Abstract
Objective
The study aimed to characterise the factors related to the X-ray dose delivered to the patient's skin during interventional cardiology procedures.
Methods
We studied 177 coronary angiographies (CAs) and/or percutaneous transluminal coronary angioplasties (PTCAs) carried out in a French clinic on the same radiography table. The clinical and therapeutic characteristics, and the technical parameters of the procedures, were collected. The dose area product (DAP) and the maximum skin dose (MSD) were measured by an ionisation chamber (Diamentor; Philips, Amsterdam, The Netherlands) and radiosensitive film (Gafchromic; International Specialty Products Advanced Materials Group, Wayne, NJ). Multivariate analyses were used to assess the effects of the factors of interest on dose.
Results
The mean MSD and DAP were respectively 389 mGy and 65 Gy cm−2 for CAs, and 916 mGy and 69 Gy cm−2 for PTCAs. For 8% of the procedures, the MSD exceeded 2 Gy. Although a linear relationship between the MSD and the DAP was observed for CAs (r=0.93), a simple extrapolation of such a model to PTCAs would lead to an inadequate assessment of the risk, especially for the highest dose values. For PTCAs, the body mass index, the therapeutic complexity, the fluoroscopy time and the number of cine frames were independent explanatory factors of the MSD, whoever the practitioner was. Moreover, the effect of technical factors such as collimation, cinematography settings and X-ray tube orientations on the DAP was shown.
Conclusion
Optimising the technical options for interventional procedures and training staff on radiation protection might notably reduce the dose and ultimately avoid patient skin lesions.
Interventional cardiology procedures such as repeated diagnostic coronary angiographies (CAs) and/or percutaneous transluminal coronary angioplasties (PTCAs) may cause radio-induced skin damage. Deterministic skin effects might occur as soon as the dose to the patient's skin exceeds 2 Gy and might correspond to a range of lesions from erythema to necrosis [1]. In relation to the risk of skin lesions, the X-ray dose depends on clinical, therapeutic and technical factors. First of all, the dose to the patient increases with increasing body mass index (BMI) [1-3]. Second, dose, fluoroscopy time and number of cine frames might differ depending on the number of treated vessels and/or stenoses [4-6], localisation and severity of lesions [2,7], stage of occlusion [7,8], tortuosity of treated vessels [6-8], number of stents and balloons [2,4,7-9], and artery approach [10,11]. With the aim of optimising the interventional cardiology procedures (i.e. minimising the X-ray dose to the patient while preserving the quality of treatment), the use of low fluoroscopy pulse rate, image intensifier field size and collimation are recommended [1,12,13]. However, the studies that concluded that there was an impact on dose of some technical parameters had mostly used descriptive or univariate analyses and/or were carried out on a phantom [2,4,14,15]. Few studies have used multivariate methods to analyse the dose variation on patients' skin [13,16]. Radiographic projections also have a major impact on skin dose, although the effect of each incidence has not yet been much described in clinical practice [14].
In order to assess the deterministic skin risks in interventional cardiology procedures, the entrance skin dose (ESD, Gy) measured on the patient's skin surface by thermoluminescent dosemeters or by radiochromic films, is commonly used as a relevant indicator. Maximum skin dose (MSD, Gy) represents the highest dose value measured among the dose distribution on the exposed skin area. MSD is the only indicator that is directly predictive of the skin lesions risk. Dose area product (DAP, Gy cm−2) is the total energy imparted to the patient. As the DAP does not depend on the patient-to-focus distance, it can be readily measured through a transmission chamber placed at the tube housing level that fully intercepts the incident primary X-ray beam. However, although DAP meters are almost routinely available on radiographic equipment, some authors demonstrated that the DAP value is not an accurate predictor of MSD and deterministic risks [16-20]. It is, however, a relevant indicator to estimate stochastic risks [1]. To monitor the dose delivered during a procedure, it is useful to characterise the technical factors that affect the skin dose according to the clinical situation.
The correlations between the MSD and the DAP during CAs and PTCAs were first described. Then, clinical, therapeutic and technical factors were analysed in order to determine their partial contribution to the dose variation.
Methods and materials
Study design and population
The study was carried out from 2004 to 2005 at the haemodynamics unit of the clinic of Saint-Gatien in Tours (France). In this catheterisation laboratory, approximately 3000 CAs and/or PTCAs were annually performed at that time. 178 interventional cardiology procedures carried out by 3 experienced cardiologists (A, B and C) were randomly selected over a period of 7 months. One procedure was excluded because of important missing data. Seven patients were treated twice. Among the 177 procedures, 112 were PTCAs, 49 were CAs and 16 were CA+PTCAs. Five angioplasties failed. Four patients underwent a PTCA plus a coronary artery bypass surgery. All the procedures were carried out on the same radiography table.
Data collection
Each procedure included several fluoroscopy sequences as well as cine frame acquisitions. DAP values from both fluoroscopy and radiography were separately collected for each X-ray beam projection using a quality-controlled transmission chamber (Diamentor, Philips, Amsterdam, Netherlands) placed at the tube housing level. In the following, the dose delivered during each sequence is named as “partial DAP” and cumulative dose recorded at the end of the procedure as “DAP”. Cumulative ESD was measured by radiochromic film (Gafchromic, International Specialty Products Advanced Materials Group, Wayne, NJ) (Figure 1) placed on the radiography table beneath the patient for each procedure. DAP values were manually collected and in most cases directly read from the monitor at the control panel.
Figure 1.
Example of an exposed Gafchromic (International Specialty Products Advanced Materials Group, Wayne, NY) film. Dark areas correspond to X-ray beam projections impacting the patient's skin during the interventional cardiology procedure.
Medical reports provided patients' characteristics (age, sex, height, weight, previous PTCA and/or coronary artery bypass surgery) and therapeutic data [artery access, localisation of lesion(s), obstruction degree of lesion(s), number and type of material for dilatation, ventriculography]. Technical parameters (number of frames, frame rate, fluoroscopy time, number of runs, type of radiography, kilovolts, milliamperes, imaging intensifier field size, use of collimation, focus–intensifier distance) were collected from the standardised radiological intervention reports. X-ray beam projection data were available from the digital imaging and communications in medicine (DICOM) header recorded on CD-roms.
