Abstract
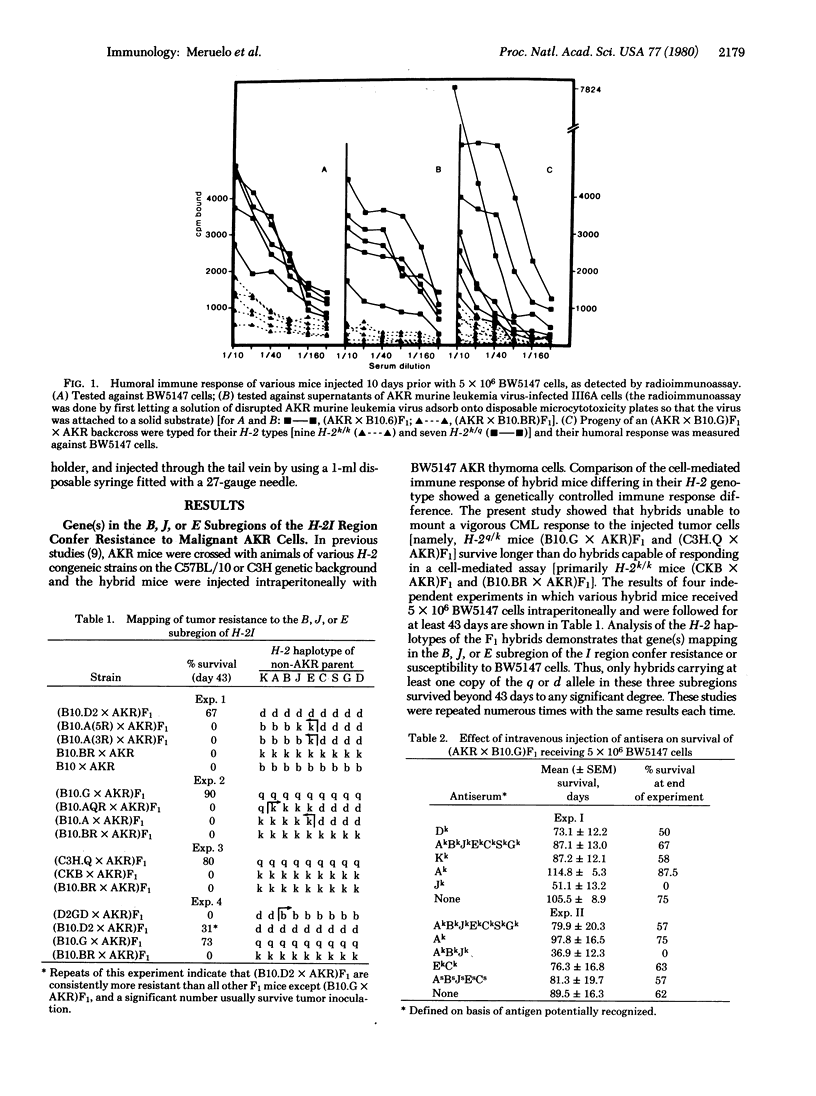
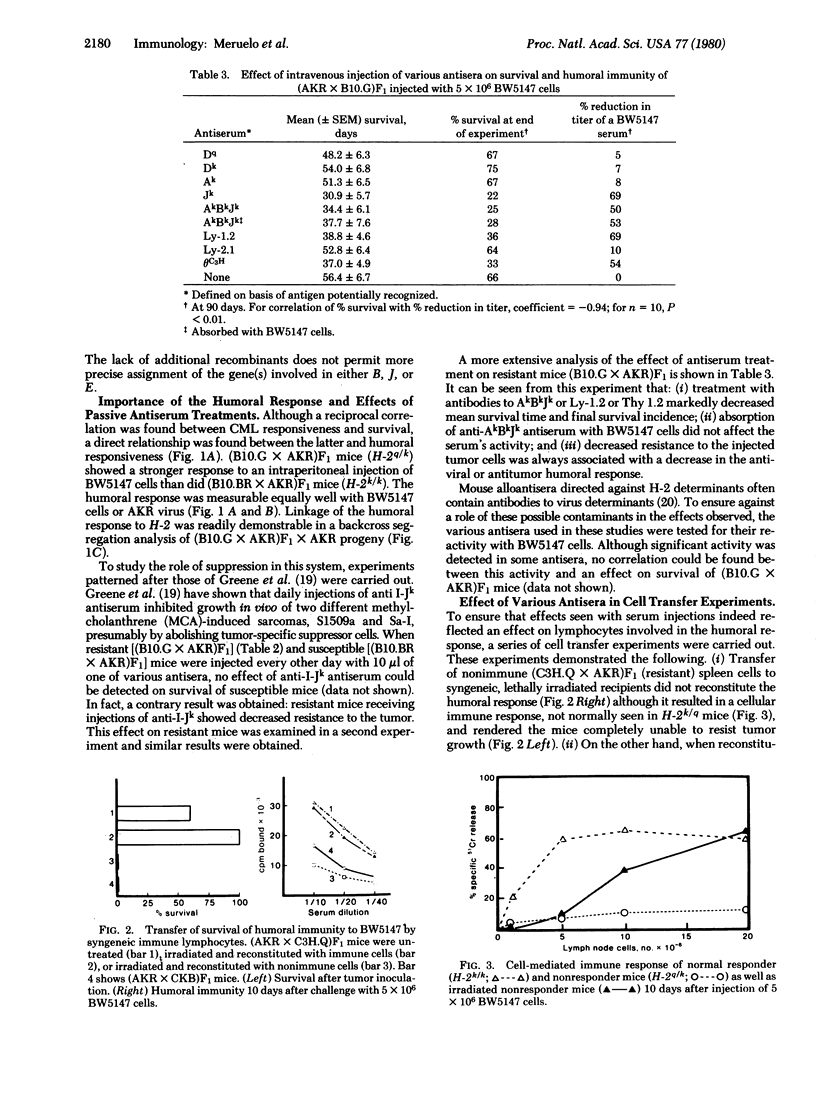
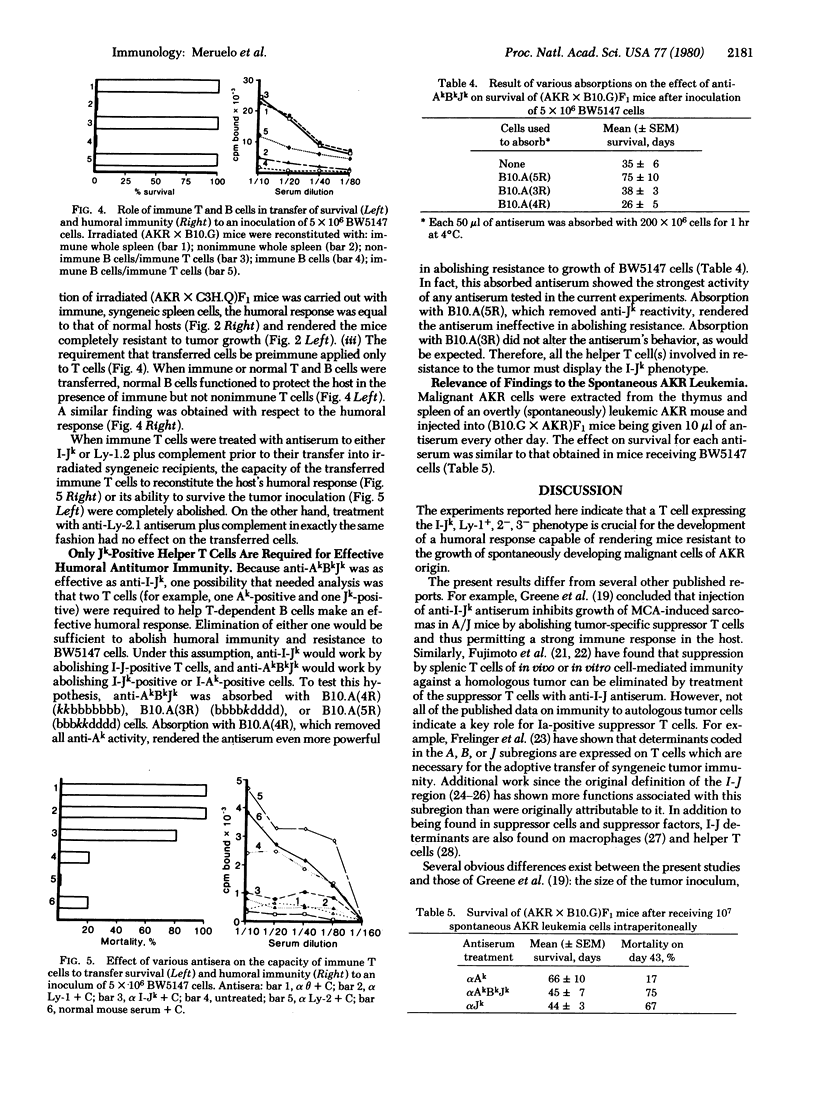
This paper provides evidence for the involvement of immune mechanisms in conferring resistance to a spontaneous AKR leukemia. It is shown that genes in the B, J, or E subregions of the H-2 complex confer resistance to a spontaneously arisen, tissue culture-adapted AKR thymoma, BW5147. A direct correlation is demonstrated between survival to injected BW5147 cells and humoral responsiveness in various hybrids obtained from crosses of AKR mice and C57BL/10 or C3H/DiSn derived congeneic strains differing at H-2. Cellular immunity appears to play no role in resistance to the proliferation of tumor cells. It is further established that development of effective humoral immunity depends on B cells and Ly-1+, 2-, 3- helper T-cells bearing the I-Jk phenotype. These findings seem directly applicable to the spontaneous disease, and results of studies using transformed cells from an overtly leukemic AKR mouse parallel those obtained using BW1547 cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki T., Boyse E. A., Old L. J. Occurrence of natural antibody to the G (gross) leukemia antigen in mice. Cancer Res. 1966 Jul;26(7):1415–1419. [PubMed] [Google Scholar]
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Doig D., Chesebro B. Anti-Friend virus antibody is associated with recovery from viremia and loss of viral leukemia cell-surface antigens in leukemic mice. Identification of Rfv-3 as a gene locus influencing antibody production. J Exp Med. 1979 Jul 1;150(1):10–19. doi: 10.1084/jem.150.