Abstract
Objective
The mechanisms that underlie the association between abdominal obesity and depression risk in older persons are not well known, but the “leptin hypothesis” of depression suggests that leptin resistance may be involved in mood regulation. We tested whether high circulatory concentration of leptin, alone and in combination with visceral adiposity, is associated with onset of depression in a sample of older persons.
Method
Participants were 1220 men and 1282 women aged 70–79 years, enrolled in the Health, Aging and Body Composition study. Plasma concentration of leptin and abdominal visceral fat ascertained by computed tomography were assessed at baseline (April 1997 – June 1998). Onset of depression was defined as a Center for Epidemiological Studies-Depression Scale 10-item score ≥ 10 and/or new antidepressant medication use at any annual visit over a 5-year follow-up.
Results
Higher leptin was associated with the risk of depression onset in men with high visceral fat (HR=1.25,95%CI=1.06–1.46, p=0.01) but not in those with normal visceral fat (HR=0.98,95%CI=0.80–1.19, p=0.80) (leptin*visceral fat p=0.04). No interaction between leptin and visceral fat was detected in the analysis focusing on women (p=0.90).
Conclusion
In older men, high leptin was associated with an increased onset of depressive symptoms especially in the presence of abdominal obesity, suggesting that underlying leptin resistance may play a role in this link. Differences in visceral fat levels and metabolic consequences may explain the absence of this association in women. These findings suggest a potential biological link between depression, obesity and their joint association with negative health outcomes.
Keywords: leptin, depression, obesity, aging
INTRODUCTION
Increasing evidence suggests a causal link between adiposity, particularly abdominal obesity, and depression (1–3). White adipose tissue produces leptin, initially identified as an anti-obesity hormone operating as a negative feedback to control energy homeostasis (4, 5). The “leptin hypothesis of depression” contends that leptin contributes to regulation of affective status (6). In animal models of depression, leptin has been shown to improve cognition and mood (7). However, clinical studies in humans have had conflicting results (6, 8) which may be partly explained by the complexity of leptin response in obese persons (9,10), who often have high, not low, levels of leptin. The lack of inhibition of food intake in obese persons is thought to be caused by a mechanism of physiological leptin resistance, similar to the one that links type 2 diabetes and insulin resistance, that blunts leptin central action despite increasing concentrations (9–11). Based on these observations, it has been hypothesized that is not the absolute serum leptin concentration but rather its impaired central action that is correlated with mood (6, 8).
Data from the Health, Aging and Body Composition study showed that visceral fat, independent of overall obesity, was a risk factor for depression onset in older men (3). We used longitudinal data from the same cohort of men and women aged 70–79 years to test whether serum leptin in older adults may represent a mechanism relating abdominal adiposity with increased risk of developing relevant depressive symptoms. Since the presence of hyperleptinemia in obese persons may be considered an indicator of leptin resistance (6), we hypothesized that risk of depression onset would be especially increased for participants with high levels of leptin and visceral fat.
METHODS AND MATERIALS
Study Population
Participants were part of the Health, Aging and Body Composition (Health ABC) study, a cohort study consisting of 3,075 initially well-functioning, 70–79-year old, black and white men and women. Participants were identified from a random sample of white Medicare beneficiaries and all age-eligible community-dwelling black residents in designated areas surrounding Memphis, Tennessee, and Pittsburgh, Pennsylvania. Participants were eligible if they reported no difficulty in walking one quarter of a mile, going up 10 steps without resting, or performing basic activities of daily living. Exclusion criteria were a history of active treatment for cancer in the prior 3 years, plans to move out of the study area in the next 3 years, or participation in a randomized trial of a lifestyle intervention. Baseline data (April 1997 – June 1998) included an in-person interview and clinic-based examination, with evaluation of body composition, diseases, and physical functioning.
For the present analyses, we initially retained 2,802 participants free of depression at baseline, as indicated by a Center for Epidemiological Studies-Depression scale (CES-D) score below the clinical cut-off and no reported use of antidepressants. We then excluded 143 participants because of missing data on baseline leptin and visceral fat, and 157 subjects without follow-up data on depressive symptoms. Those lost to follow-up, as compared to those available, were more often male and black, had poorer cognitive function, were more likely to have diabetes and cardiovascular disorders and had higher depressive symptoms. This left 2,502 participants as the primary sample. Participants were assessed annually over a median follow-up of 4.9 years (range: 0.9–5.6); 8.4% of the participants died during follow-up period.
