Abstract
Objective: Hyaluronic acid sodium salt gel 0.2% is a topical device effective in reducing skin inflammation. Facial seborrheic dermatitis, characterized by erythema and or flaking/scaling in areas of high sebaceous activity, affects up to five percent of the United States population. Despite ongoing studies, the cause of the condition is yet unknown, but has been associated with yeast colonization and resultant immune-derived inflammation. First-line management typically is with topical steroids as well as the immunosuppressant agents pimecrolimus and tacrolimus. The objective of this study was to evaluate the efficacy and safety of a topical anti-inflammatory containing low-molecular weight hyaluronic acid. Design and setting: Prospective, observational, non-blinded safety and efficacy study in an outpatient setting. Participants: Individuals 18 to 75 years of age with facial seborrheic dermatitis. Measurements: Outcome measures included scale, erythema, pruritus, and the provider global assessment, which were all measured on a five-point scale. Subjects were assessed at Baseline, Week 2, Week 4, and Week 8. Results: Interim data for 7 of 15 subjects are presented. Hyaluronic acid sodium salt gel 0.2% was shown through visual grading assessments to improve the provider global assessment by 47.62 percent from Baseline to Week 4. Reductions in scale, erythema, and pruritus were 66.67, 50, and 60 percent, respectively at Week 4. At Week 8, the provider global assessment was improved from baseline in 100 percent of subjects. Conclusion: Treatment with topical low-molecular weight hyaluronic acid resulted in improvement in the measured endpoints. Topical low-molecular weight hyaluronic acid is another option that may be considered for the treatment of facial seborrheic dermatitis in the adult population. Compliance and tolerance were excellent.
Facial seborrheic dermatitis is a very common presenting problem in dermatology patients. The papulosquamous disorder is usually found on the scalp, face, or trunk, all of which are sebum-rich areas.1 The condition is associated with an abnormal immune response and is caused by a reduction in helper T cells, phytohemagglutinin, concanavalin stimulation, and antibody titers.2 Common therapies often include a topical steroid, which can have untoward effects, such as atrophy, telangiectasias, acne, and perioral dermatitis.3 Novel, steroid-sparing agents for facial seborrheic dermatitis are a welcome addition to the treatment armamentarium of the dermatologist.
Hyaluronic acid, a neutral salt, is a naturally occurring, highly conserved polysaccharide found in the skin’s tissue.4 High molecular weight hyaluronic acid (HMWHA), a ubiquitous component of the stratum corneum, is depolymerized into low molecular weight hyaluronic acid (LMWHA) fragments in the presence of inflammation or tissue injury. LMWHA fragments are biologically active as demonstrated by their ability to trigger built-in immune defense mechanisms and promote cytokine production. The fate, and ultimately the effectiveness, of substances applied on the surface of the epidermis is dependent on their ability to penetrate the stratum corneum.2 A moist environment provided by LMWHA’s hydrophilic nature allows for affects on cellular behavior. Numerous medical applications, including wound care, have seen improvements as a result of leukocyte, fibroblast, and endothelial cell migration and activation.5 The interaction in the extracellular matrix provides a backbone for proteoglycans and an association with collagen and fibrin. Biological interactions of hyaluronic acid include effects on cell proliferation, recognition, and locomotion along with angiogenesis and inflammatory cell activity. The components break down products that actively stimulate the production of beta-defensin 2 (DEFβ2) and induce an antibacterial response allowing for healing in the skin’s epithelium.2
Methods
A single-site, prospective, observational study of hyaluronic acid sodium salt gel 0.2% (Bionect Hydrogel, Innocutis Holdings, Charleston, South Carolina) for the treatment of facial seborrheic dermatitis was conducted beginning in February 2012. All subjects were expected to have completed their Week 8 visits by September 2012. All adult subjects were enrolled in a single cohort. The sponsor, subjects, and study personnel were not blinded. The trial was conducted in accordance with the principles of Good Clinical Practices and the Declaration of Helsinki (2000). Each participant provided written informed consent prior to beginning study-related procedures. Hyaluronic acid sodium salt gel 0.2% was provided to subjects along with Cetaphil Gentle Cleanser (Galderma Laboratories, LP, Ft. Worth, Texas). Subjects were instructed to cleanse the affected area with Cetaphil Gentle Cleanser and pat dry with a soft towel before applying the provided medication. A thin layer of medication was to be gently massaged into the affected areas twice daily, in the morning and evening. Treatment was continued for four weeks. After four weeks, subjects discontinued use of the study medication and continued the cleansing regimen for an additional four weeks. Each subject was followed for eight weeks with evaluation visits at Baseline, Week 2, Week 4, and Week 8. At each visit, degrees of scale, erythema, and pruritus were evaluated, physician’s global assessment (PGA) of facial seborrheic dermatitis was performed, and medication reconciliation completed. Photographs were taken at each visit.
