Abstract
Background
Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis is hypothesized to be an important pathway linking socioeconomic position and chronic disease.
Purpose
This paper tests the association between education and the diurnal rhythm of salivary cortisol.
Methods
Up to 8 measures of cortisol (mean of 5.38 per respondent) over two days were obtained from 311 respondents, aged 18–70, drawn from the 2001–2002 Chicago Community Adult Health Study. Multi-level models with linear splines were used to estimate waking level, rates of cortisol decline, and area-under-the-curve over the day, by categories of education.
Results
Lower education (0–11 years) was associated with lower waking levels of cortisol, but not the rate of decline of cortisol, resulting in a higher area-under-the-curve for more educated respondents throughout the day.
Conclusions
This study found evidence of lower cortisol exposure among individuals with less education and thus does not support the hypothesis that less education is associated with chronic over-exposure to cortisol.
Introduction
Health disparities by socioeconomic position have been well-established in the U.S. for a variety of outcomes including mortality, cardiovascular disease, functional impairment, and dementia (1, 2). Less clear is specifically how social factors get translated into biological risk. Individuals of lower socioeconomic position are thought to face increased exposure to environmental and psychological stressors, resulting in chronic activation of stress-related biological pathways (3, 4). Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, responsible for regulating cortisol secretion, is hypothesized to be an important biological pathway mediating the effects of socioeconomic position on health outcomes. Cortisol is a key regulator of a wide array of cardiovascular, metabolic, immunologic, and other homeostatic functions, and elevated cortisol levels are associated with abdominal obesity, cardiovascular disease, immunosuppression, as well as hippocampal atrophy in the elderly (5–8).
Cortisol typically follows a strong diurnal rhythm, with high levels in the morning that peak 30–45 minutes after waking, dropping rapidly for the next several hours and declining slowly throughout the rest of the day, until a low point of around midnight (9, 10). Cortisol secretion is also pulsatile in nature, adding additional variability to measurement as the pulses are super-imposed over the underlying circadian rhythm (11). Overall, considerable variation in the diurnal pattern exists across individuals, as well as within individuals across different days (12, 13), creating challenges for measurement, especially when cortisol collection is infrequent. In modeling cortisol as an outcome, different features of the diurnal pattern have been examined including the slope of the diurnal curve from peak to trough, the size of the cortisol awakening response, levels of morning and/or evening cortisol levels, and measures of free cortisol release over the day such as area-under-the-curve.
Results from studies examining the relationship between socioeconomic position and cortisol have been mixed; see (14) for a review. Analysis of 200 British Whitehall Study participants aged 45–58 years found that average cortisol over the workday was significantly higher for lower occupational grade men but significantly lower for lower occupational grade women, with no association between socioeconomic position and levels of morning or evening cortisol (15). A study of 767 adults aged 35–65 years in Germany found lower levels of morning salivary cortisol concentrations with lower levels of education and occupational status (16). Data from 6335 participants in the 1958 British birth cohort study with saliva collected at age 45 years found that lower lifetime socioeconomic position was associated with a greater risk of extreme post-waking values and higher area-under-the-curve as measured during the first half of the day (17). A recent study of life-course socioeconomic position and cortisol in 732 Swedish adults found a higher cortisol awakening response and lower evening levels for those with lower early life socioeconomic position, with no effect for area-under-the-curve (18).
In the U.S., Cohen et al. recently found that lower income and education were associated with a flatter diurnal rhythm due to higher levels of cortisol during the evening and at bedtime in a sample of 781 middle-aged adults in the CARDIA study (19). In a different study, Cohen et al. found that lower levels of income and education were associated with higher levels of total cortisol concentration over the day in a sample of 193 adults ages 21 to 55 (20). No statistically significant differences in slopes at different times of the day by socioeconomic position were found, leading to the conclusion that the difference in overall concentration was attributable to small differences accumulating over the day. In a sample of 188 women aged 18–54 receiving welfare benefits in Michigan, higher levels of material hardship were associated with a lower morning rise in cortisol and a subsequently smaller decline throughout the day (21). A recent study of 935 participants in the Multi-Ethnic Study of Atherosclerosis similarly found that lower income respondents had lower levels of wake-up cortisol and a less steep decline across the day (22).
