Abstract
The transcription factor Nkx2-1 belongs to the homeobox-encoding family of proteins that have essential functions in prenatal brain development. Nkx2-1 is required for the specification of cortical interneurons and several neuronal subtypes of the ventral forebrain. Moreover, this transcription factor is involved in migratory processes by regulating the expression of guidance molecules. Interestingly, Nkx2-1 expression was recently detected in the mouse brain at postnatal stages. Using two transgenic mouse lines that allow prenatal or postnatal cell type-specific deletion of Nkx2-1, we show that continuous expression of the transcription factor is essential for the maturation and maintenance of cholinergic basal forebrain neurons in mice. Notably, prenatal deletion of Nkx2-1 in GAD67-expressing neurons leads to a nearly complete loss of cholinergic neurons and parvalbumin-containing GABAergic neurons in the basal forebrain. We also show that postnatal mutation of Nkx2-1 in choline acetyltransferase-expressing cells causes a striking reduction in their number. These degenerative changes are accompanied by partial denervation of their target structures and results in a discrete impairment of spatial memory.
Keywords: choline acetyltransferase, choreoathetosis, human, mouse, parvalbumin
Introduction
Mutations of homeobox genes expressed in the human forebrain may result in mental retardation, epilepsy and movement disorders (reviewed by Wigle & Eisenstat, 2008). For instance, haploinsufficiency of the transcription factor Nkx2-1 results in the so-called ‘brain-thyroid-lung syndrome’, a disease that emerges postnatally within the first few years of life (reviewed by Kleiner-Fisman & Lang, 2007). The prominent neurological symptoms include impairments of coordinated movements and a delayed development of speech (Breedveld et al., 2002; Krude et al., 2002). The specific symptoms suggest that the syndrome is caused by an abnormal development of the telencephalic parts of the basal ganglia (Krude et al., 2002; do Carmo Costa et al., 2005; Kleiner-Fisman & Lang, 2007).
During regionalization of the developing brain, multiple transcription factors are expressed in a specific temporal and spatial pattern (Schuurmans & Guillemot, 2002; Flames et al., 2007). Among them, the homeobox gene Nkx2-1 plays a critical role for cells generated in the hypothalamus and the medial ganglionic eminence (MGE), which contributes significantly to the development of various compartments of the telencephalon (Sussel et al., 1999; Marin et al., 2000; Garcia-Lopez et al., 2008). For instance, MGE-derived neurons differentiate into GABAergic interneurons that migrate tangentially to the cortical mantle and the striatum, or integrate locally to form the lateral globus pallidus (LGP; Sussel et al., 1999; Marin et al., 2000; Butt et al., 2005, 2008; Nobrega-Pereira et al., 2010). In addition, Nkx2-1 exerts an important function in controlling the migration of postmitotic interneurons by regulating guidance receptors for semaphorins (Nobrega-Pereira et al., 2008). In Nkx2-1 knockout mice, a substantial loss of cortical interneurons was observed together with severe morphological alterations of the striatum and various nuclei of the diencephalon (Kimura et al., 1996), whereas the gross brain morphology was described as normal for Nkx2-1+/− mice (Pohlenz et al., 2002). In addition, it has been shown that a postnatal synapsin I-driven deletion of the Nkx2-1 gene led to various vegetative dysfunctions (for reviews, see Son et al., 2003; Kim et al., 2006) due to alterations in the hypothalamus. However, no morphological or behavioural impairments were found in these conditional mutant mice that could be associated with telencephalic structures (Mastronardi et al., 2006).
Recently we showed that, predominantly, choline acetyltransferase (ChAT)-expressing neurons and parvalbumin (PV)-positive GABAergic neurons of the ventral forebrain maintain synthesis of Nkx2-1 throughout life (Magno et al., 2009). These findings, as well as the postnatal onset of neurological symptoms in humans, suggest that Nkx2-1 not only acts during prenatal brain development, but may also be needed for the postnatal maturation and maintenance of these neurons. We therefore generated mouse lines with cell type-specific ablation of Nkx2-1 during embryonic or postnatal development. Our results show that both prenatal and postnatal expression of Nkx2-1 is required for the maintenance of cholinergic basal forebrain neurons and their projections.
Materials and methods
Mouse lines, generation of conditional mice and genotyping
Mice expressing Cre-recombinase under the GAD67-promoter (GADcre/+) were kindly provided by H. Monyer (Heidelberg, Germany). The targeting vector used for generating these mice contained a fragment of the murine GAD67 gene spanning parts of exon 1 up to intron 6. The Cre-recombinase cDNA was placed 30 bp upstream of the translational start codon of GAD67, so that protein expression is driven by GAD67.
B6;129S6-Chattm1(cre)Lowl/J mice (ChATcre/+) were purchased from Jackson Laboratories (Charles River, Sulzfeld, Germany; stock number 6410). In these mice, Cre-recombinase expression is controlled by the endogenous gene promoter. The targeting vector was designed to insert an internal ribosome entry site-linked Cre recombinase gene (IRES-Cre) downstream of the stop codon of the ChAT gene. ChAT expression was described to be unaffected and Cre-recombinase activity was reported for all cholinergic neurons (Jackson Laboratories). The two knock-in mice were first bred with C57Bl/6 mice to obtain a uniform genetic background. Nkx2-1tm2Shk mice (Nkx2-1fl/fl) have been described in detail previously (Kusakabe et al., 2006).
To generate conditional mice with the Nkx2-1 deletion, the two Cre-lines were crossed with Nkx2-1fl/fl mice. Subsequently, Cre-positive mice of the F1 generation were bred with the homozygous floxed mice in order to delete Nkx2-1 in GAD67- and ChAT-positive cells, respectively.
