Abstract
AIM:
To evaluate the effectiveness of montelukast as add-on therapy for asthmatic patients who remain uncontrolled with low, moderate or high doses of inhaled corticosteroid monotherapy.
DESIGN:
An eight-week, multicentre, open-label, observational study.
RESULTS:
Of 320 patients enrolled, 288 (90.0%) completed the study. Of patients who had uncontrolled asthma symptoms (Canadian Asthma Consensus Guidelines Update, 2003) but were controlled according to the Asthma Control Questionnaire (ACQ score of less than 1.5), 93.9% maintained asthma control at week 8. Of patients with uncontrolled asthma at baseline for both definitions, 63.5% achieved asthma control by week 8. The mean ± SD ACQ score decreased from 1.13±0.28 to 0.57±0.50 (P<0.001) for controlled patients at baseline and from 2.38±0.73 to 1.03±0.80 (P<0.001) for patients who were uncontrolled at baseline, each representing a clinically significant improvement.
CONCLUSION:
Montelukast add-on therapy is an effective alternative to inhaled corticosteroid monotherapy.
Keywords: Asthma, Inhaled corticosteroids, Montelukast add-on
Abstract
OBJECTIF :
Évaluer l’efficacité du montélukast comme traitement d’appoint chez les patients asthmatiques dont la maladie ne peut être maîtrisée au moyen d’une monothérapie par un corticostéroïde en inhalation administré à faible dose, à dose modérée ou à dose élevée.
PROTOCOLE D’ÉTUDE :
Étude d’observation multicentrique de 8 semaines menée au su.
RÉSULTATS :
Sur les 320 patients ayant pris part à l’étude, 288 (90,0 %) l’ont terminée. Parmi les patients dont les symptômes de l’asthme n’étaient pas maîtrisés (d’après la mise à jour des lignes directrices de la Conférence canadienne de consensus sur l’asthme, 2003), mais qui l’étaient selon le Questionnaire sur la maîtrise de l’asthme (indice QMA inférieur à 1,5), 93,9 % présentaient une maîtrise de l’asthme à la 8e semaine. Sur l’ensemble des patients dont l’asthme n’était pas maîtrisé au début de l’étude d’après les 2 définitions, 63,5 % ont présenté une maîtrise de la maladie à la 8e semaine. L’indice QMA moyen ± écart type (É.T.) est passé de 1,13 ± 0,28 à 0,57 ± 0,50 (p < 0,001) chez les patients dont l’asthme était maîtrisé au départ et de 2,38 ± 0,73 à 1,03 ± 0,80 (p < 0,001) chez les patients dont l’asthme n’était pas maîtrisé au départ; ces améliorations étaient toutes deux significatives sur le plan statistique.
CONCLUSION :
Le traitement d’appoint par le montélukast est une option de rechange efficace à la monothérapie par un corticostéroïde en inhalation.
Current asthma treatment guidelines recognize the importance of the early introduction of anti-inflammatory therapy for mild asthma. This approach has evolved partly out of recognition that airway inflammation is present even in mild cases of asthma (1). In general, asthma guidelines (2) suggest that inhaled corticosteroids (ICS) are the optimal initial therapy. This is supported by a recent trial (3) showing that the early use of low-dose ICS is associated with better control of symptoms and, most importantly, with a significant 44% reduction in severe exacerbations of asthma. ICS are considered to be the first-line treatment in mild, uncontrolled asthma, while leukotriene receptor antagonists may provide an alternative treatment for asthma patients who are not controlled or not satisfied with ICS therapy (4–6).
Asthma remains poorly controlled even though a large number of patients are prescribed ICS (7). Many patients do not adequately understand the role of their medications and how to use them (8). This may partly explain the poor compliance with maintenance therapy, such as ICS therapy (9). The presence of poor asthma control may not be exclusively due to ineffectiveness of medication. Suboptimal use of the medication, failure to address aggravating factors, poor inhaler technique, poor environmental control or a lack of continuity of care all contribute to poor asthma control. Thus, treatment guidelines suggest that before modifying therapy, an assessment of adherence, control of environment and diagnosis should be completed.
Montelukast is an orally administered, once-daily leukotriene receptor antagonist that can be prescribed as monotherapy or in combination with other asthma medications, including ICS, for the treatment of asthma. Recent studies have shown that for patients whose asthma is not controlled with ICS therapy, adding a second drug rather than increasing the dose of ICS results in improved control of symptoms (10,11). The primary purpose of the Singulair Add-on Study (SAS) was to assess the effectiveness of montelukast in combination with low, moderate or high doses of ICS in patients with asthma who were not controlled, not satisfied or nonadherent with their current controller therapy. Secondary objectives of the study were to compare patient and physician satisfaction with ICS treatment versus montelukast add-on therapy, and to further assess the safety and tolerability of montelukast add-on therapy in asthmatic patients.
METHODS
Study design
The study was an eight-week, multicentre, open-label, prospective, single-cohort study. Adult patients with uncontrolled asthma while on ICS therapy were treated with montelukast add-on therapy for eight weeks. Clinical assessments were conducted at baseline (week 0) and at eight weeks. All patients were required to sign the appropriate informed consent form before their participation in the study. Before each visit, all patients were asked to refrain from using their short-acting beta-2-agonist (SABA) for 6 h. At any time, the investigators were allowed to ask the patients to come for unscheduled visits to ensure patient safety and assess adverse events. To better emulate the real-life setting and improve generalization to the target population, the study investigators were a random representative sample of general practitioners and family physicians from across Canada.