Technical aspects
The radiological device was the Integris Allura 9C system (Philips). Prior to the study, quality control tests were carried out to assess the system performances and to calibrate the DAP meter installed on the machine. For the study, three image intensifier field sizes were available: 23, 17 and 14 cm in diameter. Radiographic acquisition modes were “coronaire” (“low dose” mode), “coronaire intense” (“high dose” mode) and “VG Bloqué” (used for the ventriculography), at frame rates of 12.5 and 25 frames per second. Fluoroscopy was set at 15 frames per second for almost all interventions. Tube voltage and current (acquisition mode), automatically controlled by the system, were 90±20 kV and 760±150 mA, respectively. During the quality control tests, the dose rate varied between the low and high modes by about 30% for a 20 cm-thick phantom and about 15% for a 30 cm-thick phantom, on average, whatever the imaging intensifier field size was. The dose rate varied between imaging intensifier fields of 14 and 23 cm by about 50% for a 20 cm-thick phantom and about 60% for a 30 cm-thick one, whatever the radiography mode was.
According to the manufacturer, Gafchromic XR type R films (36×43 cm) can measure dose values ranging from 0.1 Gy to 15 Gy with a precision of 1%. A 9800 XL scanner (Microtek, Hsinchu, Taiwan) was used to read the exposed films and to assess the MSD in a specialised dosimetry laboratory (Laboratorio di Dosimetria, Servizio di Fisica Sanitaria, Ospedale Civile di Udine, Italy). After an appropriate calibration procedure, the software program enabled the isodose surfaces and the dose distribution to be visualised. MSD was defined as the mean dose over the area delimited by the 95% isodose curve, surrounding the peak skin dose that was measured on the film placed on the table at the back of the patient.
Statistical methods
As usual in dosimetry evaluation, both CA+PTCA and PTCA procedures were put together into the same category, hereafter named as “PTCA”. For the analyses, the variable “presence of occluded lesion(s)” was defined as the existence of at least one occlusion (occlusion degree ≥90%) on the treated coronary artery or arteries. The “sum of material for dilatation” was the total number of stents and balloons used to treat the stenosis or stenoses. This variable allowed the procedures to be compared according to their level of therapeutic complexity. Other studies have described a non-linear relation and significant variations of dose among slight changes in the orientation of X-ray projections [11]. Thus, we defined an accurate classification of these projections. Each projection was defined through its tube angulation—cranial (CRAN) or caudal (CAUD)—and its rotation—left anterior oblique (LAO), right anterior oblique (RAO) or postero-anterior (PA).
Correlations between the dosimetric indicators were characterised by the Pearson coefficient if a non-flagrant departure from linearity was observed. We stratified the analyses by the type of procedure (PTCA/CA), which involved different radiological protocols. However, the small sample of CAs did not allow relevant multivariate analyses for these procedures.
The main factors of interest were analysed by linear regression models. The potential explanatory variables were subjected to a preliminary selection in order to maintain an acceptable number of independent variables. Main factors of interest were first identified according to the literature and practitioners' advice. Second, because of multicollinearity issues, the following variables were excluded in order to get unbiased results: the number of lesions, the number of radioscopy sequences per procedure and the ventriculography phase. Primary variables were BMI, previous angioplasty and/or coronary artery bypass surgery (named as “antecedent”), the sum of material for dilatation, the presence of occluded lesion(s), the artery approach, the fluoroscopy time length and the total number of cine frames. Secondary explanatory variables were the age and sex of patient, the mean focus to intensifier distance and the localisation of lesions (e.g. left main, right coronary artery), and these were afterwards tested separately in the model and then together. They were not significantly related to the MSD. Subsequently, to facilitate the interpretation of the multivariate analysis with variables on different scales, the results were interpreted as the proportion of dose level variation related to changes in explanatory factors. Such dose level variations were estimated as predicted mean adjusted effects for each qualitative variable and as predicted mean adjusted dose increases between the first and the third quartiles of the distribution (the interquartile interval, IQI) of the quantitative variables.
Technical parameters variable at each sequence during a procedure could not be taken into account for the MSD analysis. Thus, their effect on dose was indirectly studied in relation to the DAP recorded at each sequence (the “partial DAP”). As the measures were repeated on a patient and related to a given practitioner, a classic regression would not be appropriate. Partial DAP measures did not require longitudinal analysis techniques, however. To take into account the structure of data, we used a linear model with random intercepts. It allowed the break-up of the DAP variance into its component parts. A random model without any explanatory variable allowed estimating the components of the dose variance related to the patients and the practitioners' variability. Next, the associations between the DAP and the technical factors were tested, having been adjusted on the subject and the practitioner “effects”, thus providing robust inferences [21,22].
The Box–Cox method was used to find the necessary transformation of the dependent variables to ensure the validity of the models [23]. The analyses were carried out using R (version 2.10; R Foundation for Statistical Computing, Vienna, Austria) and SAS (version 9.1; SAS Institute Inc., Cary, NC) statistical software.
Results
Description of the X-ray dose according to the type of interventional cardiology procedures
Table 1 describes the sample. Overall, DAP was 68 Gy cm−2, on average (median=48 Gy cm−2), and ranged from 2 to 454 Gy cm−2. DAP due to fluoroscopy represented 26% of the total DAP on average during the CAs (n=49). For the PTCAs (n=128), it represented 43%, and it varied for practitioners A, B and C at 36%, 55% and 44%, respectively.