Leptin
Measures for leptin were obtained from serum samples collected at baseline. Fasting blood samples were obtained in the morning, and after processing, the specimens were aliquoted into cryovials, frozen at −70 °C and shipped to the Health ABC Core Laboratory. Leptin concentrations were measured in duplicate using the Sensitive Human Leptin RIA Kit from Linco Research, Inc. (St. Charles, MO). The minimum concentration detectable was 0.05 ng/mL. Intraassay coefficient of variation (CVs) was 3.7%–7.5%, and interassay CVs was 3.2%–8.9%. For 155 participants, leptin concentrations were outside of the linear range of the assay and were not on the linear portion of the standard curve. These numerical values were not recorded by the laboratory and were recoded as 52 ng/mL (upper limit of the curve specified by Linco Research Inc.). In addition to continuous measures (per SD increase), sex-specific quartiles of leptin levels were constructed and dichotomous variables compared persons in the highest versus persons in quartiles 1–3. High leptin levels were respectively >10.1ng/mL for men and >28.9ng/mL for women.
Depressive symptoms
Depressive symptoms were evaluated at baseline using the Center for Epidemiological Studies-Depression scale (CES-D) (12) and at selective follow-up visits (after 2, 3, 4, and 5 years) using the CES-D10 (13), derived from a 10-item subset of the standard CES-D. The properties of the CES-D10 show satisfactory test-retest correlations and good predictive accuracy compared with the standard CES-D (13). Baseline prevalence of significant depressive symptoms was defined by the established cut-point of ≥16 on the CES-D, which is equivalent to a cut-point of ≥ 10 on the CES-D10 (13). In addition, at baseline and at follow-up (after 1, 2, 4, and 5) antidepressant use (with depression/mood as self-reported reason) was coded according to the Iowa Drug Information System (IDIS)(14). Consistent with previous papers from the Health ABC study (3, 15), depression onset was operationally defined by relevant depressive symptoms (CES-D10 score ≥10) or antidepressant use at follow-up. Following the procedures used by Vogelzangs et al. (3), two alternative definitions of depression onset were also tested. One used CES-D10 scores only and the second had the additional requirement of a minimum increase of 3 points on the CES-D10.
Abdominal visceral fat
Computed tomography (CT) scanning was performed at the fourth and fifth lumbar vertebrae to measure visceral fat (cm2) using a Somatom Plus 4 (Siemens, Erlangen, Germany) or a Picker PQ 2000S (Marconi Medical Systems, Cleveland, Ohio, US) scanner in Memphis and a 9800 Advantage scanner (General Electric, Milwaukee, Wisconsin, US) in Pittsburgh. Scans were conducted at 120 kilovolt (peak) and 200–250 mA/s with a slice thickness of 10 mm. Areas were calculated by multiplying the number of pixels of a given tissue type by the pixel area using Interactive Data Language software (Research Systems Inc, Boulder, Colorado, US). Abdominal visceral fat was manually distinguished from subcutaneous fat by tracing along the fascial plane defining the internal abdominal wall. For consistency with the previous paper by Vogelzangs et al. (3), visceral fat was considered both as a continuous measure and as a categorical variable defined by the highest sex-specific quartile (men >195.6 cm2, women >165.9 cm2).
Covariates
Covariates were a priori selected on the basis of previously reported associations with leptin and depression. The following were assessed at baseline: age, sex, race, education level and smoking status (non-smoker/former/current smoker). Alcohol consumption was assessed using a standardized questionnaire (16) and categorized as former drinker, never or <1 drink/week, 1–7 and >7 drinks/week. Physical activity during the last week was expressed as kcal/week. According to time/intensity spent on physical activities, metabolic equivalent values were assigned (17), summed and multiplied by body weight. Cognitive function was measured by the Modified Mini Mental State Examination (3MS) (18) with a maximum score of 100. Total number of chronic conditions (peripheral arterial disease, cancer, lung disease, osteoarthritis, osteoporosis, gastrointestinal disease, prostate disease, thyroid disease, Parkinson’s disease, and kidney disease) was calculated. Since leptin is associated with cardiovascular diseases (CVD: heart failure, stroke, myocardial infarction, angina pectoris, coronary angioplasty or coronary artery bypass grafting) and diabetes (19–21), these conditions were specifically addressed. Presence of diabetes and CVD was adjudicated using standardized algorithms, considering various sources of information including self-report, medications, clinical findings, and medical claims data from the former Health Care Financing Administration. Body mass index (BMI) was calculated as kg/m2 and categorized as normal (BMI < 25), overweight (25–29.99) and obese (BMI ≥ 30). Percent body fat was determined using fan-beam DXA (Hologic QDR 4500 A, Bedford, MA). Levels of IL-6 and CRP were measured in duplicate from stored serum by enzyme-linked immunosorbent assay kits (IL-6: R&D Systems, Minneapolis, MN; CRP: Calbiochem, San Diego, CA). Interassay CV were 10.3% for IL-6 and 8.0% for CRP.