Subject selection criteria
Eligible patients were 18 years of age or older with a diagnosis of asthma for at least six months, and were being treated with any dosage of an ICS as well as using an SABA on an as-needed basis. Patients had to have a forced expiratory volume in 1 s or peak expiratory flow of 80% or greater of the predicted value. In addition to the above criteria, eligible patients had to have uncontrolled asthma as defined by the Canadian Asthma Consensus Guidelines (1), fulfilling at least one of the following criteria: daytime symptoms four days or more per week; night-time symptoms one night per week or more; mild, infrequent exacerbations; absenteeism from school or work due to asthma; four doses or more per week of an SABA (apart from one dose/day before exercise); forced expiratory volume in 1 s or peak expiratory flow of 90% or less of their personal best; and diurnal variability in peak expiratory flow greater than 10% to 15%.
Patients were excluded if their asthma symptoms were controlled with their current controller therapy. Patients treated with montelukast or any of the following treatments at the time of entry into the study were excluded: a long-acting beta-2-agonist (LABA) or a combination product, prednisone, and regular use of theophylline or other asthma medications such as sodium cromoglycate or nedocromil. Patients using an antibiotic for respiratory tract infection at the time of entry into the study or who had been treated with an antibiotic within 30 days for respiratory tract infection (initiation of antibiotic treatment was permitted during the study) were also excluded. A history of cystic fibrosis or immune deficiency requiring specific therapy or any other diseases that could influence the evolution of asthma were reasons for exclusion. Patients with a history of hypersensitivity to any component of montelukast were excluded.
Treatment and follow-up
All patients were treated for eight weeks with 10 mg montelukast sodium (Singulair, Merck & Co, Inc, USA) tablets taken once daily at bedtime. Patients were assessed by the treating physician-investigators at their clinics at baseline and at eight weeks.
Outcome measures
The primary effectiveness outcome measure was the proportion of patients with asthma symptom control after eight weeks of treatment with montelukast. In the assessment of the outcome, control of asthma symptoms was defined by the Asthma Control Questionnaire (ACQ) score (12,13). The ACQ is a self-administered questionnaire and consists of seven seven-point Likert scale questions that describe the frequency and severity of asthma symptoms. The ACQ score is calculated as the mean of the seven items. The ACQ score ranges between 0 (well-controlled) and 6 (extremely poorly controlled); a score of 1.5 or higher indicates uncontrolled symptoms and a change of 0.5 or more is considered a clinically significant change in symptom control. A secondary measure of effectiveness was the absolute and per cent change in the ACQ score between the baseline and the eight-week assessment. Patient and physician satisfaction with ICS monotherapy and montelukast therapy in combination with ICS was assessed using a five-point Likert scale. Compliance with montelukast treatment was assessed at week 8 by the treating physician. Compliance was ascertained by the number of times the patient did not take the asthma medication as self-reported during the interview with the physician. Noncompliance was defined as taking less than 80% of the required asthma medication doses.
Statistical methods
Descriptive statistics were reported for patient demographics, baseline characteristics, medical history, primary trigger for asthma exacerbation, symptom distribution at baseline, and adherence to the study treatment for the study sample as a whole and by ICS dose group. Patients were classified by ICS dose used at baseline into one of the following groups: low dose, defined as 250 μg/day or less for fluticasone propionate or equivalent (500 μg/day or less for beclomethasone dipropionate and 1000 μg/day or less for budesonide); moderate dose, defined as greater than 250 μg/day up to 500 μg/day for fluticasone propionate or equivalent (greater than 500 μg/day up to 1000 μg/day for beclomethasone dipropionate and from greater than 1000 μg/day up to 2000 μg/day for budesonide); and high dose, defined as greater than 500 μg/day for fluticasone propionate or equivalent (greater than 1000 μg/day for beclomethasone dipropionate and greater than 2000 μg/day for budesonide). Between-group comparisons for ICS based on the above parameters were assessed for statistical significance with the χ2 test for categorical scales and ANOVA for continuous scales.
The proportion of patients achieving asthma control during the eight-week treatment period was calculated as the proportion of patients with an ACQ score of less than 1.5. The statistical significance of the change in ACQ score within each patient group and the study sample as a whole was assessed using Student’s t test for paired samples. The statistical significance of between-group differences with respect to the change in the ACQ score from baseline to eight weeks was assessed using one-way ANOVA. The statistical significance of the respective changes in treatment satisfaction from baseline to week 8 was assessed with the McNemar-Bowker test for paired dichotomous data. Safety was assessed by the incidence of treatment-related adverse events, which were coded and reported according to the Medical Dictionary for Regulatory Activities dictionary of terms.
Independent significant predictors of asthma control were identified using a binary logistic regression model. The dependent variable used in the model was achievement of asthma control at the eight-week assessment period. In this analysis, control of asthma symptoms was defined as an ACQ score of less than 1.5. The covariates included in the model were selected among age, body mass index, sex, duration of asthma diagnosis, primary triggers for asthma exacerbation, compliance, discontinuations, ACQ asthma control score at baseline and ICS dose group using the likelihood ratio test (14) with backward stepwise variable selection based on P<0.05.