Table 1. Overall description of coronary angiographies (CAs) and percutaneous transluminal coronary angioplasties (PTCAs) included in the study (n=177).
| Clinical, therapeutic and technical factors, and dosimetry | CA (n=49) |
PTCA (n=128) |
||||
| Mean | Median | Q1–Q3 | Mean | Median | Q1–Q3 | |
| Clinical factors | ||||||
| Age (years) | 68 | 71 | 62–76 | 70 | 71 | 62–78 |
| BMI (kg m–2) | 29 | 28 | 26–31 | 28 | 27 | 25–30 |
| Therapeutic factors | ||||||
| No dilatation material (balloons + stents) | – | – | NA | 3 | 3 | 2–5 |
| Technical factors | ||||||
| Length of fluoroscopy (min) | 5 | 4 | 3–5 | 11 | 9 | 6–15 |
| Number of run | 11 | 11 | 8–12 | 16 | 15 | 10–22 |
| Number of cine frames | 874 | 691 | 551–957 | 892 | 708 | 479–1049 |
| Focus to imaging intensifier distance (cm) | 106 | 107 | 104–110 | 108 | 108 | 103–113 |
| Dosimetry | ||||||
| MSD (mGy) (n=173) | 389 | 306 | 218–488 | 916 | 699 | 396–1068 |
| Total DAP (Gy cm−2) | 65 | 46 | 32–76 | 69 | 48 | 24–82 |
| DAP due to fluoroscopy (Gy cm−2) | 22 | 12 | 5–23 | 34 | 17 | 8–43 |
| DAP due to radiography (Gy cm−2) | 43 | 34 | 24–53 | 35 | 24 | 15–41 |
BMI, body mass index; DAP, dose area product; MSD, maximum entrance skin dose; NA, not applicable; Q1–Q3, first to third quartile.
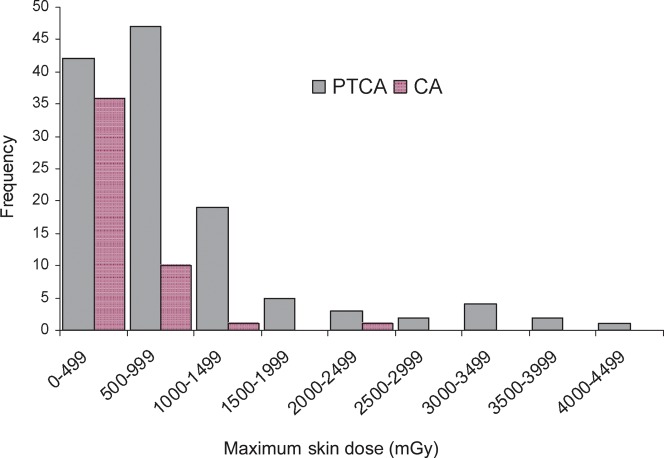
Mean MSD was 770 mGy (median=552 mGy) and ranged from 49 mGy (threshold detection of the Gafchromic film) to 4089 mGy. Mean values of 389 mGy (median=306 mGy) and 916 mGy (median=699 mGy) were observed for the CAs and the PTCAs, respectively. Of the 173 procedures with complete data, the skin dose was equal to or exceeded 2 Gy in 8% of the cases (1 CA, 2%, n=48; 12 PTCAs, 10%, n=125), whereas the skin dose was below 1 Gy in 78% of the cases (Figure 2).
Figure 2.
Distribution of maximum skin dose values (mGy) that occurred during coronary angiographies (n=48) and percutaneous transluminal coronary angioplasties (n=125).
Although DAP did not significantly differ between CAs and PTCAs (Student's test of log dose, p=0.56), MSD was higher for PTCAs (p<0.0001).
Correlation between DAP and MSD
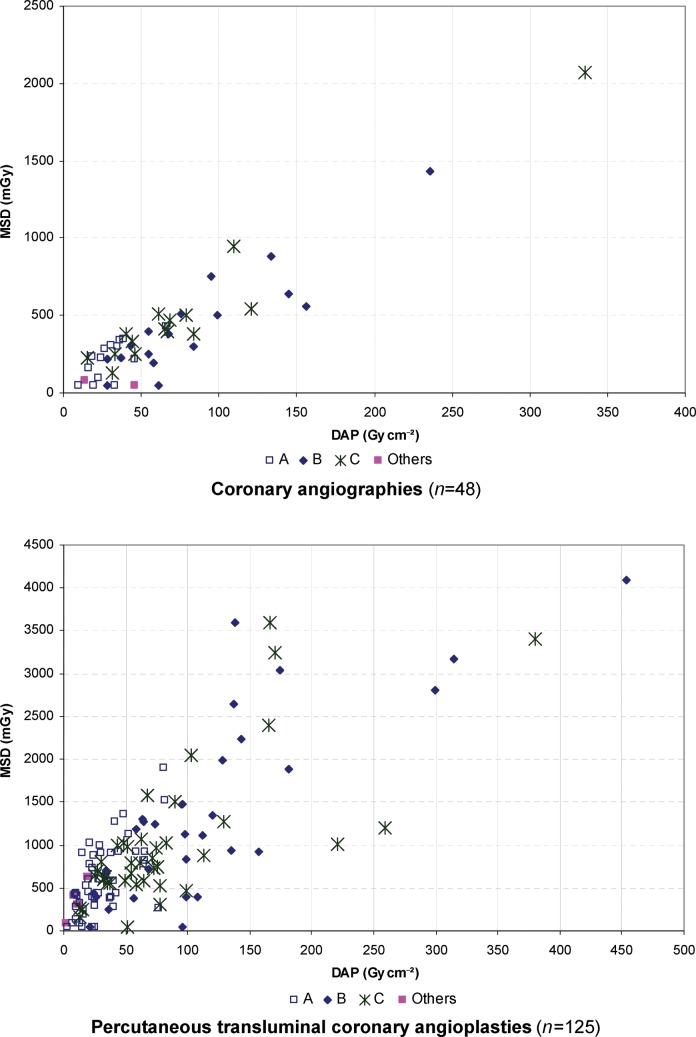
For CAs, there was a linear relationship between the MSD and the DAP (Figure 3), with a correlation coefficient of 0.93. For PTCAs, an important dispersion of the MSD values was observed with the DAP, especially for the procedures carried out by practitioners B and C (Figure 3). Such dispersion was even stronger when looking at the highest doses. For example, two PTCAs performed by practitioner B provided DAPs of 135 and 137 Gy cm−2, respectively, corresponding to MSDs of 939 and 2645 mGy. The shape of the graph remained unchanged when the results were stratified according to BMI (below or above 30 kg m−2). These results show that the prediction of the MSD through a linear function of the DAP will definitely be imprecise, particularly for high dose levels.