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A. Multigene family of endogenous retroviruses: recombinant origin of diversity. J Natl Cancer Inst. 1978 Sep;61(3):625–638. [PubMed] [Google Scholar]
- Frelinger J. A., Niederhuber J. E., Shreffler D. C. Effects of anti-Ia sera on mitogenic responses. III. Mapping the genes controlling the expression of Ia determinants on concanavalin A-reactive cells to the I-J subregion of the H-2 gene complex. J Exp Med. 1976 Oct 1;144(4):1141–1146. doi: 10.1084/jem.144.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S., Matsuzawa T., Nakagawa K., Tada T. Cellular interaction between cytotoxic and suppressor T cells against syngeneic tumors in the mouse. Cell Immunol. 1978 Jul;38(2):378–387. doi: 10.1016/0008-8749(78)90068-0. [DOI] [PubMed] [Google Scholar]
- Greene M. I., Dorf M. E., Pierres M., Benacerraf B. Reduction of syngeneic tumor growth by an anti-I-J-alloantiserum. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5118–5121. doi: 10.1073/pnas.74.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Klinman N. R., Pickard A. R., Sigal N. H., Gearhart P. J., Metcalf E. S., Pierce S. K. Assessing B cell diversification by antigen receptor and precursor cell analysis. Ann Immunol (Paris) 1976 Jun-Jul;127(3-4):489–502. [PubMed] [Google Scholar]
- LILLY F., BOYSE E. A., OLD L. J. GENETIC BASIS OF SUSCEPTIBILITY TO VIRAL LEUKAEMOGENESIS. Lancet. 1964 Dec 5;2(7371):1207–1209. doi: 10.1016/s0140-6736(64)91043-8. [DOI] [PubMed] [Google Scholar]