Missing data in the ACQ questionnaires were handled using an optimal method as described by the ACQ background, administration and analysis guidelines (12,13). To minimize the risk of bias, missing values were interpolated using either previous or subsequent completions of the questionnaire. There was only one patient for whom the ACQ score was not calculated at week 8 because only the final item of the questionnaire was reported.
In accordance with the real-life aim of the study, the intent-to-treat principle was applied for the analysis of effectiveness. Therefore, all patients, including those with protocol violations, were included in the effectiveness analysis, provided baseline and eight-week data were available. All patients who received at least one dose of the study medication were included in the safety analysis. The analyses were performed using SPSS version 12.0 for Windows (SPSS Inc, USA) with the exception of logistic regression, which was performed with Stata 10 (StataCorp, USA).
RESULTS
Patient disposition
Patient enrolment began in June 2006; the last patient was enrolled in March 2007, and the last follow-up visit was completed in July 2007. In total, 320 patients were enrolled by 41 physician investigators. Two patients were excluded from the final analysis; one patient did not receive study medication and the other patient did not complete the assessments of the baseline visit. Of the 318 patients included in the intent-to-treat population, 288 (90.6%) completed the eight-week assessment. There were 30 patients (9.4%) who discontinued before the eight-week follow-up visit. Reasons for discontinuation included the following: 17 (5.4%) were lost to follow-up, two (0.6%) discontinued due to an adverse event, nine (2.8%) had a protocol violation, one (0.3%) withdrew consent and one (0.3%) withdrew due to a diagnosis of pneumonia. These 30 patients were not included in the effectiveness analysis because eight-week follow-up data were not available.
The patient demographics and baseline characteristics for the study sample as a whole and the three patient groups are described in Table 1. The baseline characteristics were similar for the three ICS dose groups, with the exception of lower prevalence of allergic rhinitis, mean age and sinusitis in the high-dose ICS group compared with the other groups, and a lower prevalence of allergic rhinitis in the low-dose group compared with the moderate-dose group.
TABLE 1.
Demographics and baseline characteristics of patients
| Baseline characteristics |
Inhaled corticosteroid dose group
|
Total (n=318) | P | ||
|---|---|---|---|---|---|
| Low dose (n=134) | Moderate dose (n=159) | High dose (n=25) | |||
| Age, years, mean (SD) | 43.7 (15.6) | 46.0 (16.7) | 52.7 (18.2) | 45.5 (16.5) | 0.039 |
| Weight, kg, mean (SD) | 73.9 (16.8) | 75.0 (19.7) | 77.7 (19.5) | 74.8 (18.5) | 0.618 |
| Body mass index, kg/m2, mean (SD) | 27.2 (6.2) | 27.8 (6.3) | 28.2 (6.8) | 27.6 (6.3) | 0.608 |
| Sex, n (%) | |||||
| Male | 35 (26.1) | 55 (34.6) | 12 (48.0) | 102 (32.1) | 0.062 |
| Female | 99 (73.9) | 104 (65.4) | 13 (52.0) | 216 (67.9) | |
| Duration of asthma diagnosis, months, mean (SD) | 109.6 (113.6) | 118.5 (107.7) | 134.7 (97.0) | 116.0 (109.4) | 0.529 |
| Primary trigger for asthma exacerbation, n (%) | |||||
| Viral infection | 29 (21.6) | 41 (25.8) | 2 (8.0) | 72 (22.6) | 0.133 |
| Allergy to animal | 17 (12.7) | 12 (7.5) | 1 (4.0) | 30 (9.4) | 0.203 |
| Exercise-induced asthma | 7 (5.2) | 13 (8.2) | 1 (4.0) | 21 (6.6) | 0.515 |
| Exposure to extreme temperature | 16 (11.9) | 14 (8.8) | 5 (20.0) | 35 (11.0) | 0.226 |
| Seasonal allergy | 13 (9.7) | 19 (11.9) | 2 (8.0) | 34 (10.7) | 0.744 |
| Smoking history, n (%) | |||||
| Patient smokes | 35 (26.1) | 46 (28.9) | 7 (28.0) | 88 (27.7) | 0.866 |
| Member of household smokes | 33 (24.6) | 42 (26.4) | 6 (24.0) | 81 (25.5) | 0.926 |