Figure 3.
Maximum skin dose (MSD) and dose area product (DAP) relationship according to the procedure type and practitioner (A, B or C).
Clinical, therapeutic and technical factors related to the maximum skin dose
Table 2 describes the MSD for the PTCAs according to the clinical, therapeutic and technical factors.
Table 2. Maximum skin dose (mGy) according to clinical, therapeutic and technical factors during percutaneous transluminal coronary angioplasties (n=125).
| Factors | n | Mean | Median | Q1 | Q3 |
| Sex | |||||
| Male | 99 | 981 | 669 | 396 | 1199 |
| Female | 26 | 669 | 714 | 387 | 846 |
| Antecedenta | |||||
| Yes | 27 | 1126 | 719 | 389 | 1343 |
| No | 98 | 859 | 684 | 397 | 1005 |
| Practitioners | |||||
| A | 50 | 598 | 550 | 291 | 902 |
| B | 30 | 1399 | 1154 | 441 | 1989 |
| C | 40 | 1023 | 792 | 566 | 1048 |
| Others | 5 | – | – | – | – |
| Sum of material for dilatation | |||||
| 0 | 5 | – | – | – | – |
| 1–2 | 49 | 516 | 418 | 246 | 744 |
| 3–4 | 35 | 834 | 730 | 465 | 1005 |
| >4 | 36 | 1548 | 1223 | 627 | 2344 |
| Presence of occluded lesion(s) | |||||
| Yes | 49 | 977 | 796 | 441 | 1265 |
| No | 76 | 877 | 636 | 350 | 1013 |
| Artery approach | |||||
| Radial | 89 | 845 | 642 | 380 | 1027 |
| Femoral | 32 | 1058 | 763 | 413 | 1120 |
| MD | 4 | – | – | – | – |
| Cine frames rate (frame s–1) | |||||
| Only 12.5 | 100 | 840 | 636 | 378 | 1049 |
| 12.5+25.0 | 14 | 913 | 788 | 526 | 1027 |
| Only 25.0 | 11 | 1613 | 1005 | 744 | 2402 |
| Collimation | |||||
| Never | 27 | 1032 | 939 | 630 | 1265 |
| At least once | 97 | 890 | 630 | 396 | 1005 |
| MD | 1 | – | – | – | – |
| Radiography mode | |||||
| Only “low” mode | 85 | 946 | 699 | 387 | 1119 |
| “Low” + “high” modes | 24 | 975 | 902 | 455 | 1049 |
| Only “high” mode | 16 | 671 | 550 | 403 | 946 |
| Use of the largest imaging intensifier (23 cm) | |||||
| Never | 103 | 893 | 698 | 396 | 1117 |
| At least once | 22 | 1027 | 718 | 387 | 1005 |
| Ventriculography | |||||
| Yes | 9 | – | – | – | – |
| No | 116 | 916 | 725 | 397 | 1118 |
MD, missing data; Q1, first quartile; Q3, third quartile.
aPrevious angioplasty and/or coronary artery bypass surgery. Mean values are presented only when the frequency exceeds 10. Maximum skin dose was missing for four percutaneous transluminal coronary angioplasties.
In a multivariate analysis (Table 3), the patient's BMI, the sum of materials for dilation, the fluoroscopy time and the total number of cine frames were significantly related to the MSD for patients undergoing a PTCA, regardless of the practitioner. The MSD increased on average by 8–30% with the use of 3–4 and more than 4 materials for dilatation, in comparison with procedures with 1 or 2 materials, when adjusted to other factors describing a “mean procedure” (these predictions stemmed from the results presented in the Table 3). Previous angioplasty and/or coronary artery bypass surgery, the femoral artery approach and the presence of occluded lesion(s) were not significantly associated with the MSD. BMI was related to a mean dose increase of 22% across an interguartile interval (IQI) of 5.4 kg m–2, the fluoroscopy time to an increase of 162% across an IQI of 9.4 min and the total cine frames number to an increase of 118% across an IQI of 581 frames. Fluoroscopy time and cine frame acquisition were therefore the main determinants of patient skin dose. However, the MSD was also related to the patient's BMI and the therapeutic complexity, irrespective of the fluoroscopy time and the cine frames acquisition.
Table 3. Clinical, therapeutic and technical factors related to the maximum skin dose (mGy) occurring during percutaneous transluminal coronary angioplasties (n=111).
| Factors | Estimations on √(dose) scalea |
Back-transformed coefficients | p-value | |
| Coefficients | 95% CI | |||
| Constant | 37.88 | (27.13, 48.63)c | 397.59 | – |
| BMI (kg m–2) | 1.34 | (0.55, 2.14)c | 2.79 | <0.01 |
| Antecedentb (vs no) | 0.79 | |||
| Yes | 1.07 | (−7.02, 9.16) | 2.36 | |
| Sum of material for dilatation (vs 1–2) | <0.05 | |||
| 3–4 | 4.58 | (−3.98, 13.14) | 10.83 | |
| >4 | 15.72 | (4.97, 26.47)c | 78.51 | |
| Presence of occluded lesion(s) (vs no) | 0.21 | |||
| Yes | 4.28 | (−2.39, 10.95) | 9.85 | |
| Artery approach (vs femoral) | 0.73 | |||
| Radial | −1.33 | (−9.02, 6.36) | −2.77 | |
| Overall fluoroscopy duration (min) | 0.88 | (0.21, 1.55)c | 2.07 | <0.05 |
| Number of cine frames (c100) | 0.82 | (0.17, 1.48)c | 1.99 | <0.05 |
| Practitioners (vs A) | <0.001 | |||
| B | 19.30 | (10.24, 28.36)c | 113.42 | |
| C | 12.42 | (3.94, 20.89)c | 51.95 | |
BMI, body mass index; CI, confidence interval; PTCA, percutaneous transluminal coronary angioplasties.
aBoxcox transformed coefficients (λ=0.5).
bPrevious angioplasty and/or coronary artery bypass surgery.
cConfidence intervals that did not include 0.
