Abstract
RhoA upregulation has been suggested in bronchial smooth muscles (BSMs) of asthmatic rats. Here, we cloned/characterized the 5′-promoter region of the rat rhoA. A transcription-initiation site was identified at 66-bp upstream of the reference sequence, GenBank-BC061732. Luciferase assay using interleukin-13 (IL-13)–stimulated cells revealed a significant promoter activity at 238- to 166-bp upstream of the transcription-initiation site, which contains a signal transducer and activation of transcription (STAT) 6–binding region. The IL-13–induced increase in luciferase activity was inhibited by a STAT6 inhibitor, AS1517499, or a Janus kinases (JAKs) inhibitor, JAK Inhibitor-I, but not by tyrphostin-AG490, WHI-P131, or tyrphostin-AG9 (selective JAK2, JAK3, and Tyk2 inhibitors, respectively). Thus, rat BSM rhoA expression may have causal relation to the IL-13–JAK1–STAT6 signaling.
Keywords: RhoA, transcriptional regulation, bronchial smooth muscle
There is increasing evidence that RhoA/Rho-kinase is involved in the Ca2+ sensitization of airway smooth muscle contraction (1–3). When the RhoA/Rho-kinase system is activated by contractile agonists, the activity of myosin-light-chain (MLC) phosphatase is reduced and the level of phosphorylated MLC is then increased, resulting in an augmentation of contraction. Recent studies demonstrated that the agonist-induced, RhoA/Rho-kinase–mediated Ca2+ sensitization of bronchial smooth muscle (BSM) contraction is augmented in animal models of allergic asthma (1, 2). An importance of the RhoA/Rho-kinase system has also been demonstrated in human BSM (3), and the signaling of RhoA and its downstream Rho-kinases are now considered as a therapeutic target for the treatment of airway hyper-responsiveness in asthma (4).
Our previous study revealed that increased expression of RhoA protein (1) and mRNA (5) in BSM of a rat model of allergic asthma. However, the mechanism of the transcriptional regulation of the RhoA gene has not yet been clarified. Here, the transcription-initiation site of the rat RhoA gene and the promoter activity in its 5′-flanking region were determined in order to understand the transcriptional regulation of the rat RhoA gene.
Male Wistar rats were purchased from Charles River Japan, Inc. (Kanagawa) and housed in a pathogen-free facility. All animal experiments were approved by the Animal Care Committee of Hoshi University (Tokyo).
To map the transcription-initiation site of rat RhoA gene, a rapid amplification of 5′-ends (5′-RACE) was carried out using a GeneRacer™ kit (Invitrogen Life Technologies, Carlsbad, CA, USA). Briefly, 5 μg of total RNA from rat lungs were used to prepare the cDNA library: the mRNAs were firstly treated with calf intestinal phosphatase and tobacco acid pyrophosphatase to eliminate the truncated or decomposed mRNA from ligation with the GeneRacer™ RNA Oligo. Reverse transcription reaction was carried out by using oligo(dT)20 and Super-Script™ III reverse transcriptase (Invitrogen Life Technologies). The resultant first-strand cDNA library was amplified using the GeneRacer™ 5′-Primer (5′-CGACTGGAGCACGAGGACACTGA-3′, identical to the GeneRacer™ RNA Oligo) and a reverse gene-specific primer-2 (GSP2: 5′-GAGGCTGCGTGTCACAAGGCT TCAC-3′). The polymerase chain reaction (PCR) conditions were as follows: 1 μL of cDNA library, 5 μM of each primer, the 50 μL of reaction mixture containing 0.2 mM dNTPs, 10× buffer and 5 units of JumpStart™ REDTaq DNA Polymerase (Sigma, St. Louis, MO, USA) and was incubated for 2 min at 95°C followed by 30 cycles of amplification (30 s at 95°C, 30 s at 65°C, and 60 s at 72°C). An aliquot of the first PCR products was used as the template for a nested reaction by using the GeneRacer™ 5′-Nested Primer and a reverse gene-specific primer-1 (GSP1: 5′-ACTCCCGCCTTGTGTGCTCATCATTC-3′). The PCR conditions used were as described above. The PCR products were run on a 3% agarose gel, cloned into pCR4-TOPO vector, and analyzed by sequencing.