| Patient quit smoking | 29 (21.6) | 32 (20.1) | 9 (36.0) | 70 (22.0) | 0.219 |
| Member of household quit smoking | 10 (7.5) | 13 (8.2) | 3 (12.0) | 26 (8.2) | 0.756 |
| Medical history, n (%) | |||||
| Allergic rhinitis | 94 (70.1) | 91 (57.2) | 7 (28.0) | 192 (60.4) | 0.001 |
| Sinusitis | 61 (45.5) | 57 (35.8) | 4 (16.0) | 122 (38.4) | 0.013 |
| Symptom distribution at baseline, n (%) | |||||
| Daytime symptoms ≥4 days/week | 117 (87.3) | 138 (86.8) | 24 (96.0) | 279 (87.7) | 0.419 |
| Night-time symptoms ≥1 night/week | 104 (77.6) | 114 (71.7) | 20 (80.0) | 238 (74.8) | 0.420 |
| Mild infrequent exacerbations | 123 (91.8) | 142 (89.3) | 22 (88.0) | 287 (90.3) | 0.717 |
| Absenteeism due to asthma (school or work) | 35 (26.1) | 40 (25.2) | 4 (16.0) | 79 (24.8) | 0.556 |
| Short-acting beta-2-agonist ≥4 doses/week | 112 (83.6) | 135 (84.9) | 21 (84.0) | 268 (84.3) | 0.952 |
| FEV1 or PEF ≤90% of personal best | 96 (71.6) | 98 (61.6) | 18 (72.0) | 212 (66.7) | 0.163 |
| Diurnal variability in peak expiratory flow >10% to 15% | 53 (39.6) | 52 (32.7) | 11 (44.0) | 116 (36.5) | 0.344 |
FEV1 Forced expiratory volume in 1 s; PEF Peak expiratory flow
Effectiveness outcomes
At baseline, all patients reported lack of control of their asthma based upon at least one parameter of the Canadian Asthma Consensus Guidelines. There were 66 patients (20.7%) who had a baseline ACQ score of less than 1.5, indicating controlled asthma symptoms as per this scale. Of these 66 patients, 62 (93.9%) maintained an ACQ score of less than 1.5 at week 8. Among these 66 patients, 35 of 37 (94.6%) in the low-dose ICS group, 26 of 28 (92.9%) in the moderate-dose ICS group and the one patient (100.0%) in the high-dose ICS group maintained control (ACQ less than 1.5) of their asthma at eight weeks. In these 66 patients, a statistically (P<0.001) and clinically significant decrease of 0.57 in ACQ score was observed between baseline and eight weeks. A similarly significant (P<0.001) improvement in the ACQ score was observed for each subgroup of these patients treated with low- and moderate-dose ICS (Table 2 and Figure 1). Assessment of the statistical significance of change for the single patient in the high ICS dose group was not possible.
TABLE 2.
Outcome assessment stratified by the Asthma Control Questionnaire (ACQ) at baseline and inhaled corticosteroid (ICS) dose
| Asthma symptoms |
ACQ score <1.5
|
ACQ score ≥1.5
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
ICS treatment
|
P | Total |
ICS treatment
|
P | Total | |||||
| Low dose | Moderate dose | High dose | Low dose | Moderate dose | High dose | |||||
| n | 37 | 28 | 1 | – | 66 | 97 | 131 | 24 | – | 252 |
| Asthma controlled at 8 weeks, n (%) | 35 (94.6) | 26 (92.9) | 1 (100.0) | 0.962 | 62 (93.9) | 73 (75.3) | 75 (57.3) | 12 (50.0) | 0.061 | 160 (63.5) |
| Asthma not controlled at 8 weeks, n (%) | 1 (2.7) | 1 (3.8) | 0 | 2 (3.0) | 20 (20.6) | 33 (25.2) | 10 (41.7) | 63 (25.0) | ||
| Discontinued at 8 weeks, n (%) | 1 (2.7) | 1 (3.8) | 0 | – | 2 (3.0) | 4 (4.1) | 23 (17.5) | 2 (8.3) | – | 29 (11.5) |
| ACQ, mean (SD) | ||||||||||
| ACQ at baseline | 1.18 (0.30) | 1.05 (0.41) | 1.29 (NA) | 0.164 | 1.13 (0.28) | 2.16 (0.58) | 2.50 (0.77) | 2.62 (0.84) | 0.001 | 2.38 (0.73) |
| ACQ at 8 weeks | 0.69 (0.51) | 0.41 (0.43) | 0.29 (NA) | 0.068 | 0.57 (0.50) | 0.83 (0.69) | 1.12 (0.85) | 1.44 (0.73) | 0.001 | 1.03 (0.80) |
| Mean change at 8 weeks | −0.50 (0.57) | −0.63 (0.52) | −1.00 (NA) | 0.475 | −0.57 (0.55) | −1.33 (0.84) | −1.30 (0.96) | −1.19 (1.12) | 0.809 | −1.30 (0.92) |
| P (of mean change) | <0.001 | <0.001 | NA | – | <0.001 | <0.001 | <0.001 | <0.001 | – | <0.001 |
| Per cent change at 8 weeks | −35.44 (77.20) | −57.19 (47.86) | −77.78 (NA) | 0.392 | −45.28 (66.25) | −60.86 (32.70) | −52.55 (35.76) | −40.28 (33.26) | 0.027 | −54.81 (34.68) |
| P (of % change) | <0.001 | 0.009 | NA | – | <0.001 | <0.001 | <0.001 | <0.001 | – | <0.001 |
NA Nonapplicable
Figure 1).