The two Cre-lines and the mutants (GADcre/+;Nkx2-1fl/fl and ChATcre/+;Nkx2-1fl/fl) were also crossed with homozygous B6;129S4-Gt(ROSA)26Sortm1Sor/J mice (also known as ROSA26-LacZ reporter line; kindly provided by G. Willimsky, CBF, Berlin) to generate GADcre/+;R26R, ChATcre/+;R26R, GADcre/+;R26R; Nkx2-1fl/fl and ChATcre/+;R26R;Nkx2-1fl/fl mice.
All animals were housed on a 12/12-h light/dark cycle with access to food and water ad libitum and in accordance with institutional guidelines for animal welfare. Behavioural tests were performed according to the state authority of Berlin, Landesamt für Gesundheit und Soziales (LAGeSo; permit no. G 0004/09). The number of animals used for the different approaches, including their various survival times, is provided in the relevant sections of the results.
Mouse-tail biopsies were digested and processed by using an Invisorb Spin Tissue Mini kit (Invitek GmbH, Berlin, Germany). The following primers and annealing temperatures (Ta) were used to detect the Cre or floxed constructs in the genome – GAD67-Cre primers (designed by A. Vogt, Heidelberg, Germany) 5′-CCGGCGCCCCTTAGCTGTGAG-3′ and 5′-GGTTCTTGCGAACCT-CAT-CACTCG-3′ (Ta = 63 °C); ChAT-Cre primers (designed by Jackson Laboratories) 5′-GTTTGCA-GAAGCGGTGGG-3′, 5′-AGATAG-ATAATGAGAGGCTC-3′ and 5′-CCTTCTATCGCCTTCTTGACG-3′ (Ta = 55 °C); Nkx2-1-floxed primers (two Neo primers previously described by Kusakabe et al., 2006); internal primer designed by K. Paulick (MDC, Berlin-Buch) – 5′-TGCCGTGTAAACACGAGGAC-3′, 5′-GACTCTCAAGCAAGTC-CATCC-3′ and 5′-GAAGTGGCGAAAGCTAC-AGG-3′ (Ta = 55 °C).
The PCR conditions consisted of an initial activation step of 5 min at 95 °C and 35 cycles of 30 s of denaturation at 94 °C, 30 s of annealing (see above for specific Ta), and 30 or 40 s extension at 72 °C, and a final extension of 5 min at 72 °C.
Human tissue
We investigated tissue of the basal ganglia of two human brains from routine autopsies (males – 21 and 42 years old). The samples were obtained in accordance with local ethical committee guidelines. The subjects died of non-neurological causes.
The samples were sliced fresh into thick slabs (1 cm), frozen in liquid nitrogen and then stored at −80 °C. The slabs containing parts of the basal ganglia were cut with the cryostat into 100-μm-thick coronal sections that were serially collected in cold 0.1 M phosphate buffer.
Histochemistry and immunofluorescence
Brains of E14.5 and E16.5 embryos and newborns were dissected out of the skull and fixed overnight by immersion in a fixative containing 4% paraformaldehyde (PFA) and 0.1% glutaraldehyde in 0.1% phosphate buffer (PB). For fine-structural analysis the brains were further processed as described below.
All other animals (from P2 onward) assigned to morphological analyses were anaesthetized and transcardially perfused with 4% PFA in PB as described in detail (Magno et al., 2009). Animals assigned to X-gal staining were perfused with a fixative containing 4% PFA and 0.2% glutaraldehyde in PB. The brains were subsequently cut coronally (E14.5 to P2 – 100 μm; all others – 50 μm) on a vibratome and the sections were stored in PB. After treatment with 0.6% H2O2 in 0.1 M PB for 20 min, the ‘free floating’ sections were pre-incubated in a solution containing 5 or 10% normal serum of the species in which the secondary antibody was raised (see secondary antibodies below), 5% bovine serum albumin, and 0.2% Triton-X in 0.1 M PB for 1 h at room temperature. After incubation with primary antibodies (see below) the sections were transferred to solutions containing biotinylated goat anti-rabbit IgG (for PV, Nkx2-1, NeuN, p75NTR), biotinylated rabbit anti-rat IgG (for somatostatin, SOM), or biotinylated rat anti-goat IgG (for ChAT; all secondary antibodies –Vector Laboratories, Burlingame, CA, USA), diluted 1 : 250 in PB for 2 h at room temperature. Subsequent visualization of the labelling by ABC (1 : 250, Elite Vectastain ABC kit; Vector Laboratories) and DAB reactions was performed as described previously (Magno et al., 2009).
Immunofluorescence (IF) stainings were used for labelling of sections obtained from the two human brains and for double-labelling for β-galactosidase (β-gal) and ChAT or PV. These sections were labelled with primary antibodies (see below) and visualized with AlexaFluor 488- and AlexaFluor 568-conjugated secondary antibodies (Invitrogen, Karlsruhe, Germany), mounted on slides and coverslipped with DAKO fluorescent mounting medium.
For immunohistochemistry (IHC) and IF we used the following primary antibodies – polyclonal rabbit anti-PV (1 : 5000, IHC) and monoclonal rabbit anti-PV (1 : 500, IF; both from Swant, Bellinzona, Switzerland), polyclonal goat anti-ChAT (1 : 100, IHC; 1 : 500, IF) and monoclonal mouse anti-GAD67 (1 : 1000, IF; both from Chem-icon International, Temecula, CA, USA), polyclonal anti-p75NTR (1 : 1000; Promega, Madison, WI, USA), polyclonal anti-β-gal (1 : 3000; ICN/Cappel, Durham, NC, USA), polyclonal anti-TTF1 (1 : 2000, IHC; 1 : 500, IF; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and monoclonal mouse anti-NeuN (1 : 1000, IHC and IF; Chemicon).
The extent of the loss of cholinergic fibres in the mutant mice was examined using acetylcholine esterase (AChE)-histochemistry according to previous protocols (Eckenstein & Sofroniew, 1983; see Kermer et al., 1995). In addition, axons of the cholinergic basal forebrain neurons were also analysed in sections immunolabelled with antibodies against ChAT or p75NTR (see above).