A rat RhoA genomic fragment from −1217 to +21 bp (the transcription-initiation site is +1) was obtained by PCR amplification with sense and antisense primers, 5′-GGAACCCCCATTTGGAATCCTGAGC-3′ (SacI restriction site is underlined) and 5′-CGGGGAGCGCTAGCGAACGAG-3′ (NheI restriction site is underlined and mutation is in italics), respectively, using the rat genomic DNA (Clonthech, Mountain View, CA, USA) as a template. The PCR primers were designed based on the published sequence (GenBank NW_047483.1). The PCR product was digested with SacI and NheI, and the SacI-NheI fragment was inserted into the SacI-NheI site of pGL4.10 basic (Promega, Madison, WI, USA). The resultant plasmid vector contains −1195 to +10 bp of the rat RhoA gene (pGL4-D0).
The serially deleted luciferase constructs of the 5′-upstream region of rat RhoA were also prepared from the rat genomic DNA. The −675-bp (D1), −348-bp (D2), −238-bp (D3), and −166-bp (D4) fragments from the transcriptional-initiation site (+1) containing KpnI and NheI sites in the 5−- and 3−-end of the primers, respectively, were amplified by PCR and cloned into pGL4.10 basic (Promega). The primer sets used were as follows: reverse primer: 5′-CTAGCTAGCCGGGGAGCGCGA GCGAACGAG-3′ and forward primer D1: 5′-GGGGTACCCCTCACCTGGACGGTGATACAA-3′, D2: 5′-G GGGTACCCCTTCCGGGGTTAGGCCTGTCA-3′, D3: 5′-GGGGTACCCCATTCGGTGAGTATAAAATAGC-3′, and D4: 5′-GGGGTACCCCGTGATTGGTTAAGCGTCTGG-3′. The complete PCR-amplified products were subjected to DNA sequencing to verify the absence of errors.
Normal human BSM cells (hBSMCs; Cambrex Bio Science Walkersville, Inc., Walkersville, MD, USA) were cultured as previously described (6, 11). When hBSMCs were grown to 80% – 85% confluence in 96- well plates, the cells were transfected with pGL4 reporter plasmids using Lipofectamine 2000 (Invitrogen Life Technologies) according to the manufacturer’s instructions and were then cultured in serum-free medium. At 24 h after starvation, cells were treated with recombinant human interleukin-13 (IL-13) (100 ng/mL; PeproTech EC, Ltd., London, UK). After 24-h incubation with IL-13, luciferase assay was carried out using the ONE-GloTM luciferase assay system (Promega) according to the manufacturer’s instructions. In some experiments, cells were also treated with IL-13 in the presence of a selective signal transducer and activation of transcription (STAT) 6 inhibitor, 4-(benzylamino)-2-{[2-(3-chloro-4- hydroxyphenyl)ethyl]amino}pyrimidine-5-carboxamide (AS1517499, 100 nM; kindly provided from Astellas Pharma, Inc., Tokyo) (6); a non-selective Janus kinases (JAKs) inhibitor, JAK inhibitor-I (1 μM; Calbiochem, Gibbstown, NJ, USA) (7); a selective JAK2 inhibitor, tyrphostin-AG490 (50 μM; LC Laboratories, Woburn, MA, USA) (8); a selective JAK3 inhibitor, WHI-P131 (100 μM, Calbiochem) (9); a selective Tyk2 inhibitor, tyrphostin-AG9 (50 μM; Alexis Biochemicals, San Diego, CA, USA) (10); or their vehicle (0.3% DMSO).
All data were expressed as the mean with S.E.M. Statistical significance of difference was determined by the unpaired Student’s t-test or two-way analysis of variance (ANOVA) with the post hoc Bonferroni/Dunn test (Stat-View for Macintosh ver. 5.0; SAS Institute, Inc., Cary, NC, USA).