Selection of subjects. Eligibility criteria included the following: age 18 to 75, skin type I to IV, healthy men or women, mild-to-moderate facial seborrheic dermatitis diagnosis, willingness and ability to comply with regimen of using hyaluronic acid sodium salt gel 0.2% twice daily to the affected area during the study, willingness and ability to comply with all follow-up requirements of the study, and completion of the informed consent. Exclusion criteria included the following: active, localized, or systemic infection; compromised immune system; known allergy to lotions or moisturizers; unlikelihood of complying with the protocol; pregnancy or breastfeeding; use of products for seborrheic dermatitis within the last two weeks; refusal to sign the informed consent document or photo release document; refusal to comply with all follow-up requirements; treatment with systemic steroids; and current treatment with a medication that causes flushing.
Efficacy assessment. Clinical evaluation consisted of scored parameters (Table 1). The clinical evaluation was performed at Baseline, Week 2, Week 4, and Week 8. Patients who discontinued study therapy due to ineffectiveness were considered to have treatment failure.
TABLE 1.
Clinical evaluation parameters
| SCALE | 0—Absent | 1—Mild | 2—Moderate | 3—Severe | 4—Very severe |
| ERYTHEMA | 0—Absent | 1—Mild | 2—Moderate | 3—Severe | 4—Very severe |
| PRURITUS | 0—Absent | 1—Mild | 2—Moderate | 3—Severe | 4—Very severe |
| PROVIDER GLOBAL ASSESSMENT | 0—Clear | 1—Almost clear | 2—Mild | 3—Moderate | 4—Severe |
Safety assessment. Adverse events were not anticipated or experienced. In the event of an adverse event, hyaluronic acid sodium salt gel 0.2% would have been discontinued at the discretion of the investigators.
Patient-reported outcome. Subjects were each provided with an end-of-study questionnaire to complete. The questionnaire evaluated the subject’s opinion of the overall experience with hyaluronic acid sodium salt gel 0.2% as well as the patient’s opinion regarding the effectiveness of the treatment.
Statistical analysis. The study was designed as a prospective observational trial to test the efficacy and tolerability of hyaluronic acid sodium salt gel 0.2% in facial seborrheic dermatitis. Analysis will involve all subjects enrolled in the study who complete the eight-week trial period according to study protocol. Analysis will be included in the final report of this study to allow for analysis of all 15 subjects. Analysis was not completed as part of this interim report.
Results
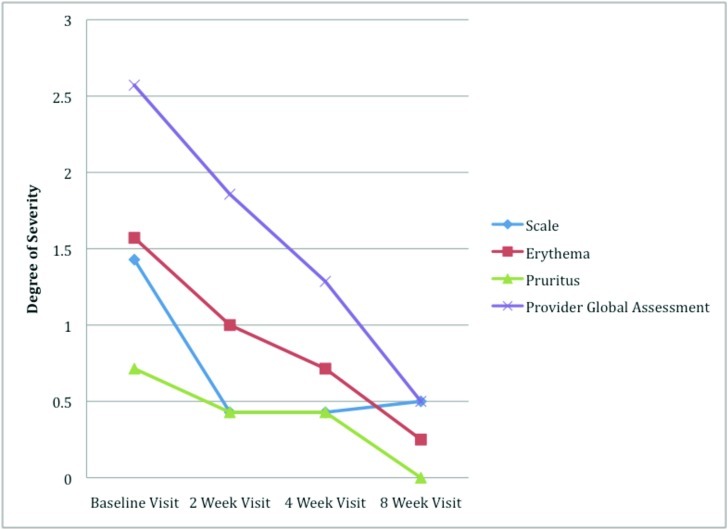
Study subjects. Nine of 15 subjects were enrolled, and four completed the study. Seven subjects completed the Week 4 visit. Two subjects voluntarily withdrew due to scheduling conflicts. All subjects received hyaluronic acid sodium salt gel 0.2%. Subjects demonstrated improvement at the Week 4 visit (Figures 1 and 2). The mean reductions in scale, erythema, and pruritus were 66.7, 50, and 60 percent, respectively (Figure 3). The PGA improved by 47.6 percent from Baseline to Week 4 and 79.2 percent from Baseline to Week 8. At Week 8, all subjects showed some level of improvement in their PGA from Baseline.
