Abstract
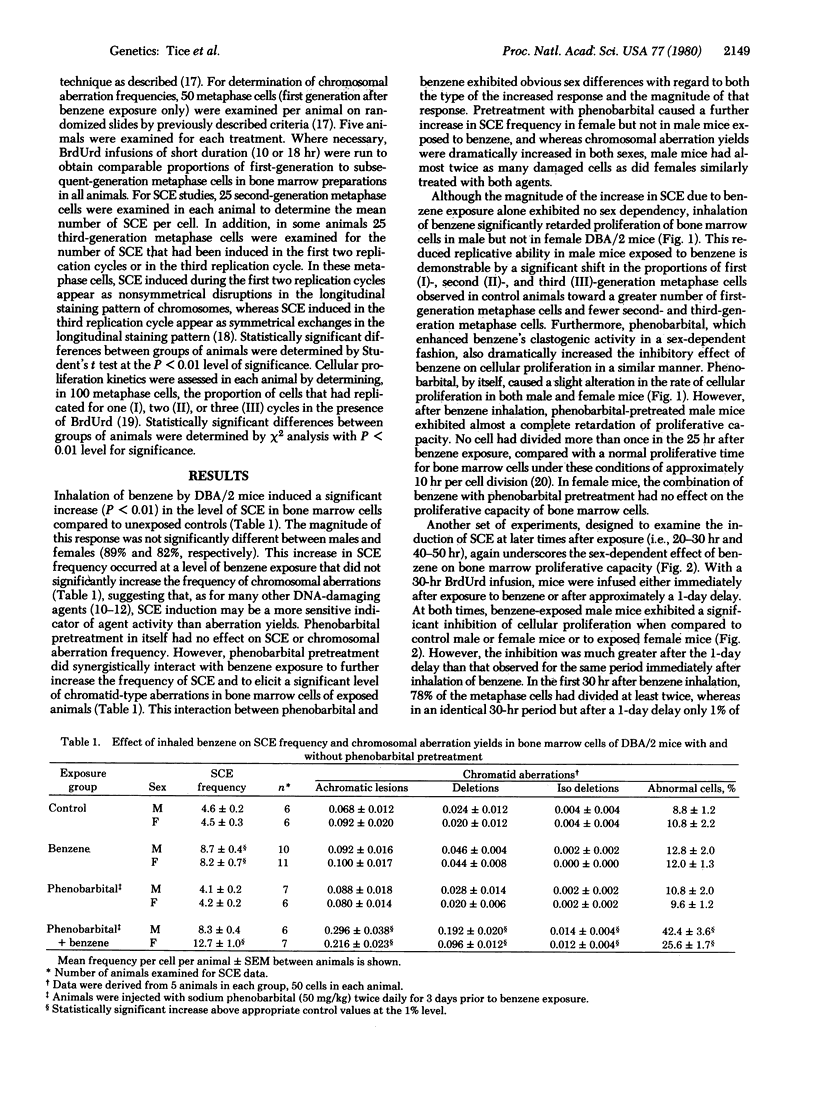
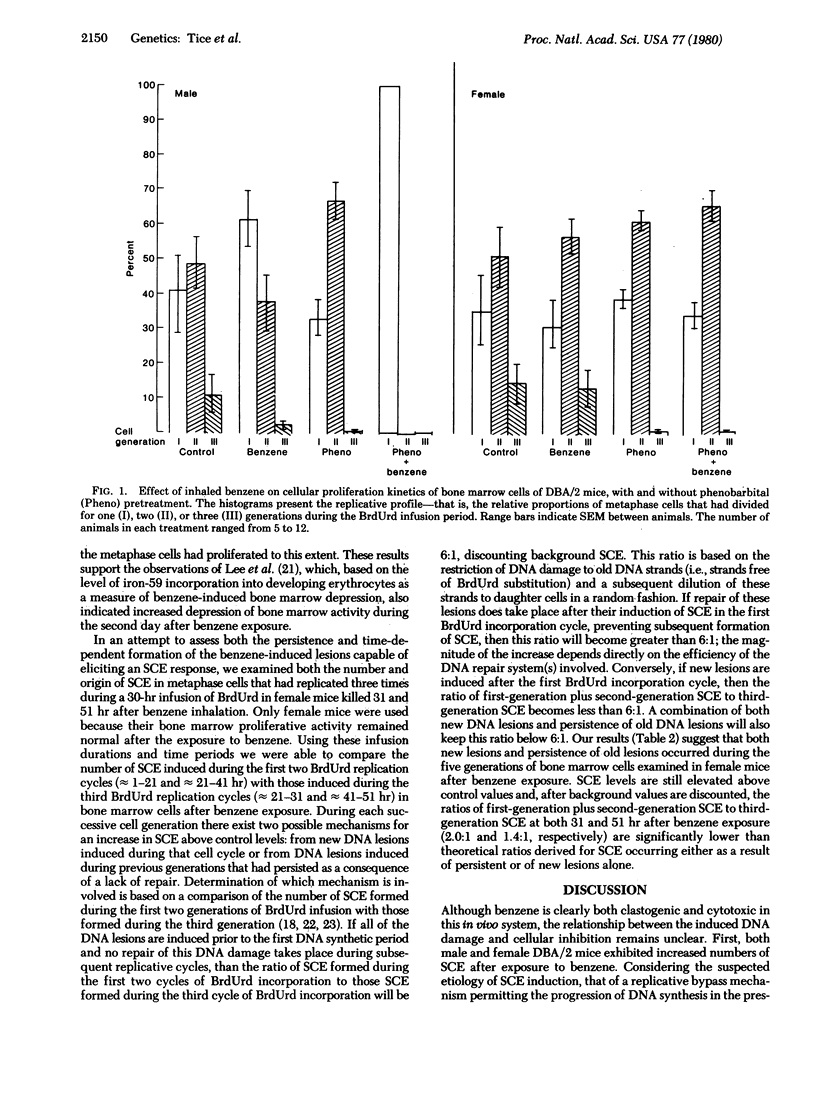
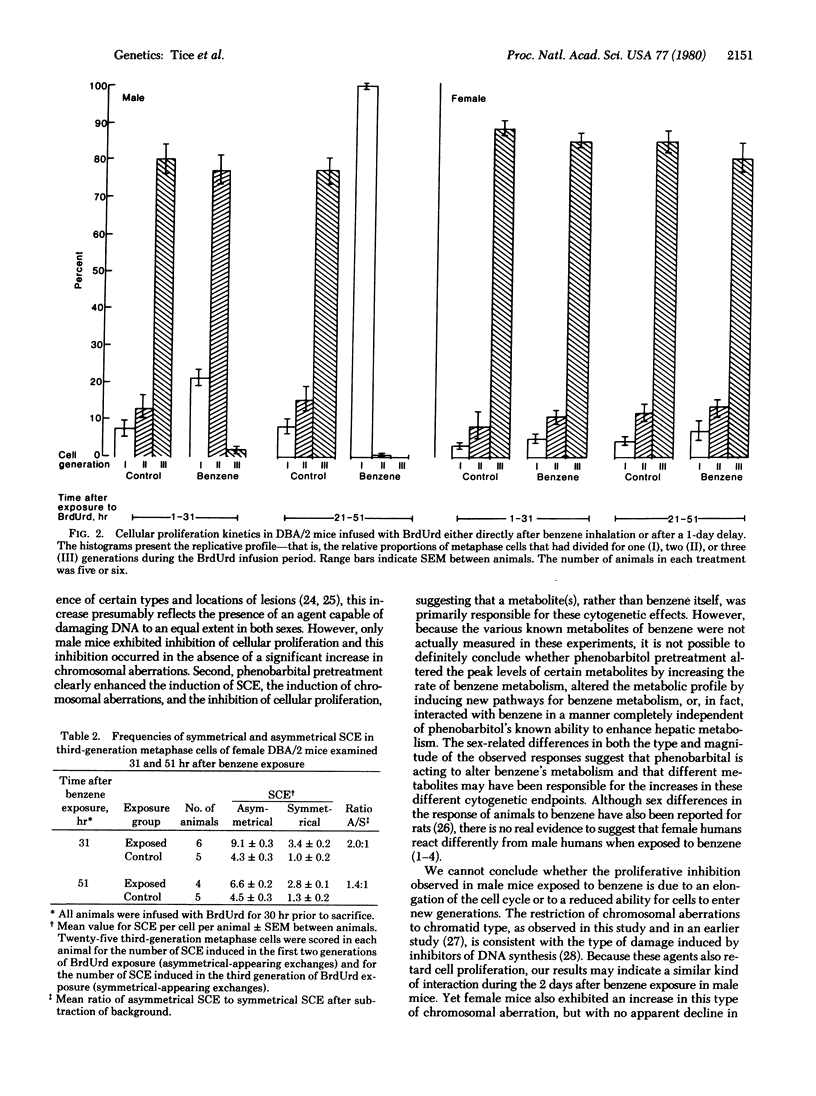
Exposure of adult male and female DBA/2 mice to 3100 ppm benzene for 4 hr significantly increased the frequency of sister chromatid exchanges in bone marrow cells of both sexes, inhibited marrow cellular proliferation (but only in male mice), and did not significantly increase the frequency of chromosomal aberrations in either sex. Phenobarbital pretreatment synergistically interacted with benzene exposure to further increase sister chromatid exchanges in female mice, induce greater inhibition of cellular proliferation in male mice, and induce a significant level of chromatid-type chromosomal aberrations in both sexes. During the second day after exposure to benzene there was increased inhibition of cellular proliferation in male mice and both new DNA damage and persistance of old DNA damage in female mice. The differences in both the type and magnitude of the response of bone marrow cellular populations, as determined by different cytogenetic end points in male and female DBA/2 mice exposed to benzene or to phenobarbital and benzene, suggest not only that a metabolite of benzene is responsible for the observed effects, but that different metabolites may be involved in different end points.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe S., Sasaki M. Chromosome aberrations and sister chromatid exchanges in Chinese hamster cells exposed to various chemicals. J Natl Cancer Inst. 1977 Jun;58(6):1635–1641. doi: 10.1093/jnci/58.6.1635. [DOI] [PubMed] [Google Scholar]
