in the early phase. Based on the intensity difference between hepatic lesions and surrounding liver parenchyma in this phase, each lesion was classified as follows: 7 lesions with a range of 2.5 to 23.5 dB were classified as hyperenhanced lesions, 4 with a range of –5.9 to –2.04 dB as hypoenhanced lesions, and 16 with a range of –1.7 to 1.6 dB as isoenhanced lesions. The maximum intensity difference in the non-tumour liver parenchyma was 2.1 dB during the late phase. Similar to the early-phase results, 2 lesions with the intensity difference of −6.6 and −2.7 dB were classified as hypoenhanced lesions and 25 lesions with a range of −1.7 to 1.5 dB were classified as isoenhanced lesions in the late phase.
Contrast enhancement of hepatic lesions was compared between qualitative subjective assessment and quantitative objective assessment. The early-phase findings were consistent in 24 lesions (88.9%) and different in the other 3 lesions; 2 lesions showed isoenhancement in the subjective assessment but hypoenhancement in the objective assessment and 1 showed hypoenhancement in the subjective assessment but isoenhancement in the objective assessment. The late-phase findings were consistent in 23 lesions (85.2%) and different in the other 4, which all showed hypoenhancement in the subjective assessment but isoenhancement in the objective assessment.
Consistent enhancement findings were detected by both early-phase sonography and artery-dominant CT imaging in 13 lesions (48.2%), while the enhancement was different in the other 14 (Table 1). The rate of detection of tumour vascularity was higher by means of ultrasound than by CT in 11 lesions (40.7%), and the detection rate was lower by means of ultrasound than by CT in 3 lesions (11.1%).
Table 1. Comparison of contrast enhancement between ultrasound and CT images of lesions.
| Results | Early-phase ultrasound findings |
||
| Hypoenhanced | Isoenhanced | Hyperenhanced | |
| Hypoenhanced on CTa | 1 | 4 | 4 |
| Isoenhanced on CTa | 3 | 12 | 3 |
aContrast enhancement on hepatic artery-dominant CT image.
Values are presented as the number of hepatic lesions.
Relationship between contrast-enhanced ultrasound findings and pathological results
Liver biopsy revealed 11 benign lesions (RN; 40.7%), 12 well-differentiated HCCs (44.5%) and 4 moderately differentiated HCCs (14.8%). All seven lesions that were hyperenhanced during the early phase were HCCs, and comprised three well-differentiated HCCs showing an isoenhanced appearance during the late phase and four moderately differentiated HCCs showing a hypoenhanced appearance (n=2) or an isoenhanced appearance (n=2) during the late phase (Tables 2 and 3, Figure 1). 20 lesions that were iso- or hypoenhanced in the early phase consisted of both benign and malignant lesions: the isoenhanced lesions included eight RNs and eight well-differentiated HCCs; and the hypoenhanced lesions included three RNs and one well-differentiated HCC (Table 2, Figure 2). HCC was more frequently associated with hyperenhancement (7/7, 100%) than iso- or hypoenhancement (9/20, 45%) during the early phase (p=0.0108).
Table 2. Relationship between ultrasound early-phase contrast enhancement and pathological results.
| Results | Pathological results |
||
| RN | Well HCC | Mod HCC | |
| Hypoenhanced on ultrasound | 3 | 1 | 0 |
| Isoenhanced on ultrasound | 8 | 8 | 0 |
| Hyperenhanced on ultrasound | 0 | 3 | 4 |
Mod HCC, moderately differentiated hepatocellular carcinoma; RN, regenerative nodule; Well HCC, well-differentiated hepatocellular carcinoma.
Values are presented as the number of hepatic lesions.
Table 3. Relationship between ultrasound late-phase contrast enhancement and pathological results.
| Results | Pathological results |
||
| RN | Well HCC | Mod HCC | |
| Isoenhanced on ultrasound | 11 | 12 | 2 |
| Hypoenhanced on ultrasound | 0 | 0 | 2 |
Mod HCC, moderately differentiated hepatocellular carcinoma; RN, regenerative nodule; Well HCC, well-differentiated hepatocellular carcinoma.