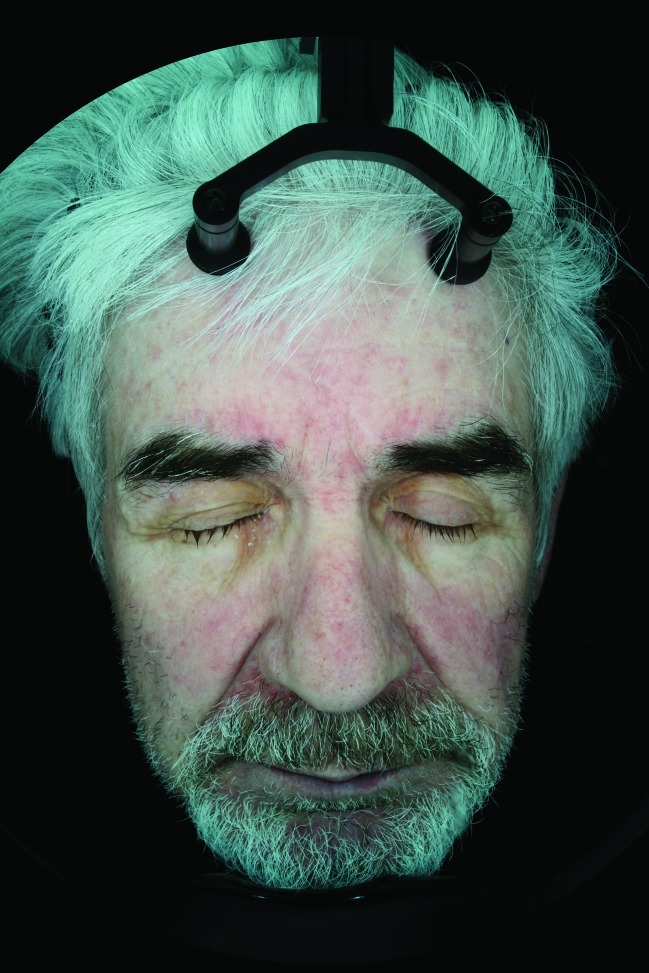
Figure 1.
Subject 001 baseline polar light image
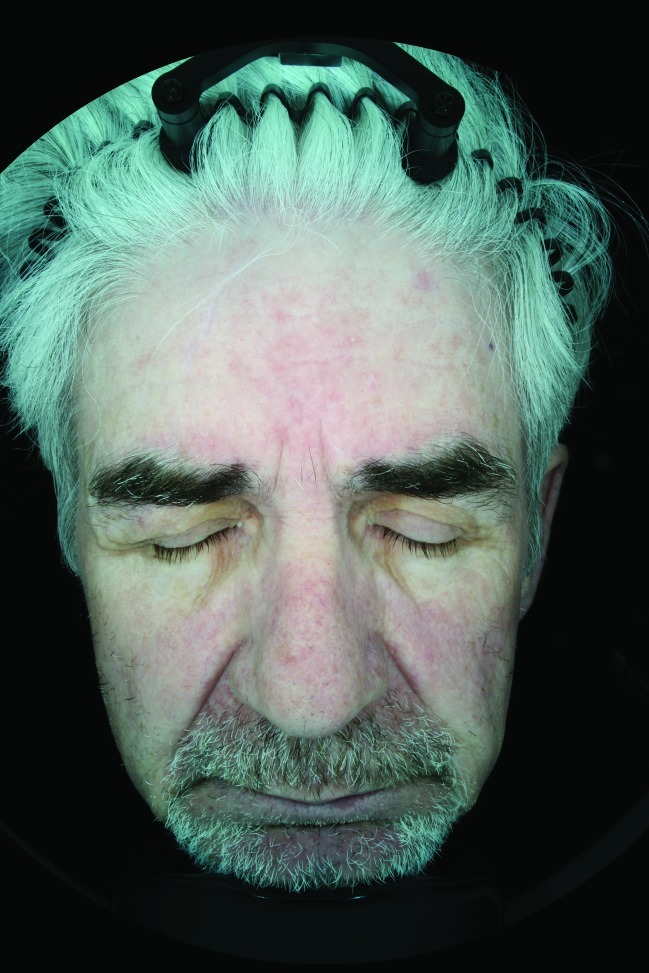
Figure 2.
Subject 001 Week 4 polar light image
Figure 3.
Degree of severity across eight weeks of treatment
Subject end-of-study questionnaires were scored. When subjects were asked their opinion regarding their overall treatment experience, the mean subject response was that they liked the product. Subjects evaluated the effectiveness of the hyaluronic acid sodium salt gel 0.2%. The mean subject response was that they agreed the hyaluronic acid sodium salt gel 0.2% was effective in treating their seborrheic dermatitis.
Efficacy. All subjects discontinued use of the hyaluronic acid sodium salt gel 0.2% at the Week 4 visit. At Week 8, after four weeks without further seborrheic dermatitis treatment, scale, erythema, and pruritus evaluation results remained the same or continued to improve in 75, 100, and 100 percent of patients, respectively. PGA remained the same or improved in 100 percent of patients during the four-week period between Week 4 and Week 8.