Statistical Analyses
Variables were reported as percentages, means ± standard deviation (SD) or as medians and interquartile range (IQR) as appropriate. Because of known differences between men and women in body composition, leptin levels and prevalence of depression in men and women, and because sex differences in the relationship between obesity and depression have been observed in the present sample (3), all results are shown for men and women separately. Differences in baseline characteristics were tested according to leptin categories using descriptive statistics. Fat levels across sexes were compared using analysis of covariance. Trajectories of depressive symptoms over time were estimated using random coefficient analyses with random intercept. This method handles missing values, different spacing of measurement observations, and correlation between multiple observations per subject. Multivariate Cox proportional hazards model were used to compare risk of depression onset over the follow-up period associated with leptin. Participants who survived without developing depressed mood were censored at the date of the last follow-up. The proportional hazards assumption was checked by including a time-to-event by leptin interaction term and was met in all analyses. To test whether visceral fat and leptin had an interacting association with depression onset we entered leptin-by-visceral fat interaction terms in the regression models including the visceral fat term. Leptin-by-race interaction terms were tested but were not statistically significant. All analyses were performed using SAS (v. 9.1, SAS Institute, Inc., Cary, NC). Significance level was set at P<0.05.
RESULTS
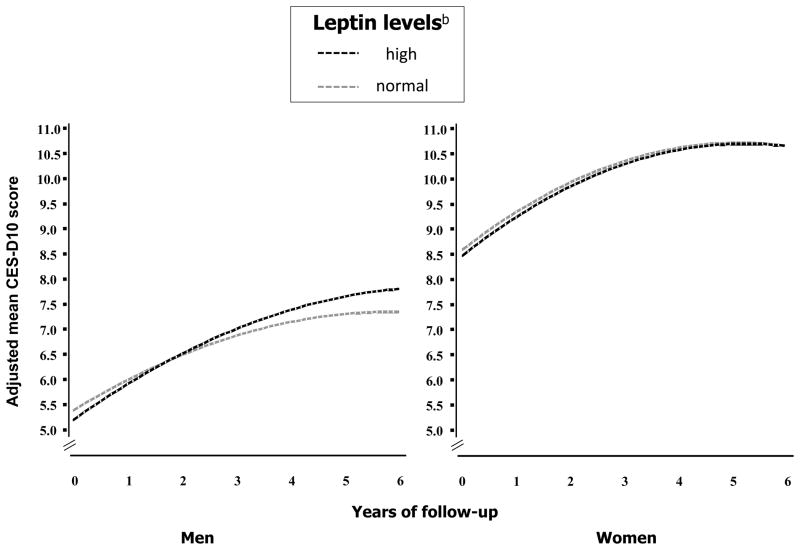
Participants’ mean age at baseline was 73.6 (±2.9) years, 51.1% were women and 40.5% were black. The median levels of leptin were 6.1 (6.6) ng/mL for men and 18.5 (19.2) ng/mL for women. Characteristics of participants according to sex and leptin groups are reported in Table 1. Overall, participants with high leptin were more likely to be smokers and obese, and to have diabetes and CVD and had a higher number of chronic diseases and higher level total percent body fat, visceral fat and inflammatory markers. In age- and race-adjusted analyses, men, as compared to women, were less likely to be obese (21.3% vs 29.1; p=0.001) and had lower mean (±SE) total percent body fat (29.3 ±0.2 vs 40.6±0.2 %; p<0.0001); in contrast, men had higher abdominal visceral fat in the whole sample (154.2±1.9 vs 133.1±1.8 cm2; p<.0001) and in the subgroup of participants with high leptin (252.1±2.8 vs 213.7±3.0cm2; p<.0001). Figure 1 shows mean CES-D10 scores over the follow-up period adjusted for age, race, education level, alcohol consumption, smoking status, physical activity, 3MS score, number of chronic diseases, CVD, diabetes, (log)IL-6, (log)CRP and visceral fat. In both men and women depressive symptoms were not different across leptin levels at baseline (leptin: p>0.40) and significantly increased over time (time: p<.0001). Men with high leptin had an increase in CES-D10 score of 0.11 points/year higher than those with normal levels (leptin*time: β=0.11, SE=0.05, p=0.02). The inclusion of BMI or total percent body fat in the models, which already included visceral fat, did not change the results and these variables were not significantly associated with change in CES-D10 scores; therefore these adjustments were not retained in the analyses.
Figure 1.
Trajectories of CES-D10 scores during follow-up according to baseline leptin.
a Estimated trajectories are adjusted for age, race, education level, alcohol consumption, smoking status, physical activity, 3MS score, number of chronic diseases, CVD, diabetes, visceral fat, (log)IL-6 and (log)CRP.
Men: main effect, high leptin: p=0.40; time: p<0.0001; time2: p<0.0001; interaction term, high leptin-by-time: p=0.02.