95% CI: confidence intervals at a=0.05.
Five PTCAs performed by practitioners (not practitioners A, B or C) were excluded from the analysis. The model was performed at mean values of continuous variables (BMI=27.8 kg m−2, radioscopy duration at 11.1 min and at 914 cine frames), r2=0.602.
The median MSD received by the patients undergoing a PTCA was 550, 1154 and 792 mGy for practitioners A (n=50), B (n=30) and C (n=40), respectively (Table 2). For a similar procedure (described by the patient's BMI and antecedent, the number of materials used for dilatation, the presence of occluded lesion(s), the artery approach, the fluoroscopy time and the number of cine frames), the MSD significantly differed according to the practitioner (Table 3). Mean dose level increased by 26% and 40% on average for practitioners B and C, respectively, as compared with practitioner A.<~?tpb=5pt?>
Technical factors related to the partial dose area-product
At each fluoroscopy sequence carried out during a PTCA, we estimated that 35.4% and 12.4% of the partial DAP variance was related to variability across the practitioners and the patients, respectively. The residual dose variance (52.2%) was the dose variability at the sequence level, linked in particular to the technical parameters for the same patient and practitioner.
Table 4 presents the results concerning the relation between the technical factors and DAP from a multivariate analysis. Beam collimation was related to a significant decrease of the DAP by 2.0 Gy cm−2 per sequence on average, when other technical parameters were kept constant. In comparison with the use of the “low dose” mode at 12.5 frames per second, raising the cine rate to 25 frames per second with the same mode was associated with an increase in DAP of 1.99 Gy cm−2. DAP increased by 2.16 Gy cm−2 with a cine rate at 25 frames per second and the “high dose” mode, and by 1.46 Gy cm−2 when keeping a cine rate of 12.5 frames per second with the “high dose” mode. We did not show that using a “high dose” mode was associated with a higher dose than using a “low dose” mode at 25 frames per second (see confidence intervals in Table 4). These cinematography settings were used, however, for only 87 sequences. DAP increased with the size of the imaging intensifier field. Because of an increase in the skin surface area exposed to radiation, however, we may expect a decrease of MSD when raising the imaging intensifier field size. Otherwise, focus to imaging intensifier distance was not associated with DAP. Finally, DAP was significantly related to the beam projections, with the highest value produced in the caudal orientation at RAO 25–55° and at LAO 35–100°, and in the cranial orientation at LAO 25–35°. Conversely, DAP was lower in PA/RAO 5–55° when adjusting other technical factors.
Table 4. Technical factors related to the DAP (Gy cm−2) at each fluoroscopy sequence delivered during 100 percutaneous transluminal coronary angioplasties performed by 3 practitioners.
| Factors | n | Estimations on log(dose) scalea |
Back-transformed coefficients | p-value | |
| Coefficients | 95% CI | ||||
| Constant | 1550 | 1.35 | (0.15, 2.56)b | 3.86 | – |
| Cinematography settings | <0.0001 | ||||
| “Low dose” mode at 12.5 fr s–1 | 1004 | 0 | reference | ||
| “Low dose” mode at 25 fr s–1 | 154 | 0.69 | (0.40, 1.00)b | 1.99 | |
| “High dose” mode at 12.5 fr s–1 | 305 | 0.38 | (0.19, 0.57)b | 1.46 | |
| “High dose” mode at 25 fr s–1 | 87 | 0.77 | (0.46, 1.08)b | 2.16 | |
| Focus–intensifier distance (cm) | 1550 | −0.00 | (−0.01, 0.01) | −1.00 | 0.30 |
| Intensifier field size (cm) | <0.0001 | ||||
| 14 | 131 | 0 | reference | ||
| 17 | 1401 | 0.43 | (0.23, 0.62)b | 1.53 | |
| 23 | 18 | 0.45 | (−0.01, 0.91) | 1.57 | |
| Collimation | <0.0001 | ||||
| No | 545 | 0 | reference | ||
| Yes | 1005 | −0.70 | (−0.82, −0.58)b | −2.01 | |
| X-ray tube orientation (vs mean effect of beam orientation) | <0.0001 | ||||
| CAUD/RAO (25–55)°C | 84 | 0.29 | (0.07, 0.51)b | 1.34 | |
| CAUD/RAO (15-25)°C | 80 | −0.15 | (−0.37, 0.06) | −1.17 | |
| CAUD/RAO (5-15)°C | 81 | −0.07 | (−0.30, 0.15) | −1.07 | |
| CAUD/PA | 112 | 0.11 | (−0.09, 0.32) | 1.12 | |
| CAUD/LAO (5-25)°C | 62 | −0.00 | (−0.33, 0.33) | −1.00 | |
| CAUD/LAO (25–35)°C | 49 | 0.21 | (−0.08, 0.50) | 1.64 | |
| CAUD/LAO (35–100)°C | 115 | 0.31 | (0.13, 0.49)b | 1.18 | |
| PA/RAO (5–55)°C | 34 | −0.53 | (−0.85, −0.22)b | −1.70 | |
| PA/PA | 30 | −0.12 | (−0.53, 0.29) | −1.13 | |
| PA/LAO (5-25)°C | 47 | −0.18 | (−0.54, 0.18) | −1.20 | |
| PA/LAO (25–35)°C | 108 | −0.09 | (−0.31, 0.14) | −1.09 | |
| PA/LAO (35–55)°C | 45 | −0.05 | (−0.34, 0.25) | −1.05 | |
| PA/LAO (55–100)°C | 117 | −0.17 | (−0.37, 0.02) | −1.20 | |
| CRAN/RAO (35–55)°C | 60 | −0.21 | (−0.47, 0.05) | −1.24 | |
| CRAN/RAO (25–35)°C | 70 | 0.06 | (−0.20, 0.33) | 1.07 | |
| CRAN/RAO (15-25)°C | 104 | 0.05 | (−0.16, 0.26) | 1.05 | |
| CRAN (35–45)°C/RAO (5-15)°C | 72 | 0.16 | (−0.09, 0.42) | 1.18 | |
| CRAN (5–35)°C/RAO (5-15)°C | 65 | −0.18 | (−0.48, 0.12) | −1.20 | |
| CRAN/PA | 72 | 0.01 | (−0.22, 0.24) | 1.01 | |
| CRAN/LAO (5-25)°C | 68 | −0.16 | (−0.43, 0.12) | −1.17 | |
| CRAN/LAO (25–35)°C | 37 | 0.50 | (0.16, 0.83)b | 1.64 | |
| CRAN/LAO (35–100)°C | 38 | 0.17 | (−0.14, 0.48) | 1.18 | |
| Sequence | 1550 | −0.01 | (−0.01, 0.00) | −1.01 | 0.08 |
95% CI, confidence interval; CAUD, caudal; CRAN, cranial; fr, frames; LAO, left anterior oblique; PA, posters-anterior; RAO, right anterior oblique.