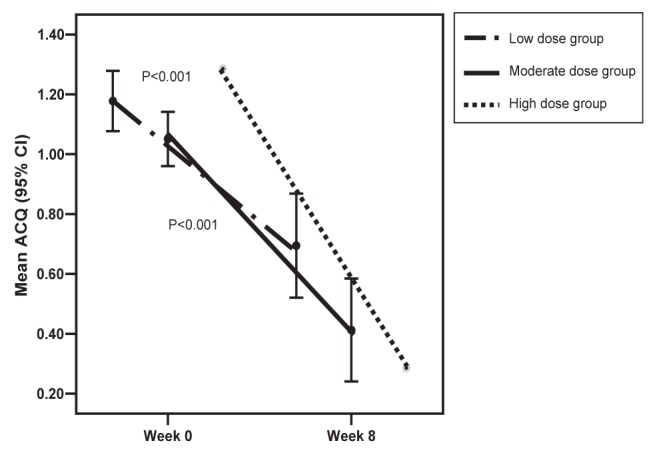
Asthma Control Questionnaire (ACQ) score by baseline inhaled corticosteroid dose groups for ACQ scores of <1.5
Of the 252 patients (79.2%) with an ACQ score of 1.5 or greater at baseline, 160 (63.5%) achieved asthma control at week 8. The proportions of these patients who had an ACQ score of less than 1.5 at eight weeks in the low-, moderate- and high-dose ICS groups were 75.3%, 57.3% and 50.0%, respectively. This difference approached statistical significance (P=0.06). For these 252 patients, a statistically and clinically significant decrease of 1.30 from baseline to eight weeks in the ACQ score was observed (P<0.001). The changes in the ACQ score for the patients in this group treated with low, moderate and high doses of ICS were also clinically and statistically significant. Although the absolute change in ACQ score was not different for the subgroups of these patients treated with low-, moderate- and high-dose ICS (P=0.809), the per cent change was significantly different, with an inverse relationship between per cent change and ICS dose category (P=0.027) (Table 2 and Figure 2).
Figure 2).
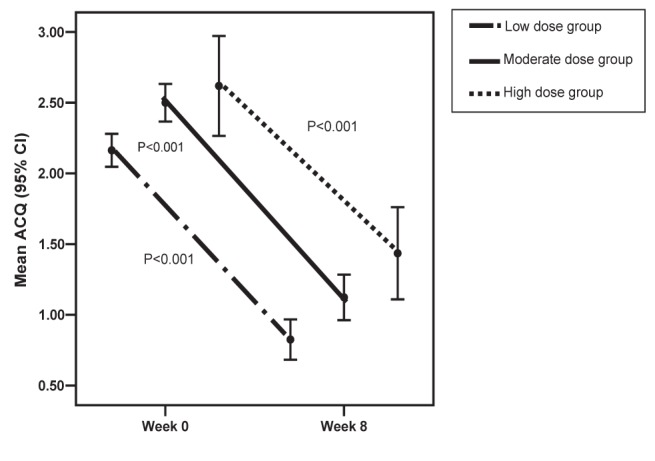
Asthma Control Questionnaire (ACQ) score by baseline inhaled corticosteroid dose groups for ACQ scores of ≥1.5
Patient and physician satisfaction with treatment
Patient ratings of satisfaction with the treatment results are summarized in Table 3. At the baseline assessment, for the low-, moderate- and high-dose ICS groups, 50.8%, 47.8% and 64.0%, respectively, of the patients were dissatisfied with their treatment and 10.5%, 10.7% and 12.0%, respectively, were satisfied. At the eight-week assessment, for the low-, moderate- and high-dose ICS groups, 77.6, 74.2% and 82.6%, respectively, were satisfied with the combination of an ICS and montelukast. Overall, 10.7% of the patients were satisfied with their ICS therapy at baseline, and 76.4% were satisfied with their montelukast add-on treatment at week 8. The change in patient satisfaction between the baseline and eight-week assessment was statistically significant (P<0.001). The investigator satisfaction rates with treatment results were similar to those obtained for patient satisfaction (Table 3).
TABLE 3.
Investigator and patient satisfaction with treatment
|
Baseline (with ICS treatment only)
|
Week 8 (with add-on montelukast)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low-dose ICS | Moderate-dose ICS | High-dose ICS | Total | Low-dose ICS | Moderate-dose ICS | High-dose ICS | Total | P* | ||||||
| Patient global satisfaction with treatment, n (%) | ||||||||||||||
| Very satisfied | 2 (1.5) | 7 (4.4) | 0 (0.0) | 9 (2.8) | 50 (38.8) | 58 (42.6) | 6 (26.1) | 114 (39.6) | <0.001 | |||||
| Satisfied | 12 (9.0) | 10 (6.3) | 3 (12.0) | 25 (7.9) | 50 (38.8) | 43 (31.6) | 13 (56.5) | 106 (36.8) | ||||||
| Neither satisfied nor dissatisfied | 52 (38.8) | 65 (40.9) | 5 (20.0) | 122 (38.4) | 22 (17.1) | 19 (14.0) | 2 (8.7) | 43 (14.9) | ||||||
| Dissatisfied | 64 (47.8) | 67 (42.1) | 13 (52.0) | 144 (45.3) | 4 (3.1) | 16 (11.8) | 1 (4.3) | 21 (7.3) | ||||||
| Very dissatisfied | 4 (3.0) | 9 (5.7) | 3 (12.0) | 16 (5.0) | 3 (2.3) | 0 (0.0) | 1 (4.3) | 4 (1.4) | ||||||
| Missing | 0 (0.0) | 1 (0.6) | 1 (4.0) | 2 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||||
| Total | 134 | 159 | 25 | 318 | 129 | 136 | 23 | 288 | ||||||
| Investigator global satisfaction with treatment, n (%) | ||||||||||||||
| Very satisfied | 3 (2.1) | 6 (3.8) | 0 (0.0) | 9 (2.8) | 46 (35.7) | 51 (37.5) | 10 (43.5) | 107 (37.2) | <0.001 | |||||
| Satisfied | 2 (1.5) | 11 (6.9) | 0 (0.0) | 13 (4.1) | 55 (42.6) | 52 (38.2) | 9 (39.1) | 116 (40.3) | ||||||
| Neither satisfied nor dissatisfied | 56 (41.8) | 49 (30.8) | 4 (16.0) | 109 (34.3) | 22 (17.1) | 24 (17.6) | 2 (8.7) | 48 (16.7) | ||||||
| Dissatisfied | 71 (53.0) | 87 (54.7) | 17 (68.0) | 175 (55.0) | 5 (3.9) | 9 (6.6) | 1 (4.3) | 15 (5.2) | ||||||
| Very dissatisfied | 2 (1.5) | 6 (3.8) | 4 (16.0) | 12 (3.8) | 0 (0.0) | 0 (0.0) | 1 (4.3) | 1 (0.3) | ||||||
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.8) | 0 (0.0) | 0 (0.0) | 1 (0.3) | ||||||