5-Bromo-4-chloro-3-indolyl-β-D-galactosidase (X-gal) staining was performed as follows. Immediately prior to staining, free-floating sections were re-fixed in 0.2% glutaraldehyde for 10 min on ice, rinsed in PB and then incubated in detergent solution (0.02% Igepal, 0.01% sodium deoxycholate and 2 mM MgCl2 in PB) for 10 min. The sections were directly transferred into the staining solution [5 mM K4Fe(CN)6, 5 mM K3Fe(CN)6, 2 mM MgCl2, 1 mg/mL X-gal in dimethylformamide, in 1× PBS] and subsequently incubated at 37 °C overnight. Thereafter, the slides were fixed in 4% PFA for 10 min. After rinsing in PB the sections were further processed for ChAT- or PV-IHC as described above and subsequently mounted on slides and coverslipped.
Stereological cell counts
Stereological cell counts (Stereo Investigator software version 4.31 – MicroBrightField, Inc., Williston, VT, USA) were performed for ChAT- and PV-immunoreactive (ir) neurons in the medial septum-vertical limb of the diagonal band complex (MSvDB), the horizontal limb of the diagonal band-substantia innominata (hDB-SI), the caudate-putamen (CPu) and the LGP. For the LGP, total neuronal cell numbers and total volume were additionally estimated by using IHC for NeuN. The optical disector/fractionator method was applied for the various regions as described in detail previously (Naumann et al., 2002). A smaller frame size (30 × 18 μm) was used for estimation of the numbers of NeuN-positive cells in the LGP. Volume calculation for the LGP was based on the following parameters –region investigated (LGP), number of sections and section thicknesses. Cell numbers of the various nuclei represent data obtained for one side of the brain.
Fine-structural analysis
Some of the brains were used for fine-structural analysis. Embryonic brains were processed as described above. Transcardial perfusion of postnatal mice was performed by using a fixative containing 4% PFA and 0.1% glutaraldehyde in 0.1 M PB. Immunocytochemical labelling of neurons and subsequent tissue handling for electron microscopy was performed as described in detail by Magno et al. (2009). Embedded tissue blocks underwent semi-thin sectioning (1–2 μm thickness) followed by ultrathin sectioning. All cuts were performed on a Reichert Ultracut S. The ultrathin sections were examined using a Zeiss EM 900 electron microscope.
In situ hybridization combined with immunohistochemistry
In situ hybridization (ISH) was performed for the transcription factors Nkx2-1 (Magno et al., 2009), Lhx7 (the digoxigenin-labelled RNA probe was kindly provided by G. Fishell, New York, USA) and Lhx6 (the digoxigenin-labelled RNA probe was kindly provided by V. Pachnis, London, UK; for detailed protocols see Magno et al., 2009). Hybridization was performed overnight at 60 °C. Some of the sections were subsequently immunostained for Nkx2-1 or SOM (1 : 2500, Chemicon) as described in detail previously (Magno et al., 2009).
Image processing
Images were captured using an Olympus digital camera (Olympus BX-51, Hamburg, Germany). Fluorescent images were obtained with a Leica confocal laser-scanning microscope. They were then processed with Adobe Photoshop CS3 (Adobe Systems Inc., San Jose, CA, USA) for general contrast and brightness enhancements and colour-level adjustments, and finally handled with Adobe Illustrator CS3 (Adobe Systems Inc.) for final compositing of the figures.
Real-time PCR
Mice used for RNA extraction were culled by cervical dislocation and the brains were stored at −80 °C until use. Total RNA was isolated from the forebrains and reverse transcribed as described previously (Magno et al., 2009). NGF, trkA, p75NTR, trkB as well as two housekeeping genes (HPRT and GAPDH) were measured with TaqMan Gene Expression Assays on an ABI PRISM 7500 Fast real-time PCR detection system (Applied Biosystems Inc., Foster City, CA, USA). To validate the amplification efficiency of the various assays, standard curves were generated. As normalization to the endogenous control genes HPRT and GAPDH led to similar results, we present only the data normalized to GAPDH expression.
Results for relative gene expression were calculated using the 2−ΔCT method. Universal TaqMan master mix was purchased from Applied Biosystems and used according to the manufacturer’s recommendations.
Behavioural tests
The Morris water maze (MWM) experiment was performed according to Bert et al. (2005). Briefly, the test was performed over 11 days and consisted of an adaptation trial (day 0), 8 days for the place version (days 1–8), the spatial probe (day 9) and the cued version (day 10). The performances were recorded by using a computerized tracking system (VideoMot version 2.3; TSE Systems, Bad Homburg, Germany). In addition, swim path (in cm) was measured and average speed was calculated (cm/s). Two weeks after the MWM test, the animals were tested on an accelerating rota-rod (Ugo Basile, Comerio, Italy) as already described in detail for rats (Bert et al., 2002).
Statistical analysis
Differences between the experimental groups (cell counts, real-time PCR) were determined by two-tailed unpaired Student’s t-tests with confidence intervals of 95% and were considered significant at P < 0.05. P, t ratio and degrees of freedom are reported for each test. All calculations were performed with GraphPad Prism version 4.00 for Microsoft Windows (GraphPad Software, San Diego, CA, USA).
As the behavioural data were not normally distributed (tested by the Shapiro–Wilk’s method) non-parametric tests were generally chosen. MWM data were analysed using Friedman one-way ANOVAs by ranks followed by post-hoc Dunnett’s method (place version, each group was analysed separately) and by Mann–Whitney rank-sum tests to detect differences between genotypes for each day of the place version and for each trial of the cued version. For the spatial probe, the time in the former platform quadrant (PQ) was compared with the mean of the three remaining quadrants (MQ) and was analysed by Wilcoxon matched-pairs tests. Data of the rota-rod test were analysed by Mann–Whitney rank-sum tests. All analyses were performed with Sigma-Plot version 11.0 (Systat Software Inc., Chicago, IL, USA). A difference was considered statistically significant at P (two-tailed) < 0.05.