aBoxox transformed coefficients (λ=0).
bConfidence interval that does not include 0. Maximum likelihood estimation criterion; standard errors in the random part are 0.59 for the practitioners intercept, 0.41 for the patients intercept and 0.83 for the residual error. Residual error variance of a previous null model was 0.79. The residual variance proportion explained by the covariates is 13.2%; the practitioners' random intercept variance decreased by 36.9% while introducing the covariates.
Technical factors according to the practitioners
The previous technical factors (beam collimation, cinematography settings, image intensifier field size, focus to imaging intensifier distance, beam projections) explained 37% of the DAP variability that was estimated across the practitioners (Table 4). Univariate analyses presented in Table 5 show that the cinematography settings, the beam collimation and the number of cine frames varied according to the practitioners. In particular, all the procedures carried out by practitioner A were performed with collimation, although it was used for only 36.7% and 80.5% of those carried out by practitioners B and C, respectively. Nevertheless, no difference was shown for the patients' BMI and the therapeutic complexity of the procedures. These results suggest that the “practitioner effect” on dose variation demonstrated above may be related to their radioprotection behaviours.
Table 5. Univariate comparisons of technical factors used during percutaneous transluminal coronary angioplasties according to the practitioners.
| A (n=52) |
B (n=30) |
C (n=41) |
|||||
| Factors | Mean | Q1–Q3 | Mean | Q1–Q3 | Mean | Q1–Q3 | p-value |
| Clinical factors | |||||||
| BMI (kg m–2) | 27.4 | 24.4–30.1 | 27.7 | 25.0–29.4 | 28.2 | 25.0–30.4 | 0.75 |
| No dilatation material | 3.8 | 2.0–6.0 | 3.2 | 1.0–5.0 | 3.4 | 1.0–5.0 | 0.10 |
| Technical factors | |||||||
| Length of fluoroscopy (min) | 11.3 | 5.9–16.1 | 12.0 | 5.4–15.8 | 10.9 | 6.7–15.3 | 0.63 |
| Number of runs | 17.1 | 12.0–22.0 | 15.5 | 9.0–23.0 | 16.8 | 10.0–22.0 | 0.07 |
| Number of cine frames | 619 | 368–746 | 1074 | 557–1489 | 1183 | 618–1689 | <0.0001 |
| % with only “low dose” mode at 12.5 fr s–1 | 40.4 | 80.0 | 41.5 | <0.01 | |||
| % without any “low dose” mode at 25 fr s–1 | 100.0 | 100.0 | 65.9 | – | |||
| % without any “high dose” mode at 12.5 fr s–1 | 53.9 | 96.7 | 87.8 | <0.0001 | |||
| % without any “high dose” mode at 25 fr s–1 | 92.3 | 93.3 | 87.8 | 0.66 | |||
| % without any collimation | 0.0 | 63.3 | 19.5 | – | |||
| % without any 14 cm intensifier field | 80.8 | 80.0 | 68.3 | 0.32 | |||
| % without any 23 cm intensifier field | 78.9 | 86.7 | 82.9 | 0.66 | |||
fr, frames; Q1–Q3: first to third quartile.
Discussion
The patient skin dose is difficult to collect routinely during an interventional cardiology procedure. The use of Gafchromic film allowed this quantity to be recorded quite precisely. The performance claimed by the manufacturer may be overestimated, however (i.e. doses below 0.2 Gy may be less reliable [9,24]). Indeed, when the X-ray beam projection is far away from the vertical position (LOA 90°, for instance, or close to it), the primary beam cannot intercept the film. Such a situation happens rather rarely within a given procedure compared with all other projections used [7% of all projections in the sample. The two proportions were on different denominators (beam projections and patients)]. Furthermore, the same radiography table was used for all the included procedures to limit the confounding factors and to facilitate the interpretation of the results.
This study demonstrated that the MSD was not only related to the fluoroscopy time and the number of cine frames. The procedure complexity and the practitioner had an independent effect on the dose, even for a comparable fluoroscopy time and cine frame number. The procedure complexity and the practitioner's attitude towards radioprotection involve other mechanisms that affect the dose level (Table 3). The impact of the practitioner may be attributable to a selection bias, but clinical and therapeutic variables (although insufficient to model the complexity of procedures as a whole [6,25]) allowed adjusting on such a potential bias. Moreover, the technical data collected in this sample allowed for studying precisely how the parameters of procedure optimisation impacted on the dose imparted to patients. To be exhaustive, other technical factors, such as focus to patient distance, fluoroscopy dose rate and fluoroscopy sequence duration, could be collected in the future.