Despite evidence that multiple days of collection are needed for reliable inter-individual comparisons in cortisol secretion (12, 13), few studies of socioeconomic position and cortisol have utilized more than one day of collection (20), and only one recent study has used multi-level models to test associations between socioeconomic position and cortisol accounting for intra and inter-individual differences in cortisol secretion (22). Moreover, inconsistencies in the literature on socioeconomic position and cortisol may result from analytical methods that fail to capture salient features of the diurnal profile. Regression-spline models with appropriately placed knots offer a potentially useful analytical strategy for such data where the rate of change is unlikely to be constant throughout the day (23). This paper utilizes multi-level modeling in combination with non-parametric spline techniques to better characterize the shape of the diurnal pattern and test whether level of education is related to cortisol secretion in a sample of 311 adults from the city of Chicago.
METHODS
Study Population
Up to four cortisol samples for each of two consecutive days were obtained from a biomarker sub-sample of 455 respondents, drawn from 80 focal neighborhoods in the larger Chicago Community Adult Health Study conducted over 2001 and 2002. The larger survey was conducted through face-to-face interviews with a multistage probability sample of 3,105 adults aged 18 or more years, living in the city of Chicago, and stratified into 343 neighborhood clusters previously defined by the Project on Human Development in Chicago Neighborhoods (24). Full details of the Chicago Community Adult Health Study design have been reported elsewhere (25). The response rate to this survey of 72 percent was one of the highest in a major population-based survey in American city in recent decades (26). Of the 3105 overall participants, all 1145 living in 80 focal neighborhoods were selected to participate in the biomarker study. Of these, 629 (55%) agreed to participate in saliva self-collection and having blood drawn by a trained phlebotomist. Younger respondents were less likely to participate in the biomarker portion of the study, but after adjustment for age there were no significant differences between those participating in the biomarker study and overall Chicago Community Adult Health Study sample in race/ethnicity, education, marital status, or functional limitations. Of the 629 participants in the biomarker study, 455 (72.3%) returned saliva samples from in-home collection. Of the 455 respondents who returned samples, 311 had one or more assayable samples within acceptable limits for cortisol values (between 0 and 2 ug) and with valid collection time records, for an effective participation rate of 27%. Hispanics were less likely to both return at all and return usable saliva samples, but no other differences by sample characteristics were noted. Half of all respondents (n=156) had 7–8 valid samples, and about a sixth of the respondents (n=53) had 2 or fewer valid samples. In all, 1674 valid cortisol measures were available from the 311 respondents who had any valid measures. Although the timing of collection varied across respondents, the distribution of sample times suggests that respondents largely adhered to the protocol. Nearly a third of the observations (n=543) were collected within one hour after awakening, with roughly half of these collected within 20 minutes of awakening. About 300 observations were collected in the period 13–16 hours after awakening, presumably before bedtime. Mean levels of cortisol between 0–20 minutes after waking, between 30–60 minutes after waking, and between 13–15 hours after waking were 0.36, 0.41, and 0.24 ug/dL respectively.
Sociodemographic and psychosocial data were collected on the full Chicago Community Adult Health Study sample during the interview portion of the study. A shortened 11-item version of the Center for Epidemiologic Studies Depression Scale (CES-D) (27) was administered to all respondents. Trained survey interviewers directly measured height and weight. All subjects gave informed consent for both the interviews and the saliva/blood collection, and these studies were approved by the University of Michigan School of Public Health Institutional Review Board. Respondents were asked to collect four saliva samples using salivettes on each of two consecutive weekdays at waking, 30 minutes after waking, 45–60 minutes before dinner, and right before going to bed. Participants were instructed not to brush their teeth, eat, drink, or smoke, until after their first saliva sample. Participants recorded information on waking and sleeping times and the time each sample was taken by the respondent on both a data collection sheet and directly on the collection tubes. Samples were returned by mail to the data tracking center at the University of Michigan and frozen at −20°C until assay, where they were thawed and spun in the laboratory immediately before assay. Samples were assayed using DPC Coat-a-Count tubes (Diagnostic Products Corporation, Los Angeles, California) and 200 μL of saliva per tube, following manufacturer’s directions. Interassay variability was 10%.