Women: main effect, high leptin: p=0.58; time: p<0.0001; time2: p<0.0001; interaction term, high leptin-by-time: p=0.68.
b High leptin: >10.1 ng/mL in men and >28.9 ng/mL in women.
Abbreviations: CES-D10, Center for Epidemiological Studies-Depression Scale 10-item.
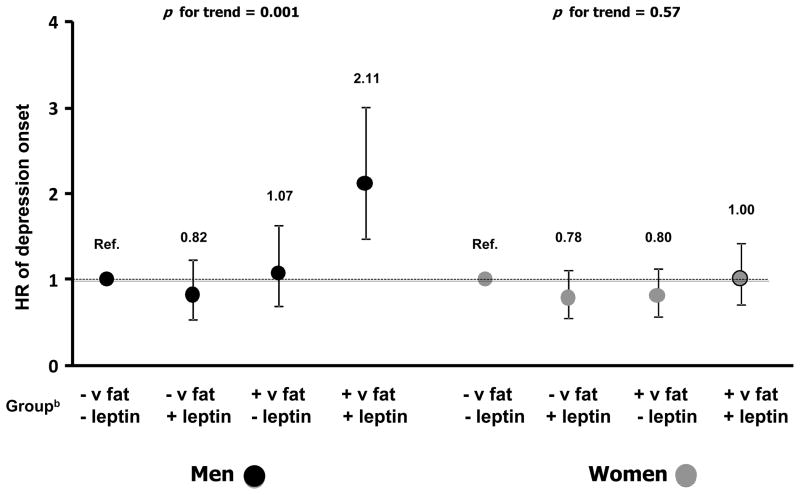
During follow-up, depression onset emerged, respectively, in 20.1% of men and 27.5% of women. Table 2 shows the risk of depression onset according to baseline leptin and in subgroups stratified by visceral fat. After full adjustment plus baseline CESD-10 scores, risk of developing depression in men was significantly associated with higher leptin (HR=1.17,95%CI=1.05–1.31, p=0.01). The strength of this association was substantially reduced after additional adjustment for visceral fat (HR=1.11,95%CI=0.97–1.26, p=0.12). In women, no significant associations were detected (HR=0.94,95%CI=0.83–1.06, p=0.32). In men, a significant leptin-by-visceral fat association was found (p=0.04). Higher leptin was significantly associated with the risk of depression onset in men with high visceral fat (HR=1.25,95%CI=1.06–1.46, p=0.01) but not in those with normal visceral fat (HR=0.98,95%CI=0.80–1.19, p=0.80). No leptin-by-visceral fat interaction was detected in the analysis focusing on women (p=0.90). Analyses distinguishing participants with high versus normal leptin obtained the same results. To further illustrate the interaction between leptin and visceral fat, Figure 2 shows that the fully adjusted HR of developing depression was higher among men with both high visceral fat and leptin as compared to all other groups.
Figure 2.
Risk of depression onset across baseline levels of leptin by visceral fat.
a HRs and 95%CIs are adjusted for age, race, education level, alcohol consumption, smoking status, physical activity, 3MS score, number of chronic diseases, CVD, diabetes, visceral fat, (log)IL-6 and (log)CRP.
b Group: + v fat = high visceral fat (>195.5 cm2 in men and >165.9 cm2 in women); − v fat = normal visceral fat; + leptin = high leptin (>10.1 ng/mL in men and >28.9 ng/mL in women); − leptin = normal leptin.
Similar results were found when alternatives operational definition of depression onset were used: in men with high visceral fat, higher leptin levels were associated with depression onset defined only by CES-D10 (70/300 cases; HR=1.24,95%CI=1.05–1.46, p=0.01) or when a requirement of a minimum 3 points increase on the CES-D10 was incorporated (72/300 cases; HR=1.24, 95%CI=1.07–1.46, p=0.01). Considering the whole sample, a significant leptin-by-sex interaction (p=0.002) was observed, confirming the differential association between leptin and depression by sex.
Finally, similar results were obtained in sensitivity analyses performed after the exclusion of 231 men and 158 women who developed incident diabetes or CVD before depression onset (data not shown).
DISCUSSION
In community-resident older men, but not women, high serum levels of leptin and abdominal obesity had an interactive effect on the onset of depressive symptoms over a 5-year period. In general, men with high leptin, as compared to those with normal levels, had a steeper increase, although of small relevance, of depressive symptoms over time. However, the impact of high serum levels on depression onset was especially evident in men with abdominal obesity. The latter may suggest that in older men leptin resistance may contribute to alterations of affective status as proposed by the “leptin hypothesis of depression” (6).