- Conney A. H., Welch R., Kuntzman R., Chang R., Jacobson M., Munro-Faure A. D., Peck A. W., Bye A., Poland A., Poppers P. J. Effects of environmental chemicals on the metabolism of drugs, carcinogens, and normal body constituents in man. Ann N Y Acad Sci. 1971 Jul 6;179:155–172. doi: 10.1111/j.1749-6632.1971.tb46897.x. [DOI] [PubMed] [Google Scholar]
- Dean B. J. Genetic toxicology of benzene, toluene, xylenes and phenols. Mutat Res. 1978;47(2):75–97. doi: 10.1016/0165-1110(78)90014-3. [DOI] [PubMed] [Google Scholar]
- Dewey W. C., Miller H. H., Leeper D. B. Chromosomal aberrations and mortality of x-irradiated mammalian cells: emphasis on repair. Proc Natl Acad Sci U S A. 1971 Mar;68(3):667–671. doi: 10.1073/pnas.68.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley T. J. Evaluation of the health effects of benzene inhalation. Clin Toxicol. 1977 Dec;11(5):531–548. doi: 10.3109/15563657708988218. [DOI] [PubMed] [Google Scholar]
- IKEDA M. ENZYMATIC STUDIES ON BENZENE INTOXICATION. J Biochem. 1964 Mar;55:231–243. doi: 10.1093/oxfordjournals.jbchem.a127876. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Bender M. A. Factors influencing the frequency of mitomycin C-induced sister-chromatid exchanges in 5-bromodeoxyuridine-substituted human lymphocytes in culture. Mutat Res. 1978 Sep;51(3):411–418. doi: 10.1016/0027-5107(78)90129-x. [DOI] [PubMed] [Google Scholar]
- Kato H. Induction of sister chromatid exchanges by chemical mutagens and its possible relevance to DNA repair. Exp Cell Res. 1974 Apr;85(2):239–247. doi: 10.1016/0014-4827(74)90123-2. [DOI] [PubMed] [Google Scholar]