Results
The transcription factor Nkx2-1 is expressed in many cholinergic and PV-positive GABAergic neurons throughout the ventral forebrain (Magno et al., 2009). We therefore examined the effects of Nkx2-1 deletion on these cell populations following GAD67-driven inactivation of the transcription factor (prenatal deletion, referred to as –GADcre/+;Nkx2-1fl/fl), and after ChAT-induced inactivation of the gene (postnatal deletion, referred to as – ChATcre/+;Nkx2-1fl/fl) and compared these data with those obtained from the two genotypes which served as controls – Nkx2-1fl/fl, as well as GADcre/+;Nkx2-1fl/+ and ChATcre/+;Nkx2-1fl/+. However, as no significant differences were found between the two controls regarding cell numbers, axonal pattern and performances in the behavioural tests, we largely present results obtained from the two Nkx2-1fl/fl animal lines as control values.
Cre-recombinase activity in GAD67- and ChAT-immunoreactive neurons of the forebrain
We first characterized Cre-recombinase activity in the two mouse lines by crossing them with the Cre-reporter ROSA26LacZ (R26R). Transcripts for GAD67 have been found as early as embryonic day (E)10 and, shortly thereafter, in the differentiation zones of the ventral forebrain (Katarova et al., 2000). Accordingly, in GADcre/+;R26R mice the typical ‘bluish’ reaction product of the X-gal staining was already observed in the prenatal forebrain long before proteins such as ChAT and PV become upregulated. As shown at postnatal day (P)2, X-gal staining could be detected in many regions of the differentiated ventral forebrain, including the CPu and septal complex (Fig. 1A and B, respectively). Throughout the subcortical brain, a dense, punctuate-like labelling was observed, which corresponds to the density of GABAergic neurons in these regions (cf. Bender et al., 1996; Magno et al., 2009).
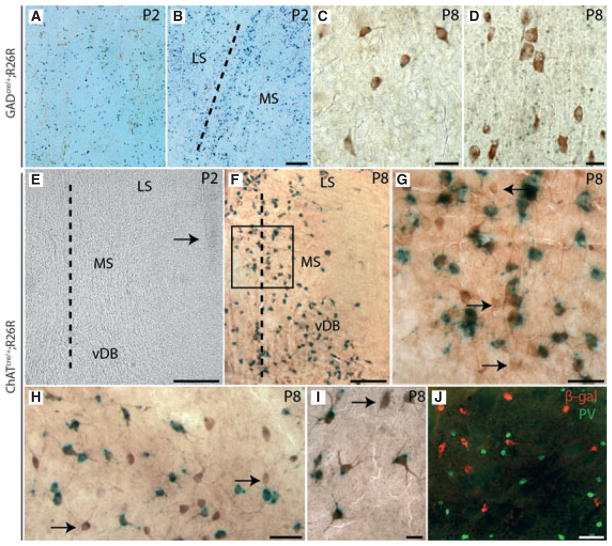
Fig. 1.
Cre-recombination in the subcortical telencephalon of GADcre/+;R26R and ChATcre/+;R26R mice. (A–D) X-gal staining is shown for GADcre/+;R26R mice at P2 (A, B) and P8 (C, D). As shown here by representative coronal sections, an intense punctuate-like labelling was observed in newborn animals throughout the telencephalon including the CPu (A) and within the septal complex (B). The border between the medial septum (MS) and the lateral septal complex (LS) is marked by a dotted line (B). From P7–8 onwards, these precipitates could be localized in GABAergic and cholinergic neurons. As shown here for the CPu (C) and the MS (D), nearly all of the ChAT-ir neurons of the ventral forebrain were also found to be positive for X-gal. Scale bars – 50 μm (A, B), 30 μm (C), 20 μm (D). (E–J) In ChATcre/+;R26R mice, no labelling for X-gal was observed prior to or shortly after birth in the telencephalon. A corresponding coronal section obtained at P2 is shown (E). The ventricle located lateral to the various septal nuclei (MS, vDB, LS) is marked by an arrow. The dotted lines (E, F) indicate the median axis of the brain. (F–J) At P8, intense green punctuate-like labelling for X-gal was observed in all cholinergic centres of the ventral forebrain including the MSvDB (F; boxed area is shown at higher magnification in G), the magnocellular complex of the hDB-SI (H), and in the CPu (I). In these regions, the overwhelming majority of X-gal-positive cells could be identified as cholinergic neurons following subsequent IHC for ChAT (brown labelling of cells; F–I). Only a small subset of ChAT-ir neurons did not present any labelling for X-gal (marked by arrows in G–I). In ChATcre/+;R26R mice, activity of Cre recombinase was found to be restricted to cholinergic neurons. As shown for the CPu, no co-localization of the two markers β-gal (red cells) and PV (green cells) could be detected in the ventral forebrain (J). Scale bars –100 μm (E, J), 200 μm (F), 50 μm (G, H) and 10 μm (I).
Cholinergic and GABAergic interneurons were described to develop from common proto-GABAergic precursors, at least in the striatum (Fragkouli et al., 2009b). Hence, at P8 the X-gal-labelled cells could be identified as PV-immunoreactive (PV-ir; not shown), but also as ChAT-ir neurons (Fig. 1C and D) in the GADcre/+;R26R mice. Close inspection of the sections revealed that the majority of these two neuronal cell types contained the activity of this Cre-driver, as demonstrated by the presence X-gal labelling in these cells (Fig. 1C and D).