The sex specificity of the present findings deserves further comment. Consistently with the established literature, we found that men, as compared to women, had lower levels of leptin and lower percentages of depression onset. Based on the above we may expect a stronger association of leptin with depression in women than men. However, the present findings highlighted the interactive effect of leptin and visceral fat in predicting depression onset. If the presence of the specific combination of both high leptin and visceral fat is important for depression to emerge, this combination may be a less important contributing factor for depression in women due to differences in visceral fat levels and related metabolic consequences. Indeed, it has been consistently shown also in the present study that women, as compared to men, have higher measures of body fat but less visceral adiposity (22,23). Visceral adiposity is associated with metabolic disturbances and increased inflammatory response that, in turn, have been linked to depression and development of leptin resistance (22–28). Finally, in women depression may have a more complex etiology and other biological (e.g. estrogen) and/or psychosocial (e.g. social support, stressful life events) (29–31) factors may have a stronger role than leptin. Nevertheless, the reasons for this sex-specific interaction remain unknown, and further research comparing men and women is needed.
Leptin is synthesized in white adipose tissue and exerts its homeostatic function by interacting with hypothalamic arcuate nucleus (32). Recently, several peripheral and extra-hypothalamic effects of leptin have been described (7). Leptin receptors are expressed in limbic substrates related to mood regulation such as the hippocampus and amygdala (32). In animals, leptin has been shown to affect hippocampal and cortical structure (33), to exert antidepressant effects and to improve learning and memory in behavioral and cellular assays (34–36). Moreover, accumulating evidence shows that leptin modulates hypothalamus-pituitary-adrenal (HPA) axis, which has been implicated in depression and obesity (37). However, the few clinical studies in depressed patients showed conflicting findings, with studies showing increased, decreased or no differences in leptin (38–45). The phenomenon of leptin resistance may explain similar conflicting results. Obese individuals commonly display hyperleptinemia associated with central leptin resistance due to impaired transport across the blood–brain barrier, reduced function of the leptin receptor and defects in leptin signal transduction (9, 10), which ultimately weaken leptin’s central effect despite increasing circulating levels. A recent study (11) showed that leptin effect were impaired in the hippocampus of diet-induced obese mice, resulting in a severe depressive state despite hyperleptinemia.
When proposing the “leptin hypothesis”, Lu (6) underscored the need for future studies to clarify the role of leptin insufficiency versus leptin resistance in depression. We believe our findings provide support for the second mechanism proposed. Thus, the key to understanding the pathophysiology of leptin may lie in its function and its impaired central action and not merely in its circulating level. Therefore, in obese depressed patients therapeutic interventions on leptin downstream pathways should target leptin central resistance rather than leptin itself (6–8,46).
Limitations of the present study should be considered. First, depression was not confirmed by a clinical diagnosis and data on psychiatric comorbidity were not available. The study design did not allow detection of depressive episodes that started and remitted between subsequent follow-up visits. Another limitation is the loss of participants to follow-up, who were slightly less healthy than the participants who remained in this study limiting the generalizability of the findings. Moreover, the detected associations could have been driven by variables related to adipokine and depression, such as diabetes and CVD (19–21). However, in this sample accounting for baseline diabetes and CVD, and excluding participants who developed these disorders during follow-up did not change the association. Moreover, adjustment for confounders decreased the possibility that factors such as physical inactivity, alcohol consumption, smoking and inflammation may have driven the observed associations. Another limitation is that the sample was not adequately sized to test for the interactive effect of leptin and visceral fat on persistent depression. Finally, the observational nature of the present study restrict the ability of drawing definite causal inferences. Further studies in larger samples well characterized in terms of psychiatric diagnoses are needed to sustain the hypothesis of a causal pathway. In conclusion, we believe our findings suggest that in older men leptin may represent a mechanism relating abdominal adiposity with depression onset. These results expands the body of evidence on the involvement of new biological factors in the pathophysiology of depression. Moreover, by demonstrating the reciprocal interaction between leptin and visceral fat, the present findings suggest a potential common shared biological link between depression, obesity and their association with negative outcomes.
Table 1.
Characteristics of the study population.