- Kato H. Spontaneous and induced sister chromatid exchanges as revealed by the BUdR-labeling method. Int Rev Cytol. 1977;49:55–97. doi: 10.1016/s0074-7696(08)61947-6. [DOI] [PubMed] [Google Scholar]
- Lutz W. K., Schlatter C. Mechanism of the carcinogenic action of benzene: irreversible binding to rat liver DNA. Chem Biol Interact. 1977 Aug;18(2):241–245. doi: 10.1016/0009-2797(77)90010-2. [DOI] [PubMed] [Google Scholar]
- Nicoll J. W., Swann P. F., Pegg A. E. Effect of dimethylnitrosamine on persistence of methylated guanines in rat liver and kidney DNA. Nature. 1975 Mar 20;254(5497):261–262. doi: 10.1038/254261a0. [DOI] [PubMed] [Google Scholar]
- Perry P., Evans H. J. Cytological detection of mutagen-carcinogen exposure by sister chromatid exchange. Nature. 1975 Nov 13;258(5531):121–125. doi: 10.1038/258121a0. [DOI] [PubMed] [Google Scholar]
- Sammett D., Lee E. W., Kocsis J. J., Snyder R. Partial hepatectomy reduces both metabolism and toxicity of benzene. J Toxicol Environ Health. 1979 Sep;5(5):785–792. doi: 10.1080/15287397909529789. [DOI] [PubMed] [Google Scholar]
- Schneider E. L., Sternberg H., Tice R. R. In vivo analysis of cellular replication. Proc Natl Acad Sci U S A. 1977 May;74(5):2041–2044. doi: 10.1073/pnas.74.5.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E. L., Tice R. R., Kram D. Bromodeoxyuridine differential chromatid staining technique: a new approach to examining sister chromatid exchange and cell replication kinetics. Methods Cell Biol. 1978;20:379–409. doi: 10.1016/s0091-679x(08)62029-4. [DOI] [PubMed] [Google Scholar]
- Shafer D. A. Replication bypass model of sister chromatid exchanges and implications for Bloom's syndrome and Fanconi's anemia. Hum Genet. 1977 Nov 10;39(2):177–190. doi: 10.1007/BF00287010. [DOI] [PubMed] [Google Scholar]