To identify the transcription-initiation site of rat RhoA gene, the 5′-RACE analysis was carried out using total RNA isolated from rat lungs. The analysis was performed using two reverse oligonucleotides derived from the 5′-untranslated region (GSP1 and GSP2; see above and Fig. 1B for details) and two adaptor primers provided with the kit for the primary and nested PCR. The primary PCR products were used as a template for nested PCR by using GeneRacer™ 5′-Nested Primer and GSP1. Agarose gel electrophoresis of these DNA fragments is shown in Fig. 1A. The transcription-initiation site was identified by sequence analysis and mapped at 66-bp (10/12 randomly selected clones) and 22-bp (2/12 clones) upstreams of the 5′-end of the reference RhoA cDNA sequence, GenBank BC061732 (Fig. 1B). The 66-bp upstream was used as the major transcription-initiation site in the present study.
Fig. 1.
Determination of transcriptional-initiation site of the rat RhoA gene using 5′-RACE. The published RhoA cDNA sequence (GenBank accession number BC061732) was used as the reference sequence. A) Amplification of 5′-end of the rat RhoA gene by PCR. The primer sets used for the amplification were as follows: lane 1: GeneRacer™5′ and GSP1 primers, lane 2: GeneRacer™5′ and GSP2 primers, and lane 3: GeneRacer™5′ nested primer and GSP1 primer (see the text). B) Sequence of the 5′-RACE PCR products. The newly isolated 5′-end was identified at 66-bp upstream of the end of the published rat RhoA cDNA.
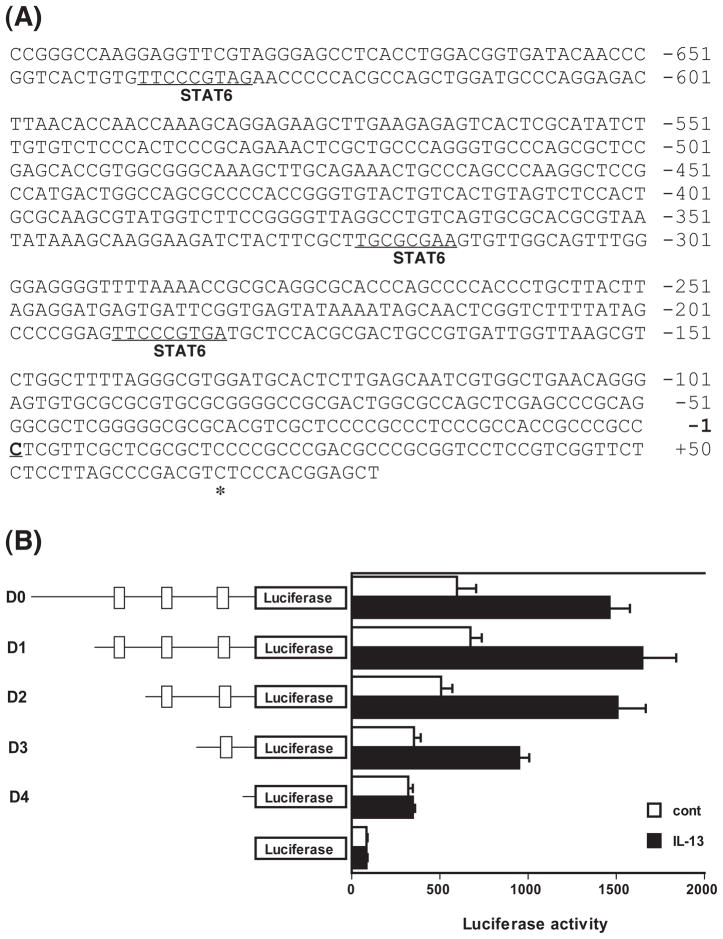
On the basis of the transcription-initiation site, a 1205- bp fragment of rat genomic DNA (from −1195 to +10 bp) was obtained by PCR. The analysis of the 5′-flanking region of the rat RhoA gene using the TFSEARCH program (http://mbs.cbrc.jp/research/db/TFSEARCH.html) revealed that it contains three STATs-binding sites: −192 to −184 bp (score 85.6), −323 to −316 bp (score 80.8), and −640 to −632 bp (score 84.6) (see Fig. 2A).
Fig. 2.