Several studies performed in rodents have shown that ChAT synthesis is switched on around P4/5 and reaches mature expression levels around P15 (Korsching, 1993; Bender et al., 1996; Naumann et al., 2002). Consistent with this, no X-gal precipitates were found until P3/4 in ChATcre/+;R26R mice (Fig. 1E). Similar stainings performed at P8 resulted in strong labelling of cellular profiles. The density of these cells resembled that of the adult staining pattern for cholinergic neurons. As shown for the MSvDB (Fig. 1F and G), hDB-SI (Fig. 1H) and CPu (Fig. 1I), most of the cells could be identified as ChAT-ir neurons. Similar to the observations made for the GADcre/+;R26R mice, only a small subset of cholinergic neurons did not contain precipitates of the X-gal reaction (cf. Fig. 1G and H). To confirm that Cre activity is restricted to the cholinergic population, we performed double immunofluorescence stainings for β-gal and PV. As shown for the CPu, no co-localization of the two markers could be detected, suggesting that Cre activity is not ectopically present in non-cholinergic cells (Fig. 1J).
Deletion of Nkx2-1 leads to substantial loss of ChAT- and PV-ir neurons in the basal forebrain
We next studied the consequences of Nkx2-1 deletion on the number of cholinergic and PV-positive neurons of the MSvDB, hDB-SI, CPu and LGP by using the optical disector/fractionator method (West et al., 1991; cf. Naumann et al., 2002). The regions of interest (described in Fig. 2P) for the cell counts are shown for three different levels of the rostro-caudal extension of the brain (modified from Paxinos, 2001).
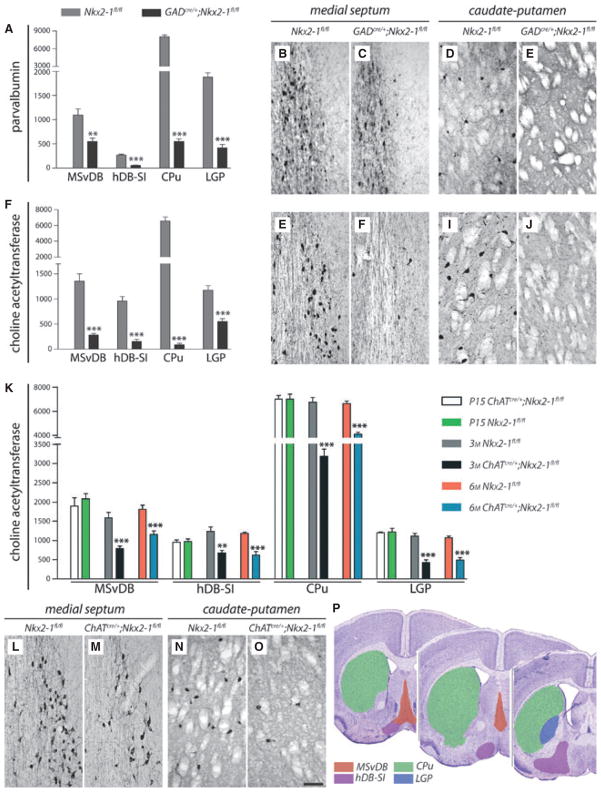
Fig. 2.
Ablation of Nkx2-1 leads to loss of ChAT- and PV-immunoreactive neurons in the subcortical telencephalon. (A) Stereological cell counts for PV-ir cells of GADcre/+;Nkx2-1fl/fl mice and Nkx2-1fl/fl controls (3 months of age) in the four regions investigated are shown. Histograms (see also F and K) show mean (+SEM) cell numbers. Asterisks indicate significant differences – **P < 0.05; ***P < 0.001. (B–E) The loss of PV-ir neurons in GADcre/+;Nkx2-1fl/fl mice is shown by representative coronal sections for the MSvDB (C) and the CPu (E). In B and D, corresponding sections of Nkx2-1fl/fl control animals are shown. (F) Stereological cell counts for ChAT-ir cells of GADcre/+;Nkx2-1fl/fl and Nkx2-1fl/fl controls (3 months of age) also revealed dramatic cell loss in the four regions investigated. (G–J) The loss of ChAT-ir neurons in GADcre/+;Nkx2-1fl/fl mutant mice is shown by representative coronal sections of the MSvDB (H) and the CPu (J). For comparison, these regions are also shown for Nkx2-1fl/fl controls (G, I). (K) Stereological cell counts for ChAT-ir neurons of ChATcre/+;Nkx2-1fl/fl mice at P15, 3 and 6 months of age of the various regions are shown. Note that at P15 the number of ChAT-ir neurons of the various regions analysed corresponds to the cell number obtained from Nkx2-1fl/fl controls. Interestingly, no further significant changes in cell number was detected for the control and mutant mice between 3 and 6 months of age. (L–O) The partial loss of ChAT-ir neurons in ChATcre/+;Nkx2-1fl/fl mice at 3 months of age is shown for the MSvDB (M) and the CPu (O) in comparison with age-matched Nkx2-1fl/fl controls (L and N). Scale bar (in O) for all images – 100 μm. (P) The four regions of interest investigated by stereological cell counts are shown at three different levels along the rostro-caudal axis of the mouse brain (modified from Paxinos, 2001). Whereas the borders of the MSvDB, the CPu and the LGP are well described in the literature, no common definition of the so-called ‘substantia innominata’ yet exists. For practical reasons we used the term ‘hDB-SI’ because the horizontal part of the diagonal band mainly contributes to this region (see left and middle sections). At more caudal level (right section), neurons of the substantia innominata and the magnocellular preoptic nucleus were also included in the estimations of the total number of cells.
Cell counts revealed a dramatic decrease of the two cell populations in GADcre/+;Nkx2-1fl/fl mutant mice at 3 months of age compared with age-matched controls (n = 7 each; two-tailed unpaired Student’s t-test, d.f. = 12; Fig. 2A–J; Table 1). Loss of neurons was found to be most prominent in the CPu (PV: −93.1%, P < 0.0001, t = 28.49; ChAT: −98.6%, P < 0.0001, t = 16.13). A comparable cell loss was detected in the MSvDB (PV: −50%, P = 0.0017, t = 4.021; ChAT: −79.1%, P < 0.0001, t = 6.935), the hDB-SI (PV: −79.5%, P < 0.0001, t = 10.86; ChAT: −84%, P < 0.0001, t = 9.533), and the LGP (PV: −77.7%, P < 0.0001, t = 13.06; ChAT: −52.6%, P < 0.0001, t = 6.947). We also performed corresponding cell counts on GADcre/+;Nkx2-1fl/fl animals at P15. However, the cell numbers of PV- and ChAT-ir neurons were reduced already at this age (data not shown) and corresponded to that of 3-month-old mutants.