Measures
Education was first categorized into three groups: 0–11, 12–15, and 16 or more years of completed education. Education was subsequently dichotomized into two categories, < 12 years of education (28% of sample), and 12 years or more (72%) after analyses showed no differences between the top two education groups with regard to cortisol output (results available upon request). Race/ethnicity was categorized into 3 categories as white, non-Hispanic black, and other. Body mass Index (BMI) was calculated as (weight in kg)/(height in m2). Age, BMI, and CES-D scores were entered into regression models as continuous variables, but were stratified for selected preliminary analyses as follows: age categorized as 18–39, 40–69, and 70+; BMI as normal (18–24.9), overweight (25–29.9), and obese (30 and greater); and respondents with a modified CES-D score in the highest quartile were identified as depressed for these analyses (28).
Statistical analysis
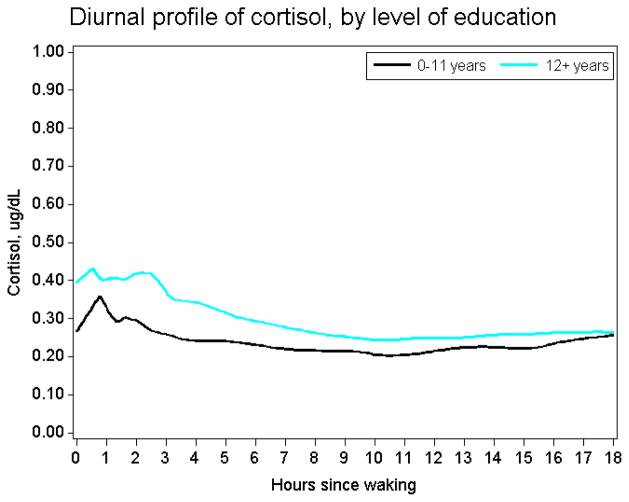
Exploratory t-tests were done to statistically compare mean levels of cortisol across education groupings; these t-tests were conducted for the full sample as well as across various stratifications (by age group, sex, race, BMI level, and depression level). Because the mean is inadequate as a summary measure of daily cortisol variations, these analyses were extended to non-parametric loess-based methods and regression spline models that characterize the entire diurnal profile of cortisol. Non-parametric loess-based models were used to obtain cortisol values as a function of time by level of education, without making a priori assumptions about the functional form of the relationship (Figure 1). Visual examination of loess plots suggested possible inflections at 40 minutes, 3, 6 and 15 hours after waking; knots were set at each of these four points in the more formal regression spline models. Inflection points are more likely be detected statistically where data density is high, which for our sample was at the beginning and end of the day. The inflection points at 3 and 6 hours after awakening occurred at a point of lower density, but were included to be conservative. Their removal from the model did not alter the basic finding that there was a significant difference in the waking level of cortisol across education groups Next, regression spline models of cortisol (μg/dL) against education, with respondent-specific random intercept and random slopes (up to second inflection), estimated by restricted maximum likelihood, were used to estimate waking level and rate of change in cortisol by years of education during the first 15 hours after waking. Slopes were allowed to vary across educational groups. Models were adjusted for age, sex, race, body-mass index, and depression score. Area-under-the-curve was estimated using the trapezoidal rule. Sensitivity analyses examined the effect of various categorizations of education.
Figure 1.