Determination of the IL-13–response region in the rat RhoA gene promoter. A) The sequence of the 5′-flanking region of the rat RhoA gene. The published 5′-end (asterisk, GenBank accession number BC061732), the newly identified translation-initiation site (underline, +1), and putative STATs-binding regions (−640 to −632 bp, −323 to −316 bp, and −192 to −184 bp) are indicated. B) Cultured human bronchial smooth muscle cells were transfected with a series of luciferase reporter plasmids containing various lengths (−1195, −675, −348, −238, and −166/+10 bp: D0, D1, D2, D3, and D4, respectively) of the rat RhoA gene promoter. Putative STATs-binding regions (−640 to −632 bp, −323 to −316 bp, and −192 to −184 bp) are indicated as boxes on the left illustrations. Luciferase assays were performed with (closed columns) or without (open columns) stimulation with 100 ng/mL of IL-13. Each column represents the mean ± S.E.M. from 5 independent experiments.
We have previously reported that IL-13 causes an upregulation of RhoA via an activation of STAT6 in hBSMCs (6). So in the present study, the reporter assay was performed using cells transfected with plasmid containing the −1195/+10-bp upstream (named D0 construct) in the absence or presence of IL-13 (100 ng/mL). The promoter activities were also measured using four 5′ progressive deletions, named D1, D2, D3, and D4 constructs (see above and Fig. 2). As shown in Fig. 2B, in the hBSMCs transfected with D0 and D1 (−1195/−676 bp) constructs, containing three STATs-binding sites, the promoter activity was approximately 2.5-fold increased by IL-13 stimulation. The deletions of −1195/−349 bp (D2, which contains two STATs-binding regions) had slightly lower basal promoter activity, while slightly higher (3-fold) IL-13–induced promoter activity was obtained as compared to D0/D1. The deletion of −1195/−239 bp (D3, which contains only the most proximal STATs-binding region) exhibited further reduced basal promoter activity, which increased approximately 2.7-fold upon stimulation with IL-13. In contrast, the deletion of −1195/−167 bp (D4, which contains no STATs-binding region) completely abolished the IL-13–induced increase of promoter activity. These findings indicate that at least the most proximal STATs-binding region is required for the IL-13–induced increase in promoter activity of the rat RhoA gene.
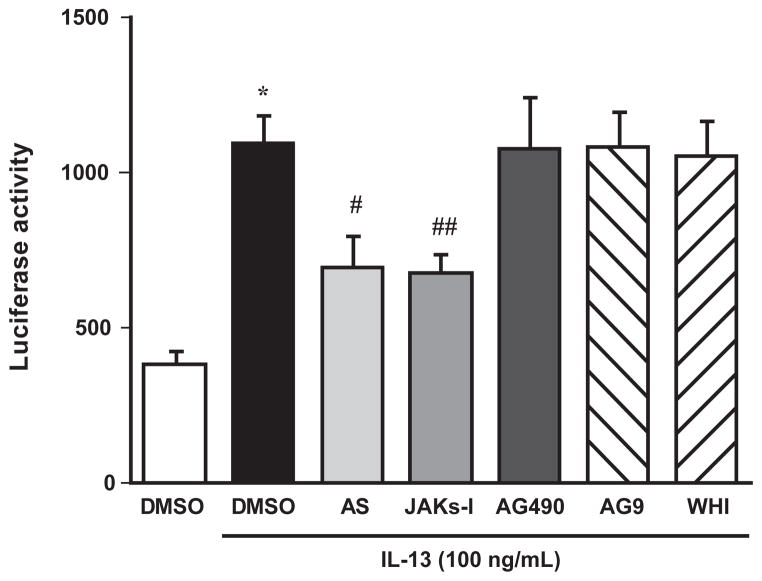
Using the D3 construct, which contains only the proximal STATs-binding site (see Fig. 2), the effects of inhibition of STAT6 and various JAKs on the IL-13–induced increase in promoter activity were examined. As shown in Fig. 3, tyrphostin-AG490 (a selective JAK2 inhibitor), WHI-P131 (a selective JAK3 inhibitor), or tyrphostin- AG9 (a selective Tyk2 inhibitor) had no effect on the IL-13–induced increase in luciferase activity. However, the IL-13–increased promoter activity was significantly inhibited by a non-selective JAKs inhibitor, JAK Inhibitor- I (Fig. 3). A significant inhibition equal to JAK Inhibitor-I was also observed when cells were treated with a selective inhibitor, AS1517499 (Fig. 3).