Table 1.
Cell counts (mean ± SEM) for ChAT- and PV-immunoreactive neurons in 3-month-old Nkx2-1fl/fl, GADcre/+;Nkx2-1fl/+ and GADcre/+;Nkx2-1fl/fl mice obtained by stereological cell counts
| Area | ChAT
|
PV
|
||||
|---|---|---|---|---|---|---|
| Nkx2-1fl/fl | GADcre/+;Nkx2-1fl/+ | GADcre/+;Nkx2-1fl/fl | Nkx2-1fl/fl | GADcre/+;Nkx2-1fl/+ | GADcre/+;Nkx2-1fl/fl | |
| MSvBD | 1353 ± 363 | 1360 ± 394 | 282 ± 73 | 1096 ± 313 | 987 ± 284 | 548 ± 197 |
| hDB-SI | 958 ± 219 | 1070 ± 47 | 153 ± 107 | 268 ± 46 | 243 ± 116 | 55 ± 27 |
| CPu | 6556 ± 1291 | 5938 ± 564 | 90.5 ± 72 | 8030 ± 734 | 7056 ± 293 | 552 ± 144 |
| LGP | 1177 ± 215 | 1209 ± 64 | 557 ± 137 | 1881 ± 216 | 1875 ± 164 | 419 ± 201 |
We then analysed the number of cholinergic neurons in the various regions of interest in ChATcre/+;Nkx2-1fl/fl mice and in the two controls (Nkx2-1fl/fl and ChATcre/+;Nkx2-1fl/+; Fig. 2K–O). At 3 months of age the number of ChAT-ir neurons (Table 2; Fig. 2K–O) was significantly reduced to about 50% in ChATcre/+;Nkx2-1fl/fl mice compared with that of Nkx2-1fl/fl controls (n = 6 each, two-tailed unpaired Student’s t-test, d.f. = 10; MSvDB: −50.2%, P = 0.0005, t = 5.026; hDB-SI: −45.4%, P = 0.0016, t = 4.268; CPu: −52.7%, P < 0.0001, t = 8.286; LGP: −62.2%, P < 0.0001, t = 6.316).
Table 2.
Cell counts (mean ± SEM) for ChAT-ir neurons in P15, 3-month- and 6-month-old Nkx2-1fl/fl and ChATcre/+;Nkx2-1fl/fl mice obtained by stereological cell counts
| Area | P15
|
3 months old
|
6 months old
|
|||
|---|---|---|---|---|---|---|
| Nkx2-1fl/fl | ChATcre/+;Nkx2-1fl/fl | Nkx2-1fl/fl | ChATcre/+;Nkx2-1fl/fl | Nkx2-1fl/fl | ChATcre/+;Nkx2-1fl/fl | |
| MSvBD | 1899 ± 430 | 2085 ± 333 | 1585 ± 354 | 790 ± 158 | 1812 ± 241 | 1155 ± 204 |
| hDB-SI | 956 ± 104 | 966 ± 176 | 1236 ± 282 | 675 ± 155 | 1185 ± 66 | 615 ± 208 |
| CPu | 6995 ± 630 | 6990 ± 1019 | 6742 ± 947 | 3187 ± 456 | 6646 ± 425 | 4077 ± 379 |
| LGP | 1199 ± 44 | 1222 ± 229 | 1109 ± 189 | 419 ± 190 | 1065 ± 102 | 487 ± 135 |
To investigate the onset and progression of the cell loss in more detail, we performed additional stereological cell counts for P15 (mutants – n = 6, controls – n = 4) and 6-month-old (n = 5 each) animals (Fig. 2K). No differences in cell numbers were observed between Nkx2-1fl/fl and ChATcre/+;Nkx2-1fl/fl mice at P15 (two-tailed unpaired Student’s t-test, d.f. = 8; MSvDB – P = 0.4613, t = 0.773; hDB-SI – P = 0.9283, t = 0.092; CPu – P = 0.9928, t = 0.009; LGP – P = 0.8549, t = 0.188; Table 2; Fig. 2K; cf. Naumann et al., 2002). Interestingly, no evidence for a progressive loss of ChAT-ir neurons in mutant mice was detected between 3 months (see above) and 6 months of age (Fig. 2K, Table 2).
To verify the specificity of the postnatal mutation of Nkx2-1 in cholinergic neurons, we also performed cell counts for PV-ir neurons in ChATcre/+;Nkx2-1fl/fl and Nkx2-1fl/fl mice. However, no alteration of this subset of GABAergic neurons was observed in the various regions of interest (data not shown). This is consistent with the lack of Cre-recombinase activity in these neurons (see above).
Inactivation of the Nkx2-1 gene leads to degenerative changes in basal forebrain neurons
In the rodent brain, many subcortical regions including the large ventral magnocellular nuclei differentiate between E13 and 18 (e.g. Bayer, 1979). The efficiency of the genetic manipulation was therefore monitored in the ventral forebrain of GADcre/+;Nkx2-1fl/fl mice by using IHC and ISH for Nkx2-1 at prenatal and postnatal stages.
Conditional prenatal Nkx2-1 ablation in GAD67-expressing cells resulted in an almost complete loss of Nkx2-1-ir cells in the various subcortical compartments of GADcre/+;Nkx2-1fl/fl mice even at prenatal stages (cf. Fig. 3A and B). As shown at E16.5, only at the level of the ventricular zone of the lateral ventricles a persistent synthesis of the transcription factor was observed (Fig. 3B). Away from the ventricular region, Nkx2-1 expression tends to fade gradually, suggesting that the mutation occurs as the cells exiting their cycle start to express GAD67 (Fig. 3C).