Values are presented as the number of hepatic lesions.
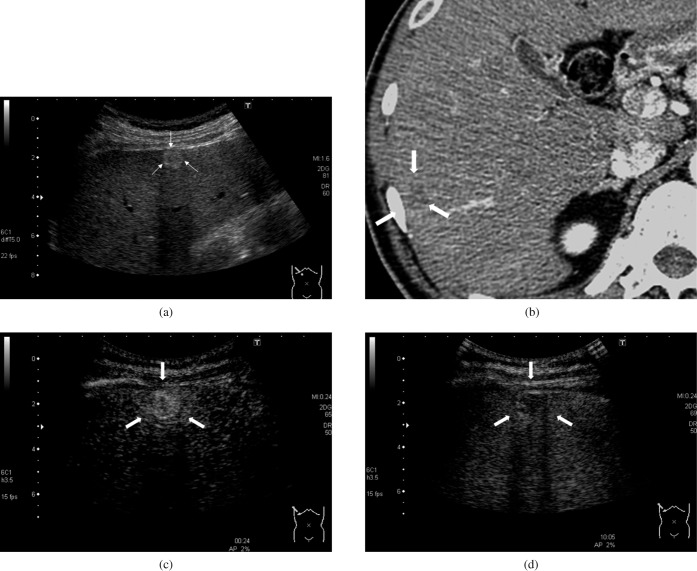
Figure 1.
A 52-year-old man with hepatitis C-related cirrhosis and moderately differentiated hepatocellular carcinoma (15.2 mm). (a) B-mode sonogram. A hyperechoic lesion was observed in the liver (arrows). (b) Contrast-enhanced CT, hepatic artery-dominant phase. The lesion showed a hypovascular appearance on the CT image (arrows). (c) Contrast-enhanced ultrasound, early phase. The intensity difference between the hepatic lesion and adjacent liver parenchyma was 22.6 dB, which was higher than the maximum intensity difference in the non-tumour parenchyma (2.0 dB). The lesion appeared as hyperenhanced (arrows). (d) Contrast-enhanced ultrasound, late phase. The intensity difference between the hepatic lesion and adjacent liver parenchyma was –6.6 dB, with the absolute value greater than the maximum intensity difference in the non-tumour parenchyma (2.1 dB). The lesion appeared as hypoenhanced lesion (arrows).
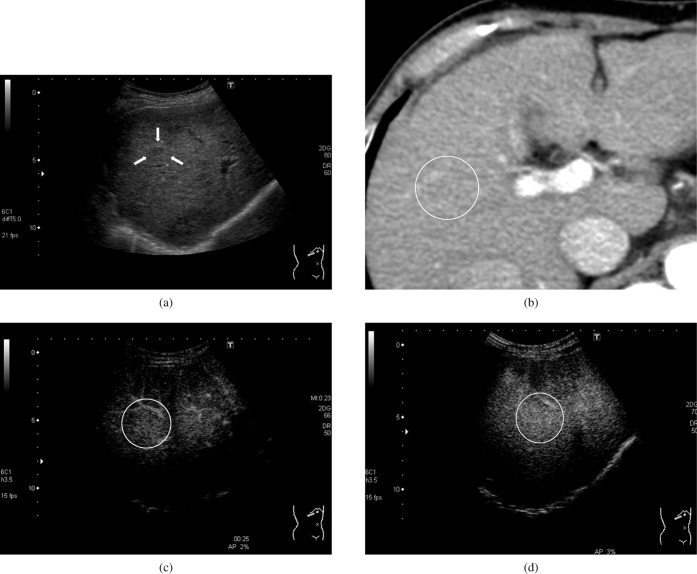
Figure 2.