Safety. As of the date of this interim data, no adverse events were reported. Tolerability of the hyaluronic acid sodium salt gel 0.2% was excellent. No subjects experienced any adverse events, such as skin reactions, and no subjects were discontinued because of an adverse event.
Discussion
This study evaluated the efficacy and tolerability of hyaluronic acid sodium salt gel 0.2% in the treatment of facial seborrheic dermatitis. All subjects were placed in a single cohort following the same fixed treatment strategy.
Using the interim data of this study, the application of hyaluronic acid sodium salt gel 0.2% twice a day after facial cleansing improved the scale, erythema, pruritus, and PGA of subjects diagnosed with facial seborrheic dermatitis. In a related study examining the efficacy of pimecrolimus cream 1% compared to vehicle, the percentage of patients with at least one adverse event reported was 32.7 and 46.8 percent in the vehicle and pimecrolimus groups, respectively.7 Hyaluronic acid sodium salt gel 0.2% had a favorable adverse event profile in comparison. Some consideration may be given to the vehicle in the aforementioned study, as it was also associated with an elevated percentage of adverse events. Given the restriction of subjects with facial seborrheic dermatitis, further study is warranted to evaluate the efficacy in other body areas.
DEFβ2 is a peptide observed in epithelial cells and is induced by bacterial infection and is widely believed to have antimicrobial properties. In the event of skin injury, LMWHA fragments are released into the skin by the process of degradation of the extracellular matrix.6 According to Gariboldi et al,2 LMWHA is a potent inducer of DEFβ2 when applied topically to the skin. These authors have also shown that skin treated with increasing concentrations of LMWHA induce a progressively higher antimicrobial effect when compared to untreated skin. The stimulation of skin innate immunity by LMWHA is a potential mechanism by which hyaluronic acid sodium salt gel 0.2% exerts its beneficial effects in reducing the signs and symptoms of seborrrheic dermatitis.
This study is weakened by its small cohort and by design as a prospective observational trial, its short follow-up interval, and unblinded nature. Additional studies with a larger number of subjects and a longer follow-up period are necessary to further delineate the safety and efficacy of hyaluronic acid sodium salt gel 0.2%.
In addition to the improvement of the visual evaluation parameters, patient responses to the hyaluronic acid sodium salt gel 0.2% were positive. Patients liked the gel and strongly agreed that it was effective in treating their facial seborrheic dermatitis.
Footnotes
DISCLOSURE:Dr. Schlesinger serves as a consultant for Innocutis Holdings, Inc. Financial support for this study was provided by Innocutis Holdings, Inc.
References
- 1.Cicek D, Kandi B, Bakar S, Turgut D. Pimecrolimus 1% cream, methylprednisolone aceponate 0.1% cream and metronidazole 0.75% gel in the treatment of seborrhoeic dermatitis: a randomized clinical study. J Dermatolog Treat. 2009;20(6):344–349. doi: 10.3109/09546630802687349. [DOI] [PubMed] [Google Scholar]
- 2.Gariboldi S, Palazzo M, Zanobbio L, et al. Low molecular weight hyaluronic acid increases the self- defense of skin epithelium by induction of ‚-defensin 2 via TLR2 and TLR4. J Immunol. 2008;181(3):2103–2110. doi: 10.4049/jimmunol.181.3.2103. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RA, Janusz CA, Janniger CK. Seborrheic dermatitis: an overview. Am Fam Physician. 20061;74(1):125–132. [PubMed] [Google Scholar]
- 4.Lee JH, Jung JY, Bang D. The efficacy of topical 0.2% hyaluronic acid gel on recurrent oral ulcers: comparison between recurrent aphthous ulcers and the oral ulcers of Behçet's disease. J Eur Acad Dermatol Venereol. 2008;22(5):590–595. doi: 10.1111/j.1468-3083.2007.02564.x. [DOI] [PubMed] [Google Scholar]
- 5.Price RD, Myers S, Leigh IM, Navsaria HA. The role of hyaluronic acid in wound healing: assessment of clinical evidence. Am J Clin Dermatol. 2005;6(6):393–402. doi: 10.2165/00128071-200506060-00006. [DOI] [PubMed] [Google Scholar]
- 6.Jiang D, Liang J, Noble PW. Hyaluronan in tissue and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 7.Warshaw E, Wohlhuter RJ, Parneix-Spake A. Results of a randomized, double-blind, vehicle-controlled efficacy trial of pimecrolimus cream 1% for the treatment of moderate to severe facial seborrheic dermatitis. J Am Acad Dermatol. 2007;57(2):257–264. doi: 10.1016/j.jaad.2006.11.007. [DOI] [PubMed] [Google Scholar]