Fig. 3.
Effects of various pharmacological inhibitors on the IL-13– induced increase in promoter activity of the rat RhoA gene. Cultured human bronchial smooth muscle cells were transfected with a luciferase reporter plasmid D3 (see Fig. 2), and luciferase assays were performed with (closed column) or without (open column) stimulation with 100 ng/mL of IL-13 in the presence or absence of various pharmacological inhibitors. AS: AS1517499 (100 nM, a selective STAT6 inhibitor), JAKs-I: JAK Inhibitor-I (1 μM, a non-selective JAKs inhibitor), AG490: tyrphostin-AG490 (50 μM, a selective JAK2 inhibitor), AG9: tyrphostin-AG9 (50 μM, a selective Tyk2 inhibitor), WHI: WHI-P131 (100 μM, a selective JAK3 inhibitor), and DMSO: dimethyl sulfoxide (0.1%, vehicle for the inhibitors). Each column represents the mean ± S.E.M. from 5 independent experiments. *P < 0.05 vs. no IL-13 treatment (open column), #P < 0.05 and ##P < 0.01 vs. DMSO–IL-13 group (closed column) by Bonferroni/Dunn’s test.
We have previously demonstrated an upregulation of RhoA in BSM of rats with antigen-induced airway hyperresponsiveness (1, 5). In the present study, we elucidated here for the first time, to our knowledge, the transcriptional regulation of the rat RhoA gene by identifying its promoter region and the transcription-initiation site. The reporter gene analyses indicated that the most proximal STATs-binding region (192- to 184-bp upstream of the transcription-initiation site) is indispensable to the induction of RhoA transcription by IL-13 stimulation. Pharmacological studies also revealed that the activation of JAK1–STAT6 signaling is involved in the transcriptional activation of the rat RhoA gene induced by IL-13.
In the present study, the 5′-RACE analysis identified the transcription-initiation site of the rat RhoA gene at 66-bp upstream of the reference mRNA sequence (Gen- Bank BC061732, Fig. 1), that is, 243-bp upstream of its translation start codon. The reporter gene analyses suggested the importance of the most proximal STATsbinding region in the IL-13–induced increase in promoter activity (Fig. 2). In Homo sapiens, the transcription-initiation site of the human RhoA gene has already been published at −276-bp upstream of its translation start codon (GenBank NM_001664). Although the promoter of the human RhoA gene is not fully understood until now to our knowledge, the analysis of the 5′-flanking region of the human RhoA gene using the TFSEARCH program indicated that it also contains STATs-binding sites: −78- to −70-bp (score 85.6), −191- to −183-bp (score 80.8), −271- to −263-bp (score 86.5), and −518- to −510-bp (score 84.6) upstream of its transcription-initiation site. It is thus possible that, in an analogous fashion, the promoter activity of the human RhoA gene might also be regulated by the IL-13–STAT6 signaling pathway. Indeed, IL-13 has an ability to cause an upregulation of RhoA via an activation of STAT6 in cultured hBSMCs (6, 11). Because antigen challenge causes an increase in IL-13 in the airways both in the rat model and patients with allergic bronchial asthma (12, 13), the rat model may be useful for the development of new drugs for the treatment of the disease.
The current findings that the IL-13–induced increase in promoter activity of the rat RhoA gene was significantly inhibited by AS1517499 (Fig. 3) also strongly support the involvement of STAT6 in its transcriptional regulation. AS1517499 is a novel selective STAT6 inhibitor synthesized by Nagashima and colleagues (14) based on the structure of a reported STAT6 inhibitor, TMC-264, discovered from the fungus Phoma (15). The previous study revealed that the AS1517499 concentration used (100 nM) completely inhibited the IL-13–induced phosphorylation of STAT6 in the cultured hBSMCs (6). However, as shown in Fig. 3, the inhibitory effect of AS1517499 on the IL-13–induced increase in promoter activity was only partial. We have previously reported that, in addition to the activation of STAT6, IL-13 is capable of activating NF-κB in the cultured hBSMCs (16). Indeed, the D3 construct (Fig. 2) also contains a putative NF-κB binding region at −197- to −188-bp upstream of the transcription-initiation site (score 83.4). An activation of NF-κB may also be involved in the increased promoter activity induced by IL-13.