A 67-year-old woman with hepatitis C-related cirrhosis and a regenerative nodule (10.6 mm). (a) B-mode. A hypoechoic lesion was observed in the liver (arrows). (b) Contrast-enhanced CT, hepatic artery-dominant phase. The lesion showed an isovascular appearance on the CT image (lesion in the white circle). (c) Contrast-enhanced ultrasound, early phase. The intensity difference between the hepatic lesion and adjacent liver parenchyma was 0.1 dB, which fell within the range of the maximum intensity difference in the non-tumour parenchyma (2.0 dB). The lesion appeared as isoenhanced (lesion in the white circle). (d) Contrast-enhanced ultrasound, late phase. The intensity difference between the hepatic lesion and adjacent liver parenchyma was 0.5 dB, which fell within the range of the maximum intensity difference in the non-tumour parenchyma (2.1 dB). The lesion appeared as isoenhanced (lesion in the white circle).
Both of the lesions that were hypoenhanced during the late phase were moderately differentiated HCCs showing a hyperenhanced appearance in the early phase (Table 3). 25 lesions isoenhanced during the late phase consisted of 11 RNs, 12 well-differentiated HCCs and 2 moderately differentiated HCCs.
The enhancement patterns of the 11 RNs included isoenhanced appearance in both the early and late phases (iso–iso pattern) in 8 lesions, and early-phase hypoenhancement combined with late-phase isoenhancement (hypo–iso pattern) in 3 lesions. Both of these enhancement patterns (i.e. either iso–iso or hypo–iso) were found in 9 malignant lesions, i.e. in 9 (75%) of the 12 well-differentiated HCCs.
Discussion
Arterial hypervascularity is a characteristic feature of HCC, and all 7 (7/27, 25.9%) hyperenhanced lesions in the current study were proven to be HCCs, even though they all showed a non-hypervascular appearance on contrast-enhanced CT. The superior detection of vascularity by ultrasound, compared with CT, has been reported in previous studies [7,20], and a lesion's non-hypervascular appearance on CT imaging is not necessarily indicative of a non-hypervascular tumour. Hyperenhancement during early-phase Sonazoid-induced sonograms is such a reliable finding that it may raise the possibility of bypassing the invasive biopsy procedure for the diagnosis of HCC in patients with CLD.
In a study by Bolondi et al [7], non-malignant lesions were found in 64.3% (9/14) of the nodules showing a non-hypervascular appearance on both contrast-enhanced ultrasound with SonoVue (Bracco, Milan, Italy) and CT. A recent report also showed that only 4 (18.2%) of 22 lesions with an iso- or hypovascular appearance on contrast-enhanced ultrasound with Definity (Lantheus, North Billerica, MA) were HCCs in patients at risk for HCC [21]. In our study, however, the incidence of HCC was 45% (9/20) in lesions found to be non-hypervascular by both contrast-enhanced ultrasound and CT, which is a higher frequency than that in previous reports [7,21]. Although this finding might be particular to the population enrolled in this study, clinicians should be aware that one should not automatically rule out malignancy in apparently non-hypervascular hepatic lesions, especially in patients with CLD.