Table 6 compares recently published dose values and correlation coefficients between the MSD and the DAP. It highlights heterogeneity in dose values that is likely to be associated with the variety of radiological equipment, the available technical parameters, the experience and radioprotection training of the practitioners and the local radiological protocols. Findings of this study were nevertheless comparable to recent publications. Among the 173 patients, 8% received an MSD between 2 and 4 Gy. In comparison, Bor et al [26] stated that 7% of 325 patients had received a dose between 2 and 6 Gy, and Bogaert et al [16] showed a proportion of 3% with a dose between 2 and 3 Gy among 318 patients.
Table 6. Comparison of recently published doses and correlation coefficients with the values in this study.
| First author | Year | Mean MSD (mGy) | Mean DAP (Gy cm−2) | Coefficient correlation (r) | Interventional cardiology procedures | n |
| This study | 389±360 | 65±58 | CA | 49 | ||
| 924±840 | 67±73 | PTCA | 112 | |||
| 770±760 | 68±69 | 0.76 | Overall | 177 | ||
| Bogaert [16] | 2009 | 310 | 56 | – | CA | 82 |
| 699 | 81.5 | PTCA | 118 | |||
| Stratis [28] | 2009 | – | 24±17 | – | CA | 108 |
| 54±47 | PTCA | 101 | ||||
| Suzuki [5] | 2006 | 2000±1600 | – | – | PCI | 97 |
| Chida [17] | 2006 | 1454±991 | 149±103 | 0.72 | PCI | 172 |
| Kuon [15] | 2003 | – | 56±26 | – | CA | 404 |
| Van de Putte [19] | 2000 | – | – | 0.77 | PCI+CC | 100 |
CA, coronary angiographies; CC, coronary catheterisation; DAP, dose area product; MSD, maximum entrance skin dose; PCI, percutaneous coronary interventions; PTCA, percutaneous transluminal coronary angioplasties; r, Pearson coefficient.
Linear correlation coefficient between the MSD and the DAP for CAs and/or PTCAs (r=0.76) was comparable to those described by Chida et al (r=0.72) [17] and by Van de Putte et al (r=0.77) [19] (Table 6). Although estimating the MSD as a linear function of DAP, as is sometimes suggested, may be acceptable for CAs, a similar approach for PTCAs would definitely not be accurate, particularly for high doses that require the best surveillance. Monitoring the skin dose during the procedure only through DAP would therefore be careless.
The study was limited by the sample size and the difficulty of modelling the therapeutic complexity. Indeed, previous works that aimed at defining a predictive score of the fluoroscopy duration revealed that other therapeutic characteristics had a significant impact: the number of complex lesions (according to American Heart Association/American College of Cardiology classification [27]), the bifurcation and ostial stenting, the severity of occlusions and the degree of the tortuosity of treated vessels [6,25]. Furthermore, our results cannot be easily extrapolated, since patients were not randomly sampled from different interventional cardiology units. Mean adjusted effects were therefore related to the specific characteristics of the sample. However, the study allowed an analysis to be made of the relative weight of each clinical and therapeutic determinant, mostly described elsewhere by only univariate analyses or in experimental conditions.
As far as optimisation of the procedures is concerned, and according to radiation protection guidelines, primary collimation, a low dose cine mode and a low cine frame rate were clearly identified as practicable options leading to significant patient dose reduction. DAP varied also according to the primary beam orientations as X-rays pass through different tissues and the dose rate was automatically controlled by the automatic exposure control system.
Conclusion
The fluoroscopy time and the number of cine frames are the leading explanatory factors of the MSD and the potential subsequent deterministic skin effect. However, this work showed that other factors should be considered in order to propose predictive models for supporting the monitoring of dose during interventional cardiology procedures.
MSD is largely the result of the technical options chosen by the operator (imaging intensifier field size, beam collimation, wedge filter, frame rate, dose rate and focus–detector distance) as their modality. The study illustrates the impact of operator behaviour on dose, which probably results in a complex attitude that integrates all theses technical options. Staff training on radioprotection is needed in catheterisation laboratories in order to optimise the use of these interlinked technical options. Computerised alarm systems or complex predictive models may also be supported to warn the practitioner when the dose may exceed specified thresholds. In any case, an increased surveillance should be encouraged with overweight patients and/or those with severe/multiple lesions.
Acknowledgments
The work has been partially supported by the Ministry of Health and the former General Department of Nuclear Safety and Radiation Protection (DGSNR). The authors wish to thank the cardiologists and the patients involved in the study.