Based on McNemar-Bowker Test for total. P values for dose stratification could not be computed due to small sample. ICS Inhaled corticosteroid
Predictors of clinical response
The logistic regression model results are summarized in Table 4. The results of the variable selection process showed that treatment with high- versus low-dose ICS (P=0.009), higher body mass index (P=0.040), patient smoking (P=0.02), viral infection as a primary trigger (P=0.012), animal allergy (P=0.014), seasonal allergy (P=0.014) and having uncontrolled symptoms at baseline (P=0.002) were significant independent predictors of having lower odds of achieving control of asthma at eight weeks.
TABLE 4.
Logistic regression model for predictors of asthma control at week 8
| Variables included in model | OR | 95% CI | SE | P |
|---|---|---|---|---|
| Montelukast in combination with ICS dose group | ||||
| Low ICS dose (reference) | 1.00 | – | – | – |
| Moderate versus low ICS dose | 0.652 | 0.334–1.274 | 0.222 | 0.211 |
| High versus low ICS dose | 0.240 | 0.083–0.696 | 0.130 | 0.009 |
| Body mass index, kg/m2 | 0.954 | 0.913–0.998 | 0.022 | 0.040 |
| Patient smoking (yes versus no) | 0.353 | 0.185–0.676 | 0.117 | 0.002 |
| Viral infection as primary trigger of exacerbation (yes versus no) | 0.382 | 0.180–0.811 | 0.147 | 0.012 |
| Allergy to animal as primary trigger of exacerbation (yes versus no) | 0.279 | 0.101–0.773 | 0.145 | 0.014 |
| Seasonal allergy as primary trigger of exacerbation (yes versus no) | 0.230 | 0.071–0.745 | 0.138 | 0.014 |
| Asthma controlled at baseline based on Asthma Control Questionnaire score (yes versus no) | 10.26 | 2.349–44.776 | 7.712 | 0.002 |
ACQ Asthma Control Questionnaire; ICS Inhaled corticosteroids
Compliance
The proportions of patients taking 80% or more of their asthma medications, including montelukast and ICS, during the eight weeks of the study in the low-, moderate- and high-dose ICS groups were 93%, 92% and 91%, respectively.
Safety
A total of 29 treatment-related nonserious adverse events (NSAEs) were reported by 23 of the 319 patients (7.2%) enrolled in the study. Of these NSAEs, two (6.9%) led to treatment discontinuation and withdrawal from the study. The most frequently reported NSAEs that were related to the study treatment were nervous system disorders reported by 13 patients (4.1%), primarily headache (n=8; 2.5%) and dizziness (n=5; 1.6%), followed by gastrointestinal disorders, reported by seven patients (2.2%), specifically diarrhea (n=2; 0.6%), dyspepsia (n=2; 0.6%), nausea (n=2; 0.6%) and abdominal pain (n=1; 0.3%). There were no serious adverse events related to the study drug reported over the course of the study.
DISCUSSION
Patients suffering from asthma experience limitations in their physical, emotional, social and professional lives. The negative impact of asthma on a patient’s life increases when asthma symptoms are not adequately controlled (15). The goals of asthma therapy are to achieve control of asthma symptoms by maintaining normal pulmonary function, preventing chronic symptoms and recurrent exacerbations, and providing optimal pharmacotherapy with minimal or no adverse events (5).
It should be noted that current guidelines recommend switching from ICS monotherapy to a single inhaler containing an ICS/LABA (2). Montelukast offers an additional treatment option in adult asthma; when added to an ICS, it has been demonstrated to be as effective as a LABA in patients previously uncontrolled on fluticasone (16). The findings of the present study further support the use of montelukast for patients who do not respond, or who are not satisfied or are noncompliant with ICS monotherapy.
In the present study, once-daily, orally administered montelukast as an add-on to a low-, moderate- or high-dose ICS was effective in achieving control or significant reduction of asthma symptoms within eight weeks of treatment for patients who had uncontrolled symptoms with ICS monotherapy. Both physician and patient satisfaction with montelukast add-on therapy were also significantly increased compared with baseline ICS treatment.
