Abstract
Reports on the MRI findings of cerebral corticosubcortical lesions in osmotic myelinolysis (OM) are rare despite several pathological descriptions of this involvement. We report two patients with subcortical lesions associated with OM that were characteristically distributed along the crowns and sides of the cerebral gyri on T2 weighted or fluid-attenuated inversion-recovery MRI. We also discuss the MRI characteristics of corticosubcortical lesions in OM.
Osmotic myelinolysis (OM) is a demyelinating disorder characterised by acutely evolving corticospinal and bulbar dysfunction following the rapid correction of hyponatraemia [1]. In the literature, MRI findings of OM consist primarily of hyperintensity on T2 weighted images (T2WI) or fluid-attenuated inversion-recovery (FLAIR) images in the central pons (central-pontine myelinolysis), midbrain, bilateral thalami and/or basal ganglia (extrapontine myelinolysis), or a combination of these [1]. Although cerebral corticosubcortical involvements have been described in pathological studies [1-3], a description of the MRI findings has been mostly limited to case reports [4-8].
We report serial MRI findings in two patients with corticosubcortical lesions associated with OM. One patient's lesions were disclosed on MRI that included diffusion-weighted imaging (DWI) and T1 weighted imaging (T1WI) with contrast enhancement that were obtained 8 days (late acute phase) and 26 days (subacute phase) after the onset of neurological symptoms. The other patient's lesions appeared on MRI 14 months (chronic phase) after symptom onset.
Case 1
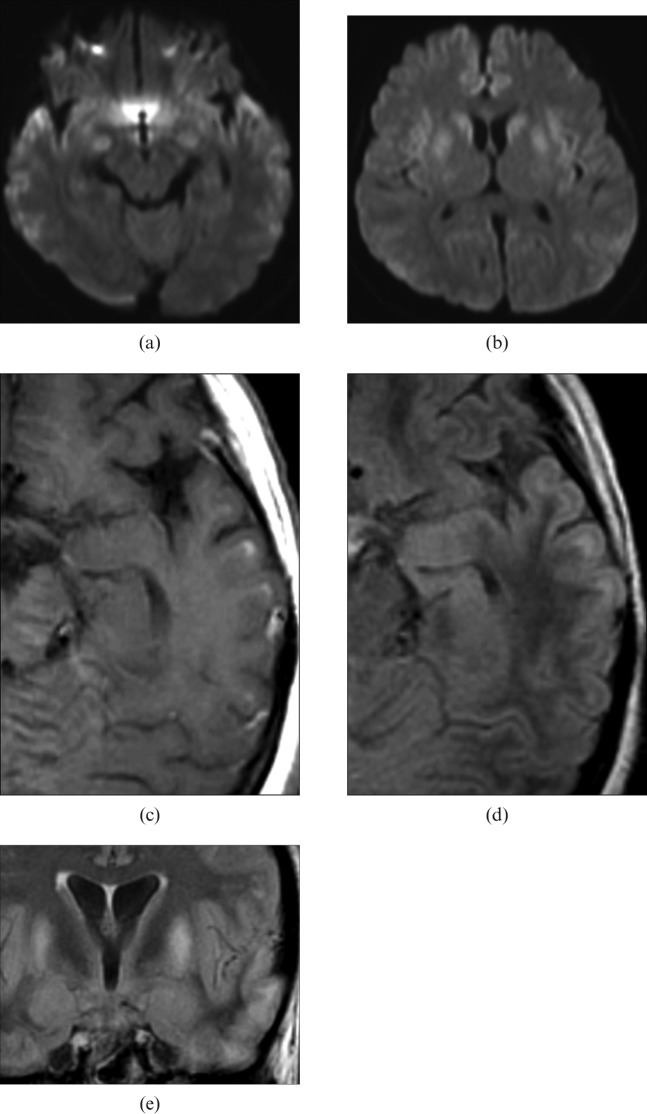
Following 1 month of chemotherapy, a 28-year-old female with advanced colon cancer developed coma and myoclonus-like involuntary movement as a result of hyponatraemia of 110 mEq l–1. This was presumably caused by water toxicosis derived from vasopressin therapy for diabetes insipidus. The clinicians tried to control the serum sodium level gradually, but it unexpectedly rapidly elevated to 178 mEq l–1 within 4 days. The patient remained comatose after returning to normonatraemia and underwent an MRI of the brain 8 days after the onset of neurological symptoms (late acute phase). DWI showed multiple hyperintense lesions in the striatum (putamen and caudate nucleus) and almost symmetrically distributed in the corticosubcortical regions (Figure 1a,b). These lesions also appeared hyperintense on T2WI and FLAIR imaging. Some of the corticosubcortical lesions showed contrast enhancement, especially those subjacent to the top (crown) of the cerebral gyri (Figure 1c). The clinical presentation and MRI findings led to a diagnosis of OM with extrapontine involvement. In the subacute phase (26 days after the onset of symptoms), the cortico-subcortical lesions became more evident on T2WI and FLAIR images (Figure 1d,e) but appeared hypointense on T1WI. The patient remains comatose.