References
- 1.International Commission on Radiological Protection (ICRP) Publication 85. Avoidance of radiation injuries from medical interventional procedures. Oxford, UK: Pergamon Elsevier Science Ltd; Ann ICRP 2000;30(2) [DOI] [PubMed] [Google Scholar]
- 2.Kuon E, Glaser C, Dahm JB. Effective techniques for reduction of radiation dosage to patients undergoing invasive cardiac procedures. Br J Radiol 2003;76:406–13 [DOI] [PubMed] [Google Scholar]
- 3.Suzuki S, Furui S, Isshiki T, Kozuma K, Endo G, Yamamoto Y, et al. Factors affecting the patient's skin dose during percutaneous coronary intervention for chronic total occlusion. Circ J 2007;71:229–33 [DOI] [PubMed] [Google Scholar]
- 4.Delichas MG, Psarrakos K, Hatziioannou K, Giannoglou G, Molyvda-Athanasopoulou E, Papanastassiou E, et al. The dependence of patient dose on factors relating to the technique and complexity of interventional cardiology procedures. Phys Med 2005;21:153–7 [DOI] [PubMed] [Google Scholar]
- 5.Suzuki S, Furui S, Kohtake H, Yokoyama N, Kozuma K, Yamamoto Y, et al. Radiation exposure to patient's skin during percutaneous coronary intervention for various lesions, including chronic total occlusion. Circ J 2006;70:44–8 [DOI] [PubMed] [Google Scholar]
- 6.Tsapaki V, Maniatis PN, Magginas A, Voudris V, Patsilinakos S, Vranzta T, et al. What are the clinical and technical factors that influence the kerma-area product in percutaneous coronary intervention? Br J Radiol 2008;81:940–5 [DOI] [PubMed] [Google Scholar]
- 7.Tsapaki V, Magginas A, Vano E, Kottou S, Papadakis E, Dafnomili P, et al. Factors that influence radiation dose in percutaneous coronary intervention. J Interv Cardiol 2006;19:237–44 [DOI] [PubMed] [Google Scholar]
- 8.Padovani R, Bernardi G, Malisan MR, Vano E, Morocutti G, Fioretti PM. Patient dose related to the complexity of interventional cardiology procedures. Radiat Prot Dosimetry 2001;94:189–92 [DOI] [PubMed] [Google Scholar]
- 9.Guibelalde E, Gonzalez L, Vano E. Suitability of resin-coated photographic paper for skin dose measurement during fluoroscopically-guided X-ray procedures. Br J Radiol 2004;77:871–5 [DOI] [PubMed] [Google Scholar]
- 10.Blanpain T, Brasselet C, Tassan-Mangina S, Deschildre A, Clément JP, Gaillot-Petit N, et al. Doses estimations of a comparative study between radial and femoral access during coronary angiograms and percutaneous coronary interventions. Radioprotection 2008;43:449–63 [DOI] [PubMed] [Google Scholar]
- 11.Georges JL, Livarek B, Gibault-Genty G, Messaoudi H, Aziza JP, Hautecoeur JL, et al. Variations of radiation dosage delivered to patients undergoing interventional cardiological procedures. A monocentric study 2002–05. Arch Mal Coeur Vaiss 2007;100:175–81 [PubMed] [Google Scholar]
- 12.Geijer H, Beckman KW, Andersson T, Persliden J. Radiation dose optimization in coronary angiography and percutaneous coronary intervention (PCI). I. Experimental studies. Eur Radiol 2002;12:2571–81 [DOI] [PubMed] [Google Scholar]
- 13.Geijer H, Beckman KW, Andersson T, Persliden J. Radiation dose optimization in coronary angiography and percutaneous coronary intervention (PCI). II. Clinical evaluation. Eur Radiol 2002;12:2813–19 [DOI] [PubMed] [Google Scholar]
- 14.Kuon E, Dahm JB, Empen K, Robinson DM, Reuter G, Wucherer M. Identification of less-irradiating tube angulations in invasive cardiology. J Am Coll Cardiol 2004;44:1420–8 [DOI] [PubMed] [Google Scholar]
- 15.Kuon E, Dorn C, Schmitt M, Dahm JB. Radiation dose reduction in invasive cardiology by restriction to adequate instead of optimized picture quality. Health Phys 2003;84:626–31 [DOI] [PubMed] [Google Scholar]
- 16.Bogaert E, Bacher K, Lemmens K, Carlier M, Desmet W, De Wagter X, et al. A large-scale multicentre study of patient skin doses in interventional cardiology: dose-area product action levels and dose reference levels. Br J Radiol 2009;82:303–12 [DOI] [PubMed] [Google Scholar]
- 17.Chida K, Saito H, Otani H, Kohzuki M, Takahashi S, Yamada S, et al. Relationship between fluoroscopic time, dose-area product, body weight, and maximum radiation skin dose in cardiac interventional procedures. Am J Roentgenol 2006;186:774–8 [DOI] [PubMed] [Google Scholar]
- 18.Domienik J, Papierz S, Jankowski J, Peruga JZ, Werduch A, Religa W. Correlation of patient maximum skin doses in cardiac procedures with various dose indicators. Radiat Prot Dosimetry 2008;132:18–24 [DOI] [PubMed] [Google Scholar]
- 19.Van dePutte S, Verhaegen F, Taeymans Y, Thierens H. Correlation of patient skin doses in cardiac interventional radiology with dose-area product. Br J Radiol 2000;73:504–13 [DOI] [PubMed] [Google Scholar]
- 20.Vano E, Gonzalez L, Ten JI, Fernandez JM, Guibelalde E, Macaya C. Skin dose and dose-area product values for interventional cardiology procedures. Br J Radiol 2001;74:48–55 [DOI] [PubMed] [Google Scholar]
- 21.Goldstein H. Multilevel statistical models. 2nd edn London, UK: Edward Arnold, 1995 [Google Scholar]
- 22.Snijders T, Bosker R. Multilevel analysis: an introduction to basic and advanced multilevel modeling. London, UK: Sage, 1999: 266 [Google Scholar]
- 23.Box GEP, Cox DR. An analysis of transformations. J R Stat Soc Series B Stat Methodol 1964;26:211–52 [Google Scholar]
- 24.Giles ER, Murphy PH. Measuring skin dose with radiochromic dosimetry film in the cardiac catheterization laboratory. Health Phys 2002;82:875–80 [DOI] [PubMed] [Google Scholar]
- 25.Bernardi G, Padovani R, Morocutti G, Vano E, Malisan MR, Rinuncini M, et al. Clinical and technical determinants of the complexity of percutaneous transluminal coronary angioplasty procedures: analysis in relation to radiation exposure parameters. Catheter Cardiovasc Interv 2000;51:1–9 [DOI] [PubMed] [Google Scholar]
- 26.Bor D, Olgar T, Toklu T, Caglan A, Onal E, Padovani R. Patient doses and dosimetric evaluations in interventional cardiology. Phys Med 2009;25:31–42 [DOI] [PubMed] [Google Scholar]
- 27.Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, King SB, Loop FD, et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). Circulation 1988;78:486–502 [DOI] [PubMed] [Google Scholar]
- 28.Stratis AI, Anthopoulos PL, Gavaliatsis IP, Ifantis GP, Salahas AI, Antonellis IP, et al. Patient dose in cardiac radiology. Hellenic J Cardiol 2009;50:17–25 [PubMed] [Google Scholar]