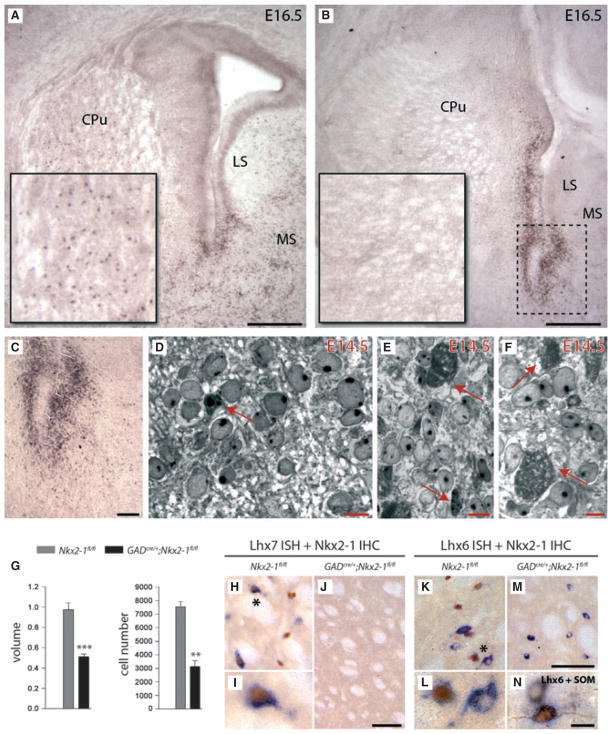
Fig. 3.
Fate of basal forebrain neurons in GADcre/+;Nkx2-1fl/fl mice. (A, B) IHC for Nkx2-1 for the regions surrounding the lateral ventricle is shown for Nkx2-1fl/fl control (A) and mutant GADcre/+;Nkx2-1fl/fl (B) mice at E16.5. Whereas in control animals intense labelling of cell profiles was predominately observed at the ventral tips of the lateral ventricles (A) and throughout the neighbouring compartments of the subcortical brain including the CPu (A; partially shown at higher magnification in the large inset), the medial septum (MS) and the lateral septum (LS; A), Nkx2-1 immunolabelling was found to be restricted to the ventricular zone in mutant mice (B, dotted frame is shown at higher magnification in C). As shown for the CPu (B, partially shown at higher magnification in the large inset), Nkx2-1-positive cell profiles could barely be detected. Scale bar for A and B – 100 μm, for C – 8 μm. (D–F) Subsequent fine-structural analysis of the subventricular zone of controls (D) and mutant mice (E, F) at E14.5 revealed that in Nkx2-1fl/fl mice only occasionally were apoptotic cell profiles observed (D, arrow). In contrast, many cell profiles showing severe signs of degeneration were detectable in mutants at this age (E, F; arrows). Scale bar for D–F – 10 μm. (G) The histograms show the results of the stereological estimations of the total volume (left) and the total number of NeuN-positive cells (right) of the LGP at 3 months of age. The data are presented as volume (mm3) and cell number + SEM, the bars show average values and significance is indicated by asterisks – **P < 0.05; ***P < 0.001. Both total volume and total number of NeuN-positive cells were significantly reduced in the mutant mice at 3 months of age. (H–N) ISH for Lhx7 or Lhx6 combined with IHC for Nkx2-1 (H–M) or SOM (N) are shown for GADcre/+;Nkx2-1fl/fl and Nkx2-1fl/fl at 4 weeks of age – ISH for Lhx6 and Lhx7 results in violet staining of the cytoplasm (H, I, K–N) while Nkx2-1-ir neurons display brown staining of cell nuclei. One of the neurons double-labelled for Nkx2-1 and Lhx7 (H, black asterisk) is shown at higher magnification (I). As shown for the CPu at 4 weeks of age, the nearly complete loss of Lhx7- and Nkx2-1-expressing cells (J) confirms the dramatic loss of cholinergic neurons in GADcre/+;Nkx2-1fl/fl mutant mice. However, in these animals, labelling for Lhx6 was partially maintained (cf. K and M), and most of these cells could be identified as SOM-ir striatal interneurons (N). In the CPu of Nkx2-1fl/fl mice cells labelled for Lhx6 and Nkx2-1 are marked by an asterisk in K (shown at higher magnification in L). Scale bars – 100 μm (K, M); 50 μm (C, F and H); 10 μm (D, G and I).
The reduction in cell numbers as observed for the GADcre/+;Nkx2-1fl/fl mice (see above) could be caused by different processes occurring in the mutated cells. For instance, it has been described that Nkx2-1 ablation in the MGE at early prenatal stages may result in a shift of the molecular profile of the mutated cells (Butt et al., 2008), or in an alteration of their migratory capacity (Nobrega-Pereira et al., 2008). Thus, a fine-structural analysis of tissue samples obtained from the ventricular zone at E14.5 was performed for GADcre/+;Nkx2-1fl/fl mice and corresponding controls (Fig. 3C and D). At this age, large accumulations of cell profiles showing severe signs of degeneration and cell debris were detectable (Fig. 3E and F), which were not observed for corresponding control animals (Fig. 3D). In these electron-dense structures, cell organelles including the cell nucleus could hardly be detected (Fig. 3E and F). Subsequent fine-structural investigation of the LGP or other subcortical compartments including the MSvDB of GADcre/+;Nkx2-1fl/fl animals at 1 or 3 months of age did not provide evidence for a degeneration of neurons (data not shown). Taken together, these data do not support the hypothesis that mutated neurons differentiate into another phenotype, but rather suggest that they degenerate locally.