Figure 1.
Case 1: diffusion-weighted images taken on the eighth day from the onset of neurological symptoms show (a) multiple spotty and laminar hyperintense lesions in bilateral corticosubcortical regions and (b) hyperintense lesions distributed bilaterally in the bilateral basal ganglia. (c) Gadolinium-enhanced T1 weighted image obtained the same day shows patchy subcortical enhancement with possible cortical swelling. (d) Transverse and (e) coronal fluid-attenuated inversion-recovery images taken on the 26th day from symptom onset show the lesions in the basal ganglia and subcortical regions with some loss of cortical swelling. On close observation, these spotty subcortical hyperintense lesions are seen to predominantly involve the corticomedullary junction beneath the crown of gyri but to spare the overlying layer of cortices in the temporal lobe.
Case 2
A 65-year-old female was admitted to the hospital with loss of consciousness and convulsion half a day after she drank a large amount of water and took psychotropic drugs in a suicide attempt. On admission, sodium serum concentration was 101 mEq l–1 and blood arterial gas and cerebrospinal fluid studies were normal. The serum sodium level was gradually corrected up to 129 mEq l–1 over 5 days in hospital, but she did not fully recover consciousness.
25 days after the onset of her neurological symptoms, an MRI showed a hyperintense lesion in the central pons on T2WI and FLAIR images, which led to a diagnosis of OM. In addition to the pontine lesion, on FLAIR images we retrospectively recognised symmetrical linear and spotty hyperintense foci in the corticosubcortical areas of the bilateral frontal and temporal lobes, which were subtle on both T1WI and T2WI. The patient's consciousness level gradually improved and she was discharged after 30 days, without neurological deficit.
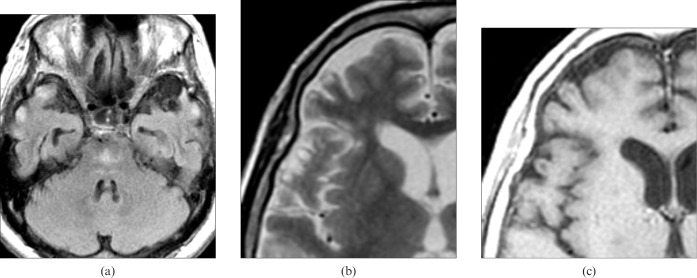
8 months after the episode of hyponatraemia, the patient began to experience gradually worsening hallucinations, and 14 months after the initial admission was re-admitted to our hospital with sensory hallucination and delirium. She demonstrated catatonic excitation and an inability to communicate. We noted neither paresis nor cranial nerve deficiency. MRI demonstrated shrinkage of the pontine lesions and atrophic changes of the cortices. FLAIR and T2WI demonstrated patchy areas of subcortical hyperintensity in both frontal and temporal lobes, which appeared hypointense on T1WI. On close observation, the lesions were seen just beneath a thin, normal-appearing external layer of the cortex in the top (crown) of the gyri (Figure 2). The psychiatric symptoms remained almost stable.
Figure 2.