| Characteristicsb | Men
|
pc | Women
|
pc | ||
|---|---|---|---|---|---|---|
| Leptina | Leptina | |||||
| Normal (n=920) | High (n=300) | Normal (n=947) | High (n=335) | |||
|
|
|
|||||
|
|
|
|||||
| Age (years) | 73.7±2.9 | 73.7±2.8 | 0.96 | 73.6±2.9 | 73.3±2.7 | 0.19 |
| Race (Black) | 40.9 | 19.7 | <.0001 | 45.0 | 46.0 | 0.76 |
| Education | 0.16 | 0.001 | ||||
| less than high school | 26.6 | 22.0 | 19.9 | 28.6 | ||
| high school | 24.5 | 29.0 | 39.6 | 39.8 | ||
| postsecondary | 48.9 | 49.0 | 40.6 | 31.6 | ||
| Alcohol intake | 0.52 | 0.63 | ||||
| never or < 1 drink/wk | 62.2 | 58.7 | 79.1 | 81.5 | ||
| 1–7 drink/wk | 25.8 | 28.9 | 17.5 | 15.5 | ||
| > 7 drink/wk | 12.0 | 12.4 | 3.5 | 3.0 | ||
| Smoking status | 0.002 | 0.04 | ||||
| non smoker | 31.3 | 29.0 | 59.4 | 53.4 | ||
| former smoker | 11.4 | 5.0 | 9.0 | 7.5 | ||
| current smoker | 57.3 | 66.0 | 31.7 | 39.1 | ||
| Physical activity (Kcal/week) | 87.0±71.4 | 79.6±68.8 | 0.12 | 85.7±71.8 | 80.1±66.9 | 0.19 |
| 3MSE | 89.5±8.9 | 90.2±7.1 | 0.002 | 91.4±7.0 | 90.6±8.7 | 0.002 |
| No. of chronic diseases | 1.4±1.0 | 1.5±1.1 | 0.09 | 0.9±0.9 | 1.1±1.1 | 0.007 |
| Diabetes | 22.8 | 37.3 | <.0001 | 14.8 | 34.0 | <.0001 |
| CVD | 20.8 | 28.7 | 0.005 | 11.5 | 16.7 | 0.01 |
| IL-6 (pg/mL) | 1.8(1.4) | 2.1(1.7) | <.0001 | 1.5(1.4) | 2.2(1.6) | <.0001 |
| CRP (ug/mL) | 1.4(1.6) | 1.8(1.9) | 0.01 | 1.6(2.1) | 2.9(3.3) | <.0001 |
| BMI | <.0001 | <.0001 | ||||
| normal | 38.0 | 4.7 | 43.2 | 3.0 | ||
| overweight | 49.5 | 47.0 | 37.1 | 41.5 | ||
| obesity | 12.5 | 48.3 | 19.8 | 55.5 | ||
| Percent Body Fat (%) | 28.1±4.5 | 33.0±4.1 | <.0001 | 39.5±5.7 | 43.9±4.3 | <.0001 |
| Abdominal Visceral fat (cm2) | 123.1±39.9 | 253.5±52.3 | <.0001 | 103.8±35.1 | 212.47±41.9 | <.0001 |
High leptin: >10.1 ng/mL in men and >28.9 ng/mL in women.
Categorical and continuous variables were reported as percentage or means ±standard deviation as appropriate. Variables with a skewed distribution were presented as median (interquartile range) and were log-transformed for the analyses.
Based on chi-square for categorical variables and indipendent t-test for continuous variables.
Abbreviations: 3MS, Modified Mini 3Mental State Examination; CVD, Cardiovascular Disorders; IL-6, Interleukin 6; CRP, C-Reactive Protein; BMI, Body Mass Index.
Table 2.
Risk of depression onset according to baseline levels of leptin.
| Risk of Depression Onset
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men
|
Women
|
|||||||||||
| LEPTIN
|
LEPTIN
|
|||||||||||
| per SD increasea
|
high (Q4) vs normalb
|
per SD increasea
|
high (Q4) vs normalb
|
|||||||||
| H.R. | 95% C.I. | P | H.R. | 95% C.I. | P | H.R. | 95% C.I. | P | H.R. | 95% C.I. | P | |
|
|
|
|
|
|||||||||
| Total sample | n = 245/1220 | n = 352/1282 | ||||||||||
| Adjustment | ||||||||||||
| sociodemographicc | 1.17 | (1.05 – 1.31) | 0.01 | 1.32 | (1.00 – 1.74) | 0.047 | 0.99 | (0.89 – 1.11) | 0.90 | 1.01 | (0.79 – 1.29) | 0.94 |
| + lifestyle and healthd | 1.17 | (1.05 – 1.31) | 0.01 | 1.29 | (0.97 – 1.71) | 0.08 | 0.94 | (0.83 – 1.06) | 0.32 | 0.94 | (0.73 – 1.21) | 0.60 |
| + visceral fat | 1.11 | (0.97 – 1.26) | 0.12 | 1.10 | (0.81 – 1.49 | 0.56 | 0.95 | (0.84 – 1.08) | 0.41 | 0.95 | (0.73 – 1.24) | 0.72 |
| leptin*visceral fat | 0.04 | 0.046 | 0.90 | 0.30 | ||||||||
| normal visceral fat | n = 170/920 | n = 254/947 | ||||||||||
| Adjustment | ||||||||||||
| sociodemographicc | 0.96 | (0.79 – 1.17) | 0.71 | 0.87 | (0.58 – 1.30) | 0.50 | 0.94 | (0.81 – 1.09) | 0.42 | 0.86 | (0.62 – 1.19) | 0.36 |
| + lifestyle and healthd | 0.98 | (0.80 – 1.19) | 0.80 | 0.84 | (0.56 – 1.27) | 0.41 | 0.91 | (0.78 – 1.07) | 0.25 | 0.81 | (0.58 – 1.15) | 0.25 |
| high visceral fat e | n = 75/300 | n = 98/335 | ||||||||||
| Adjustment | ||||||||||||
| sociodemographicc | 1.25 | (1.08 – 1.45) | 0.003 | 1.87 | (1.14 – 3.06) | 0.01 | 0.99 | (0.89 – 1.11) | 0.90 | 0.94 | (0.83 – 1.06) | 0.32 |
| + lifestyle and healthd | 1.25 | (1.06 – 1.46) | 0.01 | 1.93 | (1.14 – 3.28) | 0.02 | 1.01 | (0.79 – 1.29) | 0.94 | 0.94 | (0.73 – 1.21) | 0.60 |
Leptin. Per SD increase: 7.0 ng/mL in men and 15.1 ng/mL in women;
Leptin. High levels: >10.1 ng/mL in men and >28.9 ng/mL in women.