STAT6 is one of the major signal transducers activated by the IL13Rα1–IL4Rα receptor complex with IL-13 (17). The association of IL13Rα1 and IL4Rα chains phosphorylates and activates JAKs, and then the activated JAKs phosphorylate and activate STAT6. In mammals, the four members of the JAK family, JAK1, JAK2, JAK3, and Tyk2, are known to be associated with specific cytokine receptors. However, little is known about the major JAK activated by IL-13 in BSM cells. We thus used four inhibitors, tyrphostin-AG490, WHI-P131, and tyrphostin-AG9, which are specific for JAK2 (8), JAK3 (9), and Tyk2 (10), respectively, and JAK Inhibitor-I, which can inhibit all JAKs including JAK1 (7). Because a specific JAK1 inhibitor is currently not available, JAK Inhibitor-I (a non-specific JAKs inhibitor) was used in the present study. As shown in Fig. 3, the IL-13–induced increase in the promoter activity was significantly inhibited by JAK Inhibitor-I but not by tyrphostin-AG490, WHI-P131, or tyrphostin-AG9. These findings suggest that JAK1 is the major kinase that intermediates IL-13–STAT6 signaling in cultured hBSMCs. On the other hand, the inhibitory effect of JAK Inhibitor-I was also only partial (Fig. 3). The finding also supports an involvement of signaling other than the JAK1–STAT6 in the increased promoter activity induced by IL-13.
In summary, we have performed 5′-RACE analysis and identified the transcription-initiation site of the rat RhoA gene located at 66-bp upstream of the reference RhoA mRNA sequence, GenBank BC061732. The reporter gene analyses revealed that IL-13 has an ability to activate RhoA transcription: the most proximal STATsbinding region is indispensable to the IL-13–induced RhoA transcription. The IL-13–induced increase in the promoter activity was inhibited by AS1517499 (a selective STAT6 inhibitor) and JAK Inhibitor-I (a non-selective JAKs inhibitor), but not by tyrphostin-AG490 (a selective JAK2 inhibitor), WHI-P131 (a selective JAK3 inhibitor), or tyrphostin-AG9 (a selective Tyk2 inhibitor). These findings suggest that IL-13 is capable of up- regulating RhoA via an activation of the JAK1–STAT6 pathway in BSM cells.
Acknowledgments
This work was partly supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1.Chiba Y, Takada Y, Miyamoto S, Mitsui-Saito M, Karaki H, Misawa M. Augmented acetylcholine-induced, Rho-mediated Ca2+ sensitization of bronchial smooth muscle contraction in antigen-induced airway hyperresponsive rats. Br J Pharmacol. 1999;127:597–600. doi: 10.1038/sj.bjp.0702585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiba Y, Ueno A, Shinozaki K, Takeyama H, Nakazawa S, Sakai H, et al. Involvement of RhoA-mediated Ca2+ sensitization in antigen-induced bronchial smooth muscle hyperresponsiveness in mice. Respir Res. 2005;6:Art. No. 4. doi: 10.1186/1465-9921-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshii A, Iizuka K, Dobashi K, Horie T, Harada T, Nakazawa T, et al. Relaxation of contracted rabbit tracheal and human bronchial smooth muscle by Y-27632 through inhibition of Ca2+ sensitization. Am J Respir Cell Mol Biol. 1999;20:1190–1200. doi: 10.1165/ajrcmb.20.6.3441. [DOI] [PubMed] [Google Scholar]
- 4.Kume H. RhoA/Rho-kinase as a therapeutic target in asthma. Curr Med Chem. 2008;15:2876–2885. doi: 10.2174/092986708786242831. [DOI] [PubMed] [Google Scholar]