Case 2: MRI performed 14 months after initial admission. (a) Axial fluid-attenuated inversion-recovery image shows an ill-defined but hyperintense lesion of trident configuration in the pontine basis and multiple patchy hyperintense foci with some local atrophic changes in the bilateral temporal corticosubcortical regions. (b) T2 weighted and (c) T1 weighted images show subcortical foci beneath a normal-appearing thin layer of cortices. They are located along the top (crown) and side of the gyri and show hyperintensity on T2 weighted images and hypointensity on T1 weighted images.
Discussion
MRI in our two cases with OM demonstrated subcortical involvement that spared the superficial layer of the cortical gyri. Gocht et al [2] examined lesion distribution in 58 autopsy cases with OM and found cerebral corticosubcortical lesions in 15%. To the best of our knowledge, corticosubcortical lesions associated with OM have been unfamiliar clinically, and their imaging findings have been reported only sporadically [4-9].
Okeda et al [3] studied the pathological features of corticosubcortical lesions in autopsy cases with OM and reported two distinct subtypes of lesions: cortical and subcortical. The cortical type showed laminar cortical astrocytosis and necrosis, and the subcortical type represented demyelination in the deeper layers of the cortex and subjacent white matter of the crowns and sides of the cerebral gyri (Figure 3).
Figure 3.
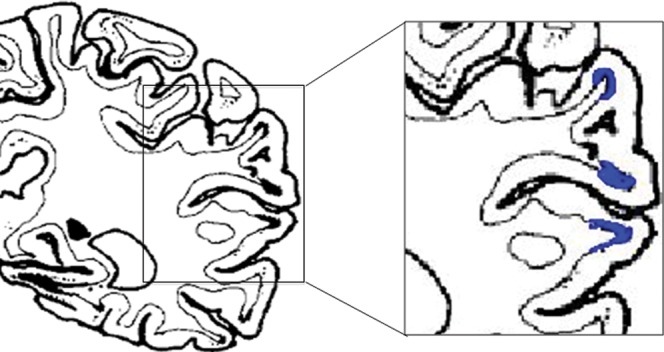
Diagram indicating location of subcortical lesions with spared cortical layer in the frontal and temporal lobes (reproduced from Okeda et al [3]). The lesions are located beneath the crown and sides of the gyri and surrounded by cortices in three directions.
Regarding the subcortical involvement, Okeda's group hypothesised that the white matter sandwiched by or intermixed with massive grey matter is susceptible to osmotic demyelination as in the pontine basis, whereas white matter bundles are intermingled with lots of pontine nuclei. Thus, myelinotoxic factors might have been derived from vascular-rich grey matter and could interact with adjacent white matter bundles to lead finally to demyelination [3].
The cortical and subcortical types of involvement have not been clearly distinguished from each other or discussed in the radiological literature with OM. To our knowledge, seven cases with corticosubcortical lesions have been described in six reports that include MRI [4-9]. Reviewing those reports, we found that, consistent with Okeda's pathological classification, the two types of corticosubcortical OM lesions can also be discriminated on MRI. Involvement was cortical in four cases [5-7,9], subcortical in two cases [4,8] and mixed in one case [5].
The cortical type showed almost symmetrical gyri form hyperintense signal on T2WI and curvilinear enhancement on contrast-enhanced T1WI, both along the cerebral cortices in the acute phase. Subacute-phase pre-contrast T1WI showed curvilinear hyperintensities along the gyri [5-7,9]. Follow-up MRI demonstrated that cortical atrophy eventually developed in the involved region in a few cases. Such characteristic features of the lesions resembled those of cortical laminar necrosis in hypoxia [10].
On T2WI and FLAIR with or without gadolinium enhancement in early clinical stage, the subcortical type also demonstrated symmetrical hyperintense signal, not in the cortices, but in the subcortical white matter; the lesions became demarcating and atrophic features developed over time. Such subcortical lesions showed no T1 shortening during the entire clinical course [4]. One case seemed to have mixed features of both types on MRI [5].