- Lilly F. The role of genetics in Gross virus leukemogenesis. Bibl Haematol. 1970;(36):213–220. doi: 10.1159/000391710. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O., Bodmer W. F. Protein clinical manifestations of primary tumors of the heart. Am J Med. 1972 Jan;52(1):1–8. doi: 10.1016/0002-9343(72)90002-2. [DOI] [PubMed] [Google Scholar]
- Meruelo D. A role for elevated H-2 antigen expression in resistance to neoplasia caused by radiation-induced leukemia virus. Enhancement of effective tumor surveillance by killer lymphocytes. J Exp Med. 1979 Apr 1;149(4):898–909. doi: 10.1084/jem.149.4.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D., Deak B., McDevitt H. O. Genetic control of cell-mediated responsiveness to an AKR tumor-associated antigen: mapping of the locus involved to the I region of the H-2 complex. J Exp Med. 1977 Nov 1;146(5):1367–1379. doi: 10.1084/jem.146.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D., McDevitt H. O. Recent studies on the role of the immune response in resistance to virus-induced leukemias and lymphomas. Semin Hematol. 1978 Oct;15(4):399–419. [PubMed] [Google Scholar]
- Meruelo D., Nimelstein S. H., Jones P. P., Lieberman M., McDevitt H. O. Increased synthesis and expression of H-2 antigens on thymocytes as a result of radiation leukemia virus infection: a possible mechanism for H-2 linked control of virus-induced neoplasia. J Exp Med. 1978 Feb 1;147(2):470–487. doi: 10.1084/jem.147.