Adjusted for age, race, education and baseline CES-D.
Additionally adjusted for alcohol intake, smoking status, physical activity, 3MSE, number of chronic diseases, diabetes, CVD, log(IL-6) and log(CRP).
Visceral fat. High levels: >195.5 cm2 in men and >165.9 cm2 in women.
Clinical points.
Obesity, in particular abdominal adiposity, is a risk factor for depression.
Leptin impaired central action (resistance) may represent a biological mechanism relating abdominal adiposity with depression onset in obese persons.
In obese depressed patients the development of therapeutic interventions on leptin downstream pathways should target leptin central resistance rather than leptin itself.
Acknowledgments
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was supported (in part) by the Intramural Research Program of the NIH, National Institute on Aging.
Role of the Sponsor: None of the sponsoring institutions interfered with the collection, analysis, presentation, or interpretation of the data reported herein.
Footnotes
Financial Disclosure: None reported.
Potential Conflicts of Interest: The authors do not have any conflict of interest in the publication of the manuscript.
References
- 1.Zhao G, ESF, Li C, et al. Waist Circumference, Abdominal Obesity, and Depression among Overweight and Obese U.S. Adults: National Health and Nutrition Examination Survey 2005–2006. BMC. 2011;11:130. doi: 10.1186/1471-244X-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 3.Vogelzangs N, Kritchevsky SB, Beekman AT, et al. Obesity and onset of significant depressive symptoms: results from a prospective community-based cohort study of older men and women. J Clin Psychiatry. 2009;71:391–399. doi: 10.4088/JCP.08m04743blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 5.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 6.Lu XY. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol. 2007;7:648–652. doi: 10.1016/j.coph.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paz-Filho G, Wong ML, Licinio J. The procognitive effects of leptin in the brain and their clinical implications. Int J Clin Pract. 2010;64:1808–1812. doi: 10.1111/j.1742-1241.2010.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zupancic ML, Mahan A. Leptin as a neuroactive agent. Psychosom Med. 2011;73:407–414. doi: 10.1097/PSY.0b013e31821a196f. [DOI] [PubMed] [Google Scholar]
- 9.Munzberg H, Myers MG., Jr Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 10.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 11.Yamada N, Katsuura G, et al. Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology. 2011;152:2634–43. doi: 10.1210/en.2011-0004. [DOI] [PubMed] [Google Scholar]
- 12.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- 13.Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 14.Pahor M, Chrischilles EA, Guralnik JM, et al. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 15.Maraldi C, Volpato S, Penninx BW, et al. Diabetes mellitus, glycemic control, and incident depressive symptoms among 70- to 79-year-old persons: the health, aging, and body composition study. Arch Intern Med. 2007;167:1137–1144. doi: 10.1001/archinte.167.11.1137. [DOI] [PubMed] [Google Scholar]
- 16.Volpato S, Pahor M, Ferrucci L, et al. Relationship of alcohol intake with inflammatory markers and plasminogen activator inhibitor-1 in well-functioning older adults: the Health, Aging, and Body Composition study. Circulation. 2004;109:607–612. doi: 10.1161/01.CIR.0000109503.13955.00. [DOI] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 19.Romero-Corral A, et al. Relationships between leptin and C-reactive protein with cardiovascular disease in the adult general population. Nat Clin Pract Cardiovasc Med. 2008;5(7):418–25. doi: 10.1038/ncpcardio1218. [DOI] [PubMed] [Google Scholar]
- 20.Wolk R, et al. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol. 2004;44(9):1819–24. doi: 10.1016/j.jacc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz MW, Porte D., Jr Diabetes obesity and the brain. Science. 2005 Jan 21;307(5708):375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 22.Beasley LE, Koster A, et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity. 2009;17:1062–1069. doi: 10.1038/oby.2008.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludescher B, Najib A, et al. Gender specific correlations of adrenal gland size and body fat distribution: a whole body MRI study. Horm Metab Res. 2007;39:515–518. doi: 10.1055/s-2007-982518. [DOI] [PubMed] [Google Scholar]