Loess-based diurnal profiles of cortisol in lower and higher education respondents
Results
Table 1 presents characteristics of the full sample and by education. Respondents with < 12 years of education had lower daily mean levels of cortisol than respondents with 12 or more years of education (0.258 μg/dL vs. 0.326 μg/dL, p-value=0.049). This difference was consistent (although not always statistically different) across most age, sex, race, BMI and depression score categories. Loess-based models indicated that those with <12 years education had uniformly lower cortisol profiles than those with >=12 years of education (Figure 1). Figure 1 also suggests potentially different morning inflection points across the two groups, falling around 30 minutes for the higher education group and 45 minutes for the lower education group. Table 2 presents regression spline models allowing for change in cortisol slopes at 40 minutes, 3, 6, and 15 hours after waking, and adjusted for age and sex. This model indicates that most of the difference between educational groups was the result of lower awakening levels of cortisol among respondents with lower education (0.295 μg/dL vs. 0.408 μg/dL, p for difference=0.039). Adjusting for multiple respondent characteristics (i.e., age, sex, race, BMI, waking time, depression) did not attenuate the educational differences in the awakening level of cortisol (remaining at 0.113 μg/dl) (Table 3). Education was not associated with the rate of cortisol decline in these models over any part of the day, either in minimally or fully adjusted models. Consequently, higher educated respondents maintained elevated cortisol levels throughout the day. Over a period of 20 hours, the estimated area-under-the-curve for respondents with lower education was 3.08 μg/dL hours, compared to 4.22 μg/dL hours in the other group. A related measure, the cortisol awakening response cortisol awakening response, calculated as the estimated area-under-the-curve from wake up time to the peak, was also lower for respondents with lower education (0.81) compared to those with > 12 years of education (1.16). However, the difference in level between higher and lower educated respondents remained constant through the day. The average difference between the peak and the trough values of cortisol over the day was almost identical across education levels, at .41 for either category.
Table 1.
Characteristics of full sample, and by education
| Full sample | By education | ||||
|---|---|---|---|---|---|
|
| |||||
| Respondent characteristics | 0–11 years of education | 12 or more years of education | Mean educational difference in average daily cortisol level (μg/dL) | p-value for difference | |
| Number of respondents | 311 | 87 | 224 | - | |
| Number of valid measures (per respondent) | 1674 (5.38) | 482 (5.54) | 1192 (5.32) | - | |
| Mean wake-up time | 6:05 | 5:48 | 6:08 | - | 0.42 |
| Mean (SD) cortisol level (μg/dL) | .306(0.26) | .258 (0.23) | .326 (0.27) | −0.06 (−0.12 – 0.01) | 0.05 |
|
| |||||
| Sex | |||||
|
| |||||
| %Female | 180 (57.6%) | 51 (58.6%) | 128 (57.1%) | −0.04 (−0.12 – 0.03) | 0.26 |
| %Male | 131 (42.4%) | 36 (41.4%) | 96 (42.9%) | −0.08 (−0.18 – 0.03) | 0.10 |
|
| |||||
| Race-ethnicity | |||||
|
| |||||
| %White | 140 (45%) | 24 (27.6%) | 116 (51.8%) | −0.10 (−0.17 – 0.02) | 0.01 |
| %Black | 123 (39.2%) | 45 (51.7%) | 77 (34.4%) | −0.01 (−0.11 – 0.09) | 0.82 |
| %Other | 48 (15.8%) | 18 (20.7%) | 31 (13.8%) | −0.10 (−0.23 – 0.02) | 0.11 |
|
| |||||
| Age | |||||
|
| |||||
| 18–39 years | 133 (42.8%) | 24 (27.6%) | 109 (48.7%) | −0.03 (−0.18 – 0.11) | 0.64 |
| 40–69 years | 144 (46.3%) | 47 (54%) | 97 (43.3%) | −0.08 (−0.14 – 0.01) | 0.03 |
| 70 years | 4 (10.9%) | 16 (18.4%) | 18 (8%) | −0.01 (−0.15 – 0.13) | 0.87 |
|
| |||||
| BMI categories | |||||
|
| |||||
| Normal (18–25) | 100 (32.2%) | 20 (23%) | 80 (35.7%) | 0 (−0.14 – 0.14) | 0.97 |
| Overweight (25–30) | 93 (29.9%) | 26 (29.9%) | 67 (29.9%) | −0.07 (−0.19 – 0.5) | 0.27 |
| Obese (>30) | 118 (37.9%) | 41 (47.1%) | 77 (34.4%) | −0.08 (-−.15 – 0.01) | 0.04 |
|
| |||||
| CES-D score | |||||
|
| |||||
| < 75th percentile | 283 (91%) | 71 (81.6%) | 212 (94.6%) | −0.05 (−0.12 – 0.02) | 0.15 |
| >= 75th percentile | 28 (9%) | 16 (18.4%) | 12 (5.4%) | −0.09 (−0.21 – 0.03) | 0.12 |
Table 2.