The distribution and serial changes of the lesions in both our cases were identical with the documented subcortical type of lesions. In our first case, DWI obtained on the eighth day from the neurological onset showed hyperintense subcortical lesions that enhanced with contrast. We presume the positive DWI findings may indicate irreversible cytotoxic damage that should have been detected earlier with conventional sequences. The characteristic involvement of subcortical lesions beneath the tops and sides of the gyri on MRI in our two cases corresponds with findings in previous pathological reports [3]. Such lesion sites may well be regarded as being surrounded by cortices in three directions, therefore allowing myelinotoxic factors deriving from vascular-rich grey matter to easily reach the subcortical white matter.
Of the cortical and subcortical types of involvement in OM, the cortical type appears radiologically and pathologically similar to cortical laminar necrosis in hypoxia; both show breakdown of the blood–brain barrier, laminar lesion distribution in the cortical layers, macrophage deposition and demyelination [9]. Calakos et al [5] suggested that hyponatraemia may increase the susceptibility of the cortical layer to associated hypoxic insult or that hypoxia could impair the normal compensatory mechanisms of the brain to alleviate the white matter involvement in hyponatraemia [5]. Roh et al [9] indicated that a complicated occurrence of several pathogenetic factors, such as OM, hypoxia and other insult, could contribute to the development of T1 shortening cortical-type lesions. In contrast to the cortical type of lesions with a probable multifactorial pathogenesis, we suspect the subcortical type of lesions could represent white matter involvement more specific to OM.
Conclusions
We report two rare cases of OM that demonstrated cerebral subcortical involvement on MRI that included DWI. Clinically, such involvement may not be very familiar, but the almost symmetrical subcortical distribution of lesions in both cerebral hemispheres may represent very characteristic involvement of OM. In particular, lesions located beneath the crown and side of the gyri that spare the adjacent cerebral cortices could be a clue to diagnosis.
References
- 1.Martin RJ. Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes. J Neurol Neurosurg Psychiatry 2004;75:iii,22–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gocht A, Colmant HJ. Central pontine and extrapontine myelinolysis: a report of 58 cases. Clin Neuropathol 1987;6:262–70 [PubMed] [Google Scholar]
- 3.Okeda R, Kitano M, Sawabe M, Yamada I, Yamada M. Distribution of demyelinating lesions in pontine and extrapontine myelinolysis—three autopsy cases including one case devoid of central pontine myelinolysis. Acta Neuropathol 1986;69:259–66 [DOI] [PubMed] [Google Scholar]
- 4.Bourgouin PM, Chalk C, Richardson J, Duang H, Vezina JL. Subcortical white matter lesions in osmotic demyelination syndrome. AJNR Am J Neuroradiol 1995;16:1495–7 [PMC free article] [PubMed] [Google Scholar]
- 5.Calakos N, Fischbein N, Baringer JR, Jay C. Cortical MRI findings associated with rapid correction of hyponatremia. Neurology 2000;55:1048–51 [DOI] [PubMed] [Google Scholar]
- 6.Lebrun C, Thomas P, Chatel M, Magnie MN, Paquis P, Chanalet S. Cortical sclerosis presenting with dementia as a sequel of rapid correction of hyponatraemia. J Neurol Neurosurg Psychiatry 1999;66:685–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takei Y, Akahane C, Ikeda S. Osmotic demyelination syndrome: reversible MRI findings in bilateral cortical lesions. Intern Med 2003;42:867–70 [DOI] [PubMed] [Google Scholar]
- 8.Odier C, Nguyen DK, Panisset M. Central pontine and extrapontine myelinolysis: from epileptic and other manifestations to cognitive prognosis. J Neurol 2010;257:1176–80 [DOI] [PubMed] [Google Scholar]
- 9.Roh JH, Kim JH, Kim SG, Park KW, Kim BJ. Cortical laminar necrosis caused by rapidly corrected hyponatremia. J Neuroimaging 2009;19:185–7 [DOI] [PubMed] [Google Scholar]
- 10.Takahashi S, Higano S, Ishii K, Sakamoto K, Iwasaki Y, Suzuki M. Hypoxic brain damage: cortical laminar necrosis and delayed changes in white matter at sequential MR imaging. Radiology 1993;189:449–56 [DOI] [PubMed] [Google Scholar]