Differences in arterial and portal blood supply between hepatic lesions and surrounding liver parenchyma account for the so-called “washed out” appearance of HCC in the portal or equivalent phase on CT, and the same logic may explain the contrast-enhanced ultrasound findings with Definity or SonoVue, which are blood-pool contrast agents that do not accumulate in the liver [21,22]. In fact, a hypoenhanced appearance on the late-phase sonogram is considered to be a sign suspicious of HCC or other malignant lesions [7,21,23,24]. This “washout” also occurs frequently in HCC lesions after the peak enhancement when using contrast agents that accumulate in the liver, e.g. Levovist (Schering, Berlin, Germany) and Sonazoid [19,25,26]. Phagocytosis by Kupffer cells is one of the theoretical mechanisms for the accumulation of microbubbles in the liver [18]. However, most HCC lesions have a relatively reduced distribution of Kupffer cells [27,28], and this may explain “washout” of HCC after the injection of Levovist or Sonazoid. Actually, both of the hepatic lesions with late-phase hypoenhancement were HCC, although the number was too small to make a definite conclusion. At this point, a problem in the evaluation of late-phase findings is that the definitive time period to use post-injection has not been determined; the term “late-phase observation” applied to phases of 5 min or more in previous reports [16,19,25,26] and we took 10 min phase images in this study. It might be substantially difficult to compare the enhancement appearance between different studies with different time definitions for the late phase. Furthermore, it remains to be clarified whether Kupffer cell function is the only reason for the late-phase enhancement patterns associated with the use of Sonazoid.
It has been proposed that an isoenhancement on the late-phase sonogram is suggestive of benign lesions, because of the same enhancement pattern in both the hepatic lesion and surrounding liver parenchyma [24-26]. As shown in our study, however, this isoenhanced appearance does not rule out the possibility of a malignant lesion, because 14 of 25 lesions (56%) with an isoenhanced appearance in the late phase proved to be HCCs. It has also been reported that some well-differentiated HCCs show an isoenhanced appearance on the imaging of delayed phase [6,27,28]. At present, ultrasound-guided biopsy may be necessary to characterise such lesions, in view of the limitations of current imaging techniques [5,29,30].
Is contrast-enhanced CT necessary for the diagnosis of hepatic lesions that can be detected by B-mode sonography? Although this question may be raised quite frequently by clinicians, an international consensus on the answer has not been achieved. However, the warning about exposure to excessive radiation from CT is receiving worldwide attention, so the unnecessary application of CT examination should be avoided. Meanwhile, diagnostic processes are also being discussed from the aspect of working efficiency, and contrast-enhanced ultrasound may have a disadvantage because it is a time-consuming examination requiring a skilled operator and an assistant for injection of the agent. Ultrasound examination is an operator-dependent procedure and there are no data documenting whether microbubble contrast agents have the potential to solve the skill gap between different operators. For this reason, under the current conditions, the best means of characterising hepatic lesions may depend on the situation at individual medical centres. However, it should be emphasised that contrast-enhanced ultrasound allows a higher detection rate for tumour vascularity than CT, based on study results published in the literature and our own findings [7,20]. Contrast-enhanced ultrasound with Sonazoid may play an important role as a first-line detailed examination useful for the characterisation of hepatic lesions initially detected by non-contrast ultrasound, thereby preventing the need for CT.
There were some limitations in our study. First, pathological results were obtained by percutaneous needle biopsy, not by surgical procedures. As the biopsy specimen reflects only a limited part of the hepatic lesion, we could not avoid the potential difficulty of inaccurate diagnosis of borderline lesions. In fact, our study did not include low- or high-grade dysplastic nodules, some of which might be treated as well-differentiated HCC. Second, our study included only a few types of hepatic lesions: HCC (at different stages of cellular differentiation) and RN. We focused on the characterisation of hepatic lesions closely related to CLD; therefore, malignancies other than HCC were excluded. Furthermore, fatty liver was also excluded because severe echogenicity of liver parenchyma might affect the intensity analysis of hepatic lesions. However, a study encompassing all types of hepatic lesions and with all kinds of diffuse liver diseases would be extremely useful. Third, intensity-based contrast assessment might be time-consuming and complex. At the same time, the authors believe that it was useful to classify the hepatic lesions because there were some discrepant cases between subjective and objective assessment. Further improvement of digital software is required to provide an automatic evaluation of contrast enhancement of hepatic lesions for routine clinical practice.