Parameter Estimates from regression spline model adjusted for age and sex (N=311)
| 0–11 years of education | 12 or more years of education | Difference (low - high education) | p for difference | |
|---|---|---|---|---|
| Estimated intercept | 0.295 (0.048) | 0.408 (0.03) | 0.113 (0.054) | 0.04 |
| Estimated slope from 0–1 hours (μg/dL/hr) | 0.064 (0.092) | 0.081 (0.049) | 0.017 (0.105) | 0.87 |
| Estimated slope from 1–3 hours (μg/dL/hr) | −0.024 (0.025) | −0.027 (0.02) | −0.003 (0.032) | 0.93 |
| Estimated slope from 3–6 hours (μg/dL/hr) | −0.034 (0.018) | −0.042 (0.016) | −0.008 (0.024) | 0.73 |
| Estimated slope from 6–15 hours (μg/dL/hr) | 0.008 (0.005) | 0.001 (0.003) | −0.007 (0.006) | 0.25 |
Table 3.
Parameter estimates from regression spline model adjusted for age, sex, race, BMI, waking time, CES-D score (N=311)
| 0–11 years of education | 12 or more years of education | Difference (low - high education) | p for difference | |
|---|---|---|---|---|
| Estimated intercept | 0.280(0.05) | 0.393 (0.034) | 0.113 (0.055) | 0.04 |
| Estimated slope from 0–1 hours (μg/dL/hr) | 0.065 (0.092) | 0.081 (0.049) | 0.016 (0.105) | 0.88 |
| Estimated slope from 1–3 hours (μg/dL/hr) | −0.025 (0.025) | −0.027 (0.02) | −0.002 (0.032) | 0.95 |
| Estimated slope from 3–6 hours (μg/dL/hr) | −0.033 (0.018) | −0.042 (0.016) | −0.009 (0.024) | 0.71 |
| Estimated slope from 6–15 hours (μg/dL/hr) | 0.008 (0.005) | 0.001 (0.003) | −0.007 (0.006) | 0.26 |
| Estimated Area-under-the curve from 0–20 hours (μg/dLhours) | 3.08 | 4.22 | −1.14 |
In sensitivity analyses (not shown), we examined various categorization schemes for education. Although in general, there was a dose-response effect of education on the regression parameters, the strongest difference was seen in the move from <=11 to 12 or more years of education, suggesting a possible threshold effect at this level.
Discussion
Elucidating the biological mechanisms linking socioeconomic position and health outcomes is an active area of research. This study looks at adult socioeconomic and cortisol in a diverse population-based sample of adults from Chicago across a wide age range, finding that lower educational levels were associated with a lowering of the overall diurnal cortisol profile. While most socioeconomic position and cortisol studies to date have relied on specialized samples that might make results difficult to generalize, the current study draws from a population-based sample. A major strength of this study is the use of non-parametric spline techniques to better identify the salient features of the diurnal pattern of secretion for this sample. An additional strength includes sampling on more than one day and the use of multi-level models to account for within and between person variation in cortisol patterns.
Limitations of this study include variation in collection times, which made one particularly time-sensitive feature of the diurnal cortisol pattern, the cortisol awakening response, difficult to detect. The time between waking and recording a “waking” cortisol sample has been shown to have important implications for the cortisol awakening response, with a delay of more than 10 minutes resulting in almost no cortisol awakening response on average (29). New methods of compliance monitoring using microchips to record the opening of collection vials could help to minimize these problems, although these technologies are currently expensive for use in population based research (10). While timing is especially important for the cortisol awakening response, over the rest of the day, variability in sample collection times was in fact advantageous for characterizing the diurnal pattern in more detail (23). From the overall focal sample of 1145 individuals, the percentage of individuals both agreeing to participate in the saliva collection and returning usable samples was quite low (27%). While our sample was slightly older and less likely to be Hispanic than the overall sample, remaining measured sociodemographic and health characteristics did not differ. It is still possible that those who did not participate or successfully return usable samples were different in ways we did not observe, which may limit the generalizability of our findings. Given the measurement error associated with cortisol patterns, our sample size of 311 may also have limited our ability to detect a more finely graded association with education. Another limitation was the use of years of education as our single measure of socioeconomic position, something necessitated by the large fraction of respondents missing income data in our sample. While using years of education as a marker of socioeconomic position likely captures more permanent attributes of lifelong socioeconomic position, it is limited in its ability to reflect more current or transitory features of socioeconomic position that may contribute to the stressors and cortisol response of our sample. Our study is also cross-sectional in nature, so we cannot rule out the possibility that genetics or early life factors influence both educational attainment and adult cortisol response. Future studies that measure both socioeconomic position and cortisol throughout the life course can help shed light on these issues.