- 24.Koster A, Stenholm S, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity. 2010;18:2354–2361. doi: 10.1038/oby.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shelton RC, Miller AH. Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Prog Neurobiol. 2010;91:275–299. doi: 10.1016/j.pneurobio.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen K, Li F, et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med. 2006;12:425–432. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- 27.Feve B, Bastard JP. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:305–311. doi: 10.1038/nrendo.2009.62. [DOI] [PubMed] [Google Scholar]
- 28.Valle M, Gascón F, Martos R, Bermudo F, Ceballos P, Suanes A. Relationship between high plasma leptin concentrations and metabolic syndrome in obese pre-pubertal children. Int J Obes Relat Metab Disord. 2003 Jan;27(1):13–8. doi: 10.1038/sj.ijo.0802154. [DOI] [PubMed] [Google Scholar]
- 29.Cushman M, Legault C, et al. Effect of postmenopausal hormones on inflammation-sensitive proteins: the Postmenopausal Estrogen/Progestin Interventions (PEPI) Study. Circulation. 1999;100:717–722. doi: 10.1161/01.cir.100.7.717. [DOI] [PubMed] [Google Scholar]
- 30.Kendler KS, Myers J, et al. Sex differences in the relationship between social support and risk for major depression: a longitudinal study of opposite-sex twin pairs. Am J Psychiatry. 2005;162:250–256. doi: 10.1176/appi.ajp.162.2.250. [DOI] [PubMed] [Google Scholar]
- 31.Maciejewski PK, Prigerson HG, et al. Sex differences in event-related risk for major depression. Psychol Med. 2001;31:593–604. doi: 10.1017/s0033291701003877. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010 Nov;167(11):1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouret SG. Neurodevelopmental actions of leptin. Brain Res. 2010 Sep 2;1350:2–9. doi: 10.1016/j.brainres.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu XY, Kim CS, et al. Leptin: a potential novel antidepressant. Proc Natl Acad Sci U S A. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garza JC, Guo M, et al. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008;283:18238–18247. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oomura Y, Hori N, et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 27:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Bornstein SR, Schuppenies A, et al. Approaching the shared biology of obesity and depression: the stress axis as the locus of gene-environment interactions. Mol Psychiatry. 2006;11:892–902. doi: 10.1038/sj.mp.4001873. [DOI] [PubMed] [Google Scholar]
- 38.Jow GM, Yang TT, et al. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord. 2006;90:21–27. doi: 10.1016/j.jad.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Kraus T, Haack M, et al. Low leptin levels but normal body mass indices in patients with depression or schizophrenia. Neuroendocrinology. 2001;73:243–247. doi: 10.1159/000054641. [DOI] [PubMed] [Google Scholar]
- 40.Antonijevic IA, Murck H, et al. Elevated nocturnal profiles of serum leptin in patients with depression. J Psychiatr Res. 1998;32:403–410. doi: 10.1016/s0022-3956(98)00032-6. [DOI] [PubMed] [Google Scholar]
- 41.Zeman M, Jirak R, et al. Leptin, adiponectin, leptin to adiponectin ratio and insulin resistance in depressive women. Neuro Endocrinol Lett. 2009;30:387–395. [PubMed] [Google Scholar]
- 42.Rubin RT, Rhodes ME, et al. Sexual diergism of baseline plasma leptin and leptin suppression by arginine vasopressin in major depressives and matched controls. Psychiatry Res. 2002;113:255–268. doi: 10.1016/s0165-1781(02)00263-9. [DOI] [PubMed] [Google Scholar]
- 43.Deuschle M, Blum WF, Englaro, et al. Plasma leptin in depressed patients and healthy controls. Horm Metab Res. 1996;28:714–717. doi: 10.1055/s-2007-979885. [DOI] [PubMed] [Google Scholar]
- 44.Atmaca M, Kuloglu M, et al. Serum leptin and cholesterol values in suicide attempters. Neuropsychobiology. 2002;45:124–127. doi: 10.1159/000054950. [DOI] [PubMed] [Google Scholar]
- 45.Lawson EA, Miller KK, et al. Leptin Levels Are Associated With Decreased Depressive Symptoms in Women Across the Weight Spectrum, Independent of Body Fat. [published online ahead of print Jul 22, 2011] Clin Endocrinol. doi: 10.1111/j.1365-2265.2011.04182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banks WA. Extrahypothalamic effects of leptin: a therapeutic for depression and dementia? Endocrinology. 2011;152:2539–41. doi: 10.1210/en.2011-1161. [DOI] [PubMed] [Google Scholar]