In conclusion, in CLD, hypervascularity on contrast-enhanced ultrasound with Sonazoid strongly suggested HCC regardless of non-hypervascularity upon CT, and late-phase hypoenhancement was also another possible finding indicative of HCC. However, characterisation of hepatic lesions with other enhancement patterns was difficult using our technique. Although this is a similar result to other studies, it should be validated by further larger series. Furthermore, an additional evaluation is needed before contrast-enhanced ultrasound with Sonazoid can be considered sufficiently useful such that needle biopsy of hepatic lesions can be avoided.
References
- 1.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208–36 [DOI] [PubMed] [Google Scholar]
- 2.Tanaka H, Nouso K, Kobashi H, Kobayashi Y, Nakamura S, Miyake Y, et al. Surveillance of hepatocellular carcinoma in patients with hepatitis C virus infection may improve patient survival. Liver Int 2006;26:543–51 [DOI] [PubMed] [Google Scholar]
- 3.Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol 2001;16:553–9 [DOI] [PubMed] [Google Scholar]
- 4.Larcos G, Sorokopud H, Berry G, Farrell GC. Sonographic screening for hepatocellular carcinoma in patients with chronic hepatitis or cirrhosis: an evaluation. AJR Am J Roentgenol 1998;171:433–5 [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona–2000 EASL Conference. J Hepatol 2001;35:421–30 [DOI] [PubMed] [Google Scholar]
- 6.Hayashi M, Matsui O, Ueda K, Kawamori Y, Kadoya M, Yoshikawa J, et al. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. AJR Am J Roentgenol 1999;172:969–76 [DOI] [PubMed] [Google Scholar]
- 7.Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, et al. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology 2005;42:27–34 [DOI] [PubMed] [Google Scholar]
- 8.Libbrecht L, Desmet V, Roskams T. Preneoplastic lesions in human hepatocarcinogenesis. Liver Int 2005;25:16–27 [DOI] [PubMed] [Google Scholar]
- 9.Borzio M, Fargion S, Borzio F, Fracanzani AL, Croce AM, Stroffolini T, et al. Impact of large regenerative, low grade and high grade dysplastic nodules in hepatocellular carcinoma development. J Hepatol 2003;39:208–14 [DOI] [PubMed] [Google Scholar]
- 10.Kremkau FW. Diagnostic ultrasound: principles and instruments. 4th edn Philadelphia, PA: W.B. Saunders: 1993 [Google Scholar]
- 11.Rettenbacher T. Focal liver lesions: role of contrast-enhanced ultrasound. Eur J Radiol 2007;64:173–82 [DOI] [PubMed] [Google Scholar]
- 12.Quaia E. Microbubble ultrasound contrast agents: an update. Eur Radiol 2007;17:1995–2008 [DOI] [PubMed] [Google Scholar]
- 13.Lencioni R, Piscaglia F, Bolondi L. Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma. J Hepatol 2008;48:848–57 [DOI] [PubMed] [Google Scholar]
- 14.Numata K, Morimoto M, Ogura T, Sugimori K, Takebayashi S, Okada M, et al. Ablation therapy guided by contrast-enhanced sonography with Sonazoid for hepatocellular carcinoma lesions not detected by conventional sonography. J Ultrasound Med 2008;27:395–406 [DOI] [PubMed] [Google Scholar]
- 15.Luo W, Numata K, Morimoto M, Nozaki A, Nagano Y, Sugimori K, et al. Three-dimensional contrast-enhanced sonography of vascular patterns of focal liver tumors: pilot study of visualization methods. AJR Am J Roentgenol 2009;192:165–73 [DOI] [PubMed] [Google Scholar]
- 16.Maruyama H, Ishibashi H, Takahashi M, Imazeki F, Yokosuka O. Effect of signal intensity from the accumulated microbubbles in the liver for differentiation of idiopathic portal hypertension from liver cirrhosis. Radiology 2009;252:587–94 [DOI] [PubMed] [Google Scholar]