- 5.Chiba Y, Sakai H, Wachi H, Sugitani H, Seyama Y, Misawa M. Upregulation of rhoA mRNA in bronchial smooth muscle of antigen-induced airway hyperresponsive rats. J Smooth Muscle Res. 2003;39:221–228. doi: 10.1540/jsmr.39.221. [DOI] [PubMed] [Google Scholar]
- 6.Chiba Y, Todoroki M, Nishida Y, Tanabe M, Misawa M. A novel STAT6 inhibitor AS1517499 ameliorates antigen-induced bronchial hypercontractility in mice. Am J Respir Cell Mol Biol. 2009;41:516–524. doi: 10.1165/rcmb.2008-0163OC. [DOI] [PubMed] [Google Scholar]
- 7.Thompson JE, Cubbon RM, Cummings RT, Wicker LS, Frankshun R, Cunningham BR, et al. Photochemical preparation of a pyridone containing tetracycle: a Jak protein kinase inhibitor. Bioorg Med Chem Lett. 2002;12:1219–1223. doi: 10.1016/s0960-894x(02)00106-3. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchiya Y, Takahashi N, Yoshizaki T, Tanno S, Ohhira M, Motomura W, et al. A Jak2 inhibitor, AG490, reverses lipin-1 suppression by TNF-alpha in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2009;382:348–352. doi: 10.1016/j.bbrc.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Kumar N, Mishra J, Narang VS, Waters CM. Janus kinase 3 regulates interleukin 2-induced mucosal wound repair through tyrosine phosphorylation of villin. J Biol Chem. 2007;282:30341– 30345. doi: 10.1074/jbc.C600319200. [DOI] [PubMed] [Google Scholar]
- 10.Sugimoto N, Nakahira M, Ahn HJ, Micallef M, Hamaoka T, Kurimoto M, et al. Differential requirements for JAK2 and TYK2 in T cell proliferation and IFN-gamma production induced by IL-12 alone or together with IL-18. Eur J Immunol. 2003;33:243–251. doi: 10.1002/immu.200390027. [DOI] [PubMed] [Google Scholar]
- 11.Chiba Y, Nakazawa S, Todoroki M, Shinozaki K, Sakai H, Misawa M. Interleukin-13 augments bronchial smooth muscle contractility with an up-regulation of RhoA protein. Am J Respir Cell Mol Biol. 2009;40:159–167. doi: 10.1165/rcmb.2008-0162OC. [DOI] [PubMed] [Google Scholar]
- 12.Bodey KJ, Semper AE, Redington AE, Madden J, Teran LM, Holgate ST, et al. Cytokine profiles of BAL T cells and T-cell clones obtained from human asthmatic airways after local allergen challenge. Allergy. 1999;54:1083–1093. doi: 10.1034/j.1398-9995.1999.00889.x. [DOI] [PubMed] [Google Scholar]
- 13.Prieto J, Lensmar C, Roquet A, van der Ploeg I, Gigliotti D, Eklund A, et al. Increased interleukin-13 mRNA expression in bronchoalveolar lavage cells of atopic patients with mild asthma after repeated low-dose allergen provocations. Respir Med. 2000;94:806–814. doi: 10.1053/rmed.2000.0826. [DOI] [PubMed] [Google Scholar]
- 14.Nagashima S, Yokota M, Nakai E, Kuromitsu S, Ohga K, Takeuchi M, et al. Synthesis and evaluation of 2-{[2-(4-hydroxyphenyl)-ethyl]amino}pyrimidine-5-carboxamide derivatives as novel STAT6 inhibitors. Bioorg Med Chem. 2007;15:1044–1055. doi: 10.1016/j.bmc.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai M, Nishio M, Yamamoto K, Okuda T, Kawano K, Ohnuki T. TMC-264, a novel inhibitor of STAT6 activation produced by Phoma sp. TC 1674. J Antibiot (Tokyo) 2003;56:513–519. doi: 10.7164/antibiotics.56.513. [DOI] [PubMed] [Google Scholar]
- 16.Goto K, Chiba Y, Misawa M. IL-13 induces translocation of NFkappaB in cultured human bronchial smooth muscle cells. Cytokine. 2009;46:96–99. doi: 10.1016/j.cyto.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]