This paper’s findings contribute to the inconsistent findings in the literature concerning the association between socioeconomic position and the diurnal pattern of cortisol secretion (14). To our knowledge, this study is the first to identify lower total free cortisol release among those with lower socioeconomic position as measured by area- under-the-curve. Previous work has found a higher area-under-the-curve among those with lower socioeconomic position or no association. In the CARDIA study, a higher area-under-the-curve was found as a result of similar morning levels but higher evening cortisol levels for those with lower socioeconomic position (19), which appears to also be the case in the analysis of Li et al. with respect to morning and mid-day values (17). In another study, Cohen et al. found non-significantly higher levels of cortisol with lower socioeconomic position for each of seven cortisol measurements throughout the day, attributing the area-under-the-curve difference to small difference accumulating over the day (20). The Cohen study found no significant association between socioeconomic position and slope, suggesting a uniformly higher pattern for those with lower socioeconomic position, in contrast to the uniformly lower pattern found here. Recent results from the Multi Ethnic Study of Atherosclerosis found no association between income and wealth and area-under-the-curve, but those with the lowest levels of income and wealth had lower wake-up cortisol levels compared with the highest group, consistent with our results (22).
Our findings of an overall lower diurnal cortisol pattern for those with less education are consistent with the findings of Brandtstadter, et al, who found that lower socioeconomic position was associated with lower levels of morning and afternoon cortisol in a sample of German adults, with no differences found in evening levels (16). Steptoe, et al also reported a uniformly lower pattern for women with lower socioeconomic position in the Whitehall II studies, but the pattern was reversed for men (15). Other studies have shown a blunted but higher diurnal pattern of cortisol secretion in those with lower socioeconomic position. Ranjit, et al found no differences in waking levels but flatter slopes over the course of the day for women reporting increased material hardship (21).
Overall, existing discrepancies in the literature on socioeconomic position and salivary cortisol likely result from great variation in the collection and methods of analysis of salivary cortisol data. Intra-individual variation in patterns of cortisol secretion is known to be high, especially around time of awakening, making inter-individual comparisons based on a single day of collection likely to be noisy (13). Of previous studies examining socioeconomic position and diurnal patterns of salivary cortisol secretion, only three collected measures for more than one day (20, 22, 30), and only the recent Multi Ethnic Study of Atherslerosis study (22) utilized a multi-level approach to separate out variability within or across individuals (13).
These findings also highlight the lack of clarity in the literature on socioeconomic position and cortisol with respect to current research on cortisol and health outcomes. While earlier theoretical and animal models focused on chronic overexposure to cortisol as pathogenic, increasing evidence suggests that cortisol deviations in either direction are potentially harmful to health, and the importance of cortisol elevations or declines depends on the condition of interest (31). The same can be said for the relationship between chronic stress and cortisol output, which for many years assumed that HPA activity always increased with stress. More recently, critical features of chronic stress that impact the direction of the HPA activity-stress relationship have emerged, including the time elapsed since stressor onset and the controllability of the stressor, among others(31). While some work has begun investigating more specific elements of socioeconomic position and chronic stress with regards to cortisol such as job demands, job control, and relationship functioning (29, 32–36), overall efforts to link socioeconomic position to specific stressors and their implications for cortisol is lagging behind similar work in psychology and psychoneuroendocrinology. The net result of this lack of theoretical clarity has been to interpret any differences in the profile of cortisol secretion by socioeconomic position as supportive of the hypothesis that cortisol dysregulation is a link between low socioeconomic position and poor health outcomes, even if the direction of the socioeconomic differences in cortisol are found to be in the opposite direction in different studies.