- Snyder R., Kocsis J. J. Current concepts of chronic benzene toxicity. CRC Crit Rev Toxicol. 1975 Jun;3(3):265–288. doi: 10.3109/10408447509079860. [DOI] [PubMed] [Google Scholar]
- Snyder R., Lee E. W., Kocsis J. J. Binding of labeled benzene metabolites to mouse liver and bone marrow. Res Commun Chem Pathol Pharmacol. 1978 Apr;20(1):191–194. [PubMed] [Google Scholar]
- Solomon E., Bobrow M. Sister chromatid exchanges--a sensitive assay of agents damaging human chromosomes. Mutat Res. 1975 Nov;30(2):273–278. [PubMed] [Google Scholar]
- Tice R. R., Bender M. A., Ivett J. L., Drew R. T. Cytogenetic effects of inhaled ozone. Mutat Res. 1978 Nov;58(2-3):293–304. doi: 10.1016/0165-1218(78)90022-8. [DOI] [PubMed] [Google Scholar]
- Tice R., Chaillet J., Schneider E. L. Evidence derived from sister chromatid exchanges of restricted rejoining of chromatid subunits. Nature. 1975 Aug 21;256(5519):642–644. doi: 10.1038/256642a0. [DOI] [PubMed] [Google Scholar]
- Tice R., Schneider E. L., Rary J. M. The utilization of bromodeoxyuridine incorporation into DNA for the analysis of cellular kinetics. Exp Cell Res. 1976 Oct 15;102(2):232–236. doi: 10.1016/0014-4827(76)90037-9. [DOI] [PubMed] [Google Scholar]
- Tsutsui T., Umeda M., Maizumi H., Saito M. Comparison of mutagenicity and inducibility of DNA single-strand breaks and chromosome aberrations in cultured mouse cells by potent mutagens. Gan. 1977 Oct;68(5):609–617. [PubMed] [Google Scholar]
- Vigliani E. C., Forni A. Benzene and leukemia. Environ Res. 1976 Feb;11(1):122–127. doi: 10.1016/0013-9351(76)90115-8. [DOI] [PubMed] [Google Scholar]