In the current study, the predictors for achieving asthma control with montelukast as an add-on to an ICS have also been identified as risk factors for asthma (17–19). Although montelukast add-on therapy was effective in controlling asthma symptoms in the patients treated with low-, moderate- and high-dose ICS, those treated with high-dose ICS had decreased odds of achieving control. This is compatible with previous studies of patients with mild asthma in which higher therapeutic effectiveness was observed in patients with lower asthma severity and lower treatment levels (20). This has important implications for patient care and may support the use of add-on montelukast therapy in the management of patients with mild asthma.
Overall, montelukast once-daily was well tolerated and safe during the eight-week treatment period of the study. The adverse events that occurred were predominantly mild and resolved with no long-term effects. There were no treatment-related serious adverse events reported.
Potential weaknesses of the present study include the single-cohort design without a parallel control group. The aim of the study, however, was to assess the effectiveness of montelukast as an add-on to ICS in patients who were either not controlled, non-compliant or not satisfied with ICS monotherapy. In day to day practice, any of these scenarios would be an invitation to consider changing current treatment. Therefore, the relevant question is whether the addition of montelukast can improve asthma control in these patients and how it compares to alternative approaches. In addition, from a methodological perspective, the baseline (pre-treatment) status of these patients provides the control for the evaluation of effectiveness. Another potential weakness of the study is the open-label design. However, this is necessary for emulating a real-life setting in which blinding of treatment used is not applicable. In addition, the study period was relatively short, and long-term effects on asthma control and especially asthma exacerbations could not be reliably measured.
The strengths of the current study include the high potential for generalization of the study results to the Canadian target population; this was achieved by enrolling patients from a representative sample of Canadian general practitioners and family physicians. The use of standardized and validated questionnaires to assess the severity of asthma enhanced the internal validity of the study and allowed direct comparison of the results to those obtained in other clinical trials. By using less stringent inclusion criteria, in comparison to those employed in controlled clinical trials, and by including in the analysis all patients regardless of their compliance and adherence to the protocol, the results are more generalizable to the real-life settings compared with randomized, controlled trials. Finally, the logistic regression analysis assessing predictors of response to treatment identified known risk factors as significant factors associated with improved response to treatment. This observation further validates the results of the study and its relevance to the general target populations of asthma patients not responding to ICS monotherapy.
Montelukast add-on therapy is an effective and well-tolerated alternative to ICS treatment monotherapy in adults with asthma who are uncontrolled or unsatisfied with current ICS therapy.
Acknowledgments
Financial disclosures and potential conflicts of interest: S Foucart is an employee in Clinical Research at Merck Frosst Canada Ltd; D Haine, E Psaradellis and J Sampalis are employees of JSS Medical Research Inc (CRO); RA McIvor, Dr S Coyle and Dr JM Fitzgerald are consultants for the SAS study. Dr McIvor has received honoraria for continuing medical education (CME) and attending Advisory Board meetings for pharmaceutical companies with respect to asthma management, including AstraZeneca, GlaxoSmithKline, Merck Frosst, Novartis and Nycomed. Dr Coyle has received honoraria for CME and attending advisory board meetings for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Merck Frosst, Biovail, Pfizer and Abbott Laboratories. Dr JM Fitzgerald has received fees for advisory board attendance and membership of CME lecture panels sponsored by a number of companies, including AstraZeneca, GlaxoSmithKline, Nycomed, Merck Frosst and Novartis, who market drugs used in the treatment of asthma. He has also received travel assistance to attend the European Respiratory Society meeting in September 2008, and received research funding for asthma-related research from these pharmaceutical companies, which was paid directly to the University of British Columbia. All investigators received grants related to the conduct of the study including but not limited to patient recruitment and case report form completion on a prorated basis. List of investigators: Chouinard, Guy; Gonzalez, Yolanda; Grigorian, Anahid; Fournier, Pierre; Sylvain-Lucien, Joseph; Luu, Thanh Phi; Naim, Maurice; Vartanian, Astghik; Villeneuve, Lucien; Axler, John; Bodok-Nutzati, Rebecca; Bartlett, John; Browne, Noel; Che, Claudius; Csanadi, Michael; DeSouza, Selwyn; Ismail, Shiraz; Joshi, Shelendra; Lawless, John; Ng, Ken; Olsheski, Wayne; O’Mahony, Michael; Pang, Patrick; Rabb, Lucy; Riche, Cyril; Rockman, Gerald; Ubani, Nelson; Verdonk, Robert; William, Hany; Wong, Albert; Kerr, Pauline; Biala, Barbara; Cham, Patrick; du Preez, Miranda; Kiellerman, Ewa Jussak; Wong, Wilfred T; Look, J Michael; Kelly, John; Hosie, Andrew; Cook, Alan; Fera, Tharwat; Chow, Walter; Luke, Edward; Ezekiel, Daniel; James, Christopher; Nirwan, Amarjit; Morgan, David; Bergstrom, Craig; Horner, Richard H; Collette, Ronald; Charbonneau, Jacques; Thériault, Lyne; Serfaty, Samuel; Leclair, Marcel; Barrière, Ginette; Laporte, Hélène; Despard, Caroline; Dhillon, Ripple; Ho, Micheal; Hsieh, Daniel; Lotfallah, Talaat; Rideout, Gary; Vaithianathan, Kandiah; Nuttall, Richard; Wong, Leo; Lin, Betty Ping; Trudel, François; Zackon, Harold; Schulz, Jan; Faiers, Alan; Kim, Matthew; Schumacher, Albert; Ying, James; Fruchtermann, Lucien; Hong, Tommy; De Rubeis, Andrea; Savard, Claude; Tran, Vu Kiet; Sunerh, Pal; Houde, Danielle; Belle-Isle, Jasmin; Saksena, Rajni; Gagnon, Robert; Shields, Jean; Hartford, Brian; Bukovy, Brent; Young, Michelle; Wheeler, Bruce; Jablonski, Ted.