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Herzenberg L. A., Okumura K., Herzenberg L. A., McDevitt H. O. A new I subregion (I-J) marked by a locus (Ia-4) controlling surface determinants on suppressor T lymphocytes. J Exp Med. 1976 Sep 1;144(3):699–712. doi: 10.1084/jem.144.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Shrefler D. C. Cross-reactivity between H-2K and H-2D products. I. Evidence for extensive and reciprocal serological cross-reactivity. J Exp Med. 1975 Feb 1;141(2):374–391. doi: 10.1084/jem.141.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Klein P. A. Anomalous reactions of mouse alloantisera with cultured tumor cells. II. Cytotoxicity is caused by antibodies to leukemia viruses. J Immunol. 1975 Nov;115(5):1261–1268. [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. SPECIFICITY OF ISOANTISERA AGAINST LEUKAEMIC AND THYMIC LYMPHOCYTES. Nature. 1963 Dec 28;200:1332–1333. doi: 10.1038/2001332b0. [DOI] [PubMed] [Google Scholar]
- Tada T., Takemori T., Okumura K., Nonaka M., Tokuhisa T. Two distinct types of helper T cells involved in the secondary antibody response: independent and synergistic effects of Ia- and Ia+ helper T cells. J Exp Med. 1978 Feb 1;147(2):446–458. doi: 10.1084/jem.147.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Taniguchi M., David C. S. Properties of the antigen-specific suppressive T-cell factor in the regulation of antibody response of the mouse. IV. Special subregion assignment of the gene(s) that codes for the suppressive T-cell factor in the H-2 histocompatibility complex. J Exp Med. 1976 Sep 1;144(3):713–725. doi: 10.1084/jem.144.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]