- 17.Marelli C. Preliminary experience with NC100100, a new ultrasound contrast agent for intravenous injection. Eur Radiol 1999;9:S343–6 [DOI] [PubMed] [Google Scholar]
- 18.Watanabe R, Matsumura M, Munemasa T, Fujimaki M, Suematsu M. Mechanism of hepatic parenchyma-specific contrast of microbubble-based contrast agent for ultrasonography: microscopic studies in rat liver. Invest Radiol 2007;42:643–51 [DOI] [PubMed] [Google Scholar]
- 19.Maruyama H, Takahashi M, Ishibashi H, Okugawa H, Okabe S, Yoshikawa M, et al. Ultrasound-guided treatments under low acoustic power contrast harmonic imaging for hepatocellular carcinomas undetected by B-mode ultrasonography. Liver Int 2009;29:708–14 [DOI] [PubMed] [Google Scholar]
- 20.Giorgio A, Ferraioli G, Tarantino L, de Stefano G, Scala V, Scarano F, et al. Contrast-enhanced sonographic appearance of hepatocellular carcinoma in patients with cirrhosis: comparison with contrast-enhanced helical CT appearance. AJR Am J Roentgenol 2004;183:1319–26 [DOI] [PubMed] [Google Scholar]
- 21.Jang HJ, Kim TK, Burns PN, Wilson SR. Enhancement pattern of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology 2007;244:898–906 [DOI] [PubMed] [Google Scholar]
- 22.Morel DR, Schwieger I, Hohn L, Terrettaz J, Llull JB, Cornioley YA, et al. Human pharmacokinetics and safety evaluation of SonoVue, a new contrast agent for ultrasound imaging. Invest Radiol 2000;35:80–5 [DOI] [PubMed] [Google Scholar]
- 23.Nicolau C, Catalá V, Vilana R, Gilabert R, Bianchi L, Solé M, et al. Evaluation of hepatocellular carcinoma using SonoVue, a second generation ultrasound contrast agent: correlation with cellular differentiation. Eur Radiol 2004;14:1092–9 [DOI] [PubMed] [Google Scholar]
- 24.von Herbay A, Vogt C, Willers R, Häussinger D. Real-time imaging with the sonographic contrast agent SonoVue: differentiation between benign and malignant hepatic lesions. J Ultrasound Med 2004;23:1557–68 [DOI] [PubMed] [Google Scholar]
- 25.Dietrich CF, Ignee A, Trojan J, Fellbaum C, Schuessler G. Improved characterization of histologically proven liver tumours by contrast enhanced ultrasonography during the portal venous and specific late phase of SHU 508A. Gut 2004;53:401–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryant TH, Blomley MJ, Albrecht T, Sidhu PS, Leen EL, Basilico R, et al. Improved characterization of liver lesions with liver-phase uptake of liver-specific microbubbles: prospective multicenter study. Radiology 2004;232:799–809 [DOI] [PubMed] [Google Scholar]
- 27.Tanaka M, Nakashima O, Wada Y, Kage M, Kojiro M. Pathomorphological study of Kuppfer cells in hepatocellular carcinoma and hyperplastic nodular lesions in the liver. Hepatology 1996;24:807–12 [DOI] [PubMed] [Google Scholar]
- 28.Imai Y, Murakami T, Yoshida S, Nishikawa M, Ohsawa M, Tokunaga K, et al. Super paramagnetic iron oxide enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histological grading. Hepatology 2000;32:205–12 [DOI] [PubMed] [Google Scholar]
- 29.Caturelli E, Solmi L, Anti M, Fusilli S, Poselli P, Andriulli A, et al. Ultrasound guided fine needle biopsy for early hepatocellular carcinoma complicating liver cirrhosis: a multicenter study. Gut 2004;53:1356–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durand F, Regimbeau JM, Belghiti J, Sauvanet A, Vilgrain V, Terris B, et al. Assessment of the benefit and risk of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 2001;35:254–8 [DOI] [PubMed] [Google Scholar]