Footnotes
STUDY: Registered at clinicaltrials.gov (Identifier NCT 00755794).
FUNDING: This study was funded by Merck Frosst Ltd.
REFERENCES
- 1.Lemiere C, Bai T, Balter M, et al. Adult Asthma Consensus Guidelines Update 2003. Can Respir J. 2004;11(Suppl A):9A–18A. doi: 10.1155/2004/271362. [DOI] [PubMed] [Google Scholar]
- 2.Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–78. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 3.Pauwels RA, Pedersen S, Busse WW, et al. Early intervention with budesonide in mild persistent asthma: A randomised, double-blind trial. Lancet. 2003;361:1071–6. doi: 10.1016/S0140-6736(03)12891-7. [DOI] [PubMed] [Google Scholar]
- 4.Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA), 2006. <www.ginasthma.org> (Version current 2006).
- 5.National Asthma Education and Prevention Program: Expert Panel Report: Guidelines for the diagnosis and management of asthma. Update on selected topics – 2002. J Allergy Clin Immunol. 2002;110(Suppl 1):S141–S219. [PubMed] [Google Scholar]
- 6.Malmstrom K, Rodriguez-Gomez G, Guerra J, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med. 1999;130:487–95. doi: 10.7326/0003-4819-130-6-199903160-00005. [DOI] [PubMed] [Google Scholar]
- 7.Cowie RL, Underwood MF, Mack S. The impact of asthma management guideline dissemination on the control of asthma in the community. Can Respir J. 2001;8(Suppl A):41A–5A. doi: 10.1155/2001/213953. [DOI] [PubMed] [Google Scholar]
- 8.Boulet LP. Perception of the role and potential side effects of inhaled corticosteroids among asthmatic patients. Chest. 1998;113:587–92. doi: 10.1378/chest.113.3.587. [DOI] [PubMed] [Google Scholar]
- 9.Cochrane MG, Bala MV, Downs KE, Mauskopf J, Ben-Joseph RH. Inhaled corticosteroids for asthma therapy: Patient compliance, devices, and inhalation technique. Chest. 2000;117:542–50. doi: 10.1378/chest.117.2.542. [DOI] [PubMed] [Google Scholar]
- 10.Pauwels RA, Lofdahl CG, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group 2. N Engl J Med. 1997;337:1405–11. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 11.Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Allen & Hanburys Limited UK Study Group 1. Lancet. 1994;344:219–24. doi: 10.1016/s0140-6736(94)92996-3. [DOI] [PubMed] [Google Scholar]
- 12.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–7. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 13.Juniper EF, O’Byrne PM, Roberts JN. Measuring asthma control in group studies: Do we need airway calibre and rescue beta2-agonist use? Respir Med. 2001;95:319–23. doi: 10.1053/rmed.2001.1034. [DOI] [PubMed] [Google Scholar]
- 14.Neter J, Kutner MH, Wasserman W, Nachtsheim CJ. Applied Linear Statistical Models. 4th edn. Chicago: McGraw-Hill; 1996. [Google Scholar]
- 15.Braman SS. The global burden of asthma. Chest. 2006;130(1 Suppl):4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 16.Bjermer L, Bisgaard H, Bousquet J, et al. Montelukast and fluticasone compared with salmeterol and fluticasone in protecting against asthma exacerbation in adults: One year, double blind, randomised, comparative trial. BMJ. 2003;327:891. doi: 10.1136/bmj.327.7420.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Nieuwenhof L, Schermer T, Eysingk P, et al. Can the asthma control questionnaire be used to differentiate between patients with controlled and uncontrolled asthma symptoms? A pilot study. Fam Pract. 2006;23:674–81. doi: 10.1093/fampra/cml041. [DOI] [PubMed] [Google Scholar]
- 18.Lavoie KL, Bacon SL, Labrecque M, Cartier A, Ditto B. Higher BMI is associated with worse asthma control and quality of life but not asthma severity. Respir Med. 2006;100:648–57. doi: 10.1016/j.rmed.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 19.de Vries MP, van den BL, Lince S, Muris JW, Thoonen BP, van Schayck CP. Factors associated with asthma control. J Asthma. 2005;42:659–65. doi: 10.1080/02770900500264903. [DOI] [PubMed] [Google Scholar]
- 20.Laforest L, Van Ganse E, Devouassoux G, et al. Influence of patients’ characteristics and disease management on asthma control. J Allergy Clin Immunol. 2006;117:1404–10. doi: 10.1016/j.jaci.2006.03.007. [DOI] [PubMed] [Google Scholar]