In the current paper, the finding that lower socioeconomic position is associated with a uniformly lower cortisol profile could potentially be explained in two ways: 1.) lower socioeconomic position is not associated with higher levels of chronic stress and subsequent increased HPA-activation as commonly proposed; 2.) lower socioeconomic position is associated with higher levels of chronic stress, but the long term chronic stress reflected in lower educational attainment results in a blunted diurnal rhythm and hypocortisolism, or chronic under-production of cortisol. The current study is consistent with both of these scenarios, and understanding which one better reflects reality will rely on continued progress in understanding the relationships between chronic stress and HPA-axis functioning (31). While the current study has improved upon previous studies of socioeconomic position and cortisol associations that did not model the important within and between person differences in cortisol secretion, future investigations of socioeconomic position and cortisol patterns should test more specific hypotheses about how chronic and acute stressors associated with socioeconomic position impact HPA-axis activity.
Acknowledgments
This research was supported, in part, by Grant P50HD38986 and R01HD050467 from the National Institute of Child Health and Human Development.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health: No easy solution. Journal of the American Medical Association. 1993;269:3140–5. [PubMed] [Google Scholar]
- 2.Kaplan GAHM, Syme SL, Minkler M, Winkleby M. Socioeconomic status and health. Am J Prev Med. 1987;3:125–29. [Google Scholar]
- 3.Kristenson M, Eriksen HR, Sluiter JK, Starke D, Ursin H. Psychobiological mechanisms of socioeconomic differences in health. Social Science & Medicine. 2004;58:1511–22. doi: 10.1016/S0277-9536(03)00353-8. [DOI] [PubMed] [Google Scholar]
- 4.Almeida DM, Neupert SD, Banks SR, Serido J. Do Daily Stress Processes Account for Socioeconomic Health Disparities? J Gerontol B Psychol Sci Soc Sci. 2005;60:S34–9. doi: 10.1093/geronb/60.special_issue_2.s34. [DOI] [PubMed] [Google Scholar]
- 5.Walker BR. Glucocorticoids and Cardiovascular Disease. Eur J Endocrinol. 2007;157:545–59. doi: 10.1530/EJE-07-0455. [DOI] [PubMed] [Google Scholar]
- 6.Cacioppo JT, Kiecolt-Glaser JK, Malarkey WB, Laskowski BF, Rozlog LA, Poehlmann KM, Burleson MH, Glaser R. Autonomic and Glucocorticoid Associations with the Steady-State Expression of Latent Epstein-Barr Virus. Hormones and Behavior. 2002;42:32–41. doi: 10.1006/hbeh.2002.1801. [DOI] [PubMed] [Google Scholar]
- 7.Roberge C, Carpentier AC, Langlois M-F, Baillargeon J-P, Ardilouze J-L, Maheux P, Gallo-Payet N. Adrenocortical dysregulation as a major player in insulin resistance and onset of obesity. Am J Physiol Endocrinol Metab. 2007;293:E1465–78. doi: 10.1152/ajpendo.00516.2007. [DOI] [PubMed] [Google Scholar]
- 8.Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NPV, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 9.Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free Cortisol Levels after Awakening: A Reliable Biological Marker for the Assessment of Adrenocortical Activity. Life Sciences. 1997;61:2539–49. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- 10.Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. PNAS. 2006;103:17058–63. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young EA, Abelson J, Lightman SL. Cortisol pulsatility and its role in stress regulation and health. Frontiers in Neuroendocrinology. 2004;25:69–76. doi: 10.1016/j.yfrne.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology. 2007;32:80–6. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Hruschka DJ, Kohrt BA, Worthman CM. Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;30:698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Dowd JB, Simanek AM, Aiello AE. Socio-economic status, cortisol and allostatic load: a review of the literature. Int J Epidemiol. 2009 doi: 10.1093/ije/dyp277. dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steptoe A, Kunz-Ebrecht S, Owen N, Feldman PJ, Willemsen G, Kirschbaum C, Marmot M. Socioeconomic Status and Stress-Related Biological Responses Over the Working Day. Psychosom Med. 2003;65:461–70. doi: 10.1097/01.psy.0000035717.78650.a1. [DOI] [PubMed] [Google Scholar]
- 16.Brandtstadter J, Baltes-Gotz B, Kirschbaum C, Hellhammer D. Developmental and personality correlates of adrenocortical activity as indexed by salivary cortisol: Observations in the age range of 35 to 65 years. Journal of Psychosomatic Research. 1991;35:173–85. doi: 10.1016/0022-3999(91)90072-v. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Power C, Kelly S, Kirschbaum C, Hertzman C. Life-time socio-economic position and cortisol patterns in mid-life. Psychoneuroendocrinology. 2007;32:824–33. doi: 10.1016/j.psyneuen.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson PE, Janlert U, Theorell T, Hammarstrom A. Life-course socioeconomic trajectories and diurnal cortisol regulation in adulthood. Psychoneuroendocrinology. 2010;35:613–23. doi: 10.1016/j.psyneuen.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic Status, Race, and Diurnal Cortisol Decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- 20.Cohen S, Doyle WJ, Baum A. Socioeconomic Status Is Associated With Stress Hormones. Psychosom Med. 2006;68:414–20. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- 21.Ranjit N, Young EA, Kaplan GA. Material hardship alters the diurnal rhythm of salivary cortisol. Int J Epidemiol. 2005;34:1138–43. doi: 10.1093/ije/dyi120. [DOI] [PubMed] [Google Scholar]
- 22.Hajat A, Diez-Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N, Castro C, Watson K, Sanchez B, Kirschbaum C. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2010;35:932–43. doi: 10.1016/j.psyneuen.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranjit N, Young EA, Raghunathan TE, Kaplan GA. Modeling cortisol rhythms in a population-based study. Psychoneuroendocrinology. 2005;30:615–24. doi: 10.1016/j.psyneuen.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Raudenbush SW, Sampson RJ. Ecometrics: Toward a Science of Assessing Ecological Settings, With Application to the Systematic Social Observation of Neighborhoods. Sociological Methodology. 1999;29:1–41. [Google Scholar]
- 25.Morenoff JD, House JS, Hansen BB, Williams DR, Kaplan GA, Hunte HE. Understanding social disparities in hypertension prevalence, awareness, treatment, and control: The role of neighborhood context. Social Science & Medicine. 2007;65:1853–66. doi: 10.1016/j.socscimed.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galea S, Tracy M. Participation Rates in Epidemiologic Studies. Annals of Epidemiology. 2007;17:643–53. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–93. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 29.Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–28. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 30.Decker SA. Salivary cortisol and social status among Dominican men. Horm Behav. 2000;38:29–38. doi: 10.1006/hbeh.2000.1597. [DOI] [PubMed] [Google Scholar]
- 31.Miller GE, Chen E, Zhou ES. If it goes up, must it come down?. Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 32.Steptoe A, Siegrist J, Kirschbaum C, Marmot M. Effort--Reward Imbalance, Overcommitment, and Measures of Cortisol and Blood Pressure Over the Working Day. Psychosom Med. 2004;66:323–9. doi: 10.1097/01.psy.0000126198.67070.72. [DOI] [PubMed] [Google Scholar]
- 33.Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13:653–76. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- 34.Kunz-Ebrecht SR, Kirschbaum C, Steptoe A. Work stress, socioeconomic status and neuroendocrine activation over the working day. Social Science & Medicine. 2004;58:1523–30. doi: 10.1016/S0277-9536(03)00347-2. [DOI] [PubMed] [Google Scholar]
- 35.Evolahti A, Hultcrantz M, Collins A. Women’s work stress and cortisol levels: A longitudinal study of the association between the psychosocial work environment and serum cortisol. Journal of Psychosomatic Research. 2006;61:645–52. doi: 10.1016/j.jpsychores.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 36.Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]