To confirm further that Nkx2-1-expressing cells are not replaced by other cell types, we determined the total volume and number of neurons of the LGP, a structure known for its very high number of neurons expressing both PV and Nkx2-1 (Magno et al., 2009; Nobrega-Pereira et al., 2010). Close inspection of slices processed for IHC already indicated that the LGP of GADcre/+;Nkx2-1fl/fl mice was significantly smaller in size. Accordingly, both the total volume (Fig. 3G, right; n = 6 each, two-tailed unpaired Student’s t-test, d.f. = 10; GADcre/+;Nkx2-1fl/fl = 0.51 mm3 vs. Nkx2-1fl/fl = 0.97 mm3; −47.6% P < 0.0001, t = 6.645) and the total number of NeuN-positive neurons (Fig. 3G, left; n = 3 each, two-tailed unpaired Student’s t-test, d.f. = 4; GADcre/+;Nkx2-1fl/fl = 3146.3 vs. Nkx2-1fl/fl = 7543.3; −58.3% P = 0.0015, t = 7.735) of the mutated LGP were substantially reduced compared with controls. Thus, this region was obviously not populated by other neuronal cell types after deletion of Nkx2-1.
Developing cholinergic and GABAergic basal forebrain neurons can be further characterized by the expression of transcription factors acting downstream of Nkx2-1, namely Lhx7 and Lhx6 (Sussel et al., 1999; Du et al., 2008). As shown for the CPu of GADcre/+;Nkx2-1fl/fl mutants at 3 months of age, no cells positive for either Lhx7 mRNA or Nkx2-1 could be detected (cf. Fig. 3H–J). A similar loss of cholinergic neurons was observed in the neighbouring regions, including the septal complex and the LGP (data not shown). In contrast, Lhx6 labelling was found to be partially maintained in the CPu of mutant mice (Fig. 3M), and most Lhx6-positive cells could be double-labelled by subsequent IHC for SOM (Fig. 3N). SOM-positive striatal neurons are known to downregulate Nkx2-1 expression as soon as they exit their cell cycle (Marin et al., 2000), and they are therefore not affected by the mutation. These data also point to the possibility that prenatal deletion of Nkx2-1 in postmitotic cells leads to their degeneration rather than causing cell-fate shift and/or migratory defects.
The cholinergic phenotype of ventral forebrain neurons develops within the first 2 weeks after birth (Gnahn et al., 1983; Mobley et al., 1986; Sofroniew et al., 1987), and mature cell numbers are detectable around P12–15 (cf. Bender et al., 1996; Naumann et al., 2002). As shown by our cell counts, in postnatal ChATcre/+;Nkx2-1fl/fl mice a substantial loss of cholinergic basal forebrain neurons was detected at 3 months of age. These changes were therefore preferentially examined by using fine-structural methods. Among the neurons lacking signs of degeneration (cf. Fig. 4A and B) we found a large number of very dark-coloured neurons in semi-thin tissue preparations (Fig. 4B). At ultrastructural level, these cells appeared electron-dense and thus organelles and membranes could barely be detected (cf. Fig. 4C–E). However, many of these neurons still present intact nuclear membranes and, at least partially, input synapses (Fig. 4E). These fine-structural characteristics indicate long-lasting degenerative processes rather than apoptotic cell death (cf. Naumann et al., 1992b). As revealed by the cell counts for ChATcre/+;Nkx2-1fl/fl mice (Fig. 2K), about 50% of the ChAT-ir neurons remain detectable after ChAT-Cre driven ablation of Nkx2-1. These cells do not show signs of degeneration and, as shown for the CPu, they do not express Nkx2-1, at least during the various postnatal stages analysed (Fig. 4F). By using confocal microscopy, subsequent analysis of cells in the forebrain of ChATcre/+;R26R;Nkx2-1fl/fl mice revealed that almost all remaining ChAT-positive neurons were also labelled for β-gal (Fig. 4G–I).
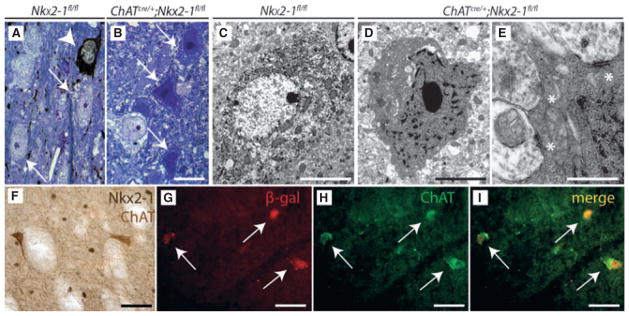
Fig. 4.
Fate of basal forebrain neurons in ChATcre/+;Nkx2-1fl/fl mice. (A, B) As shown by semithin sections following IHC for ChAT, some cholinergic neurons in ChATcre/+;Nkx2-1fl/fl mice underwent severe degenerative processes following ablation of Nkx2-1. Several unlabelled neurons (A, thin arrows) and one ChAT-ir neuron (A, arrowhead) in the MS of a 4-week-old Nkx2-1fl/fl mouse are shown. In the mutant mice, a dramatic increase in the number of dark-coloured neurons (B, arrows) was observed. (C–E) Further fine-structural analysis revealed that many ventral forebrain neurons including those of the MS show signs of severe degenerative changes, including darkening of the cytoplasm and condensation of the chromatin (D, E). Notably, these heavily damaged neurons partially still bore input synapses (E; white asterisks). Similar changes were never observed in corresponding regions of control animals (C). Scale bars represent 20 μm (A and B), 10 μm (C), 6 μm (D) and 2 μm (E). (F) As shown here for the CPu of a ChATcre/+;Nkx2-1fl/fl mouse at 4 weeks of age, the remaining ChAT-ir neurons (in light brown; note dendritic processes) do not express Nkx2-1 (dark brown nuclei). (G–I) Confocal microscopy of double-labelled sections of ChATcre/+;R26R;Nkx2-1fl/fl mice at 4 weeks of age. As shown for the CPu, double immunofluorescence for β-gal and ChAT demonstrates that the overwhelming majority of the remaining ChAT-ir neurons are positive for β-gal. Scale bars represent 50 μm (F) and 30 μm (G–I).
Target denervation and altered expression of neurotrophin receptors in Nkx2-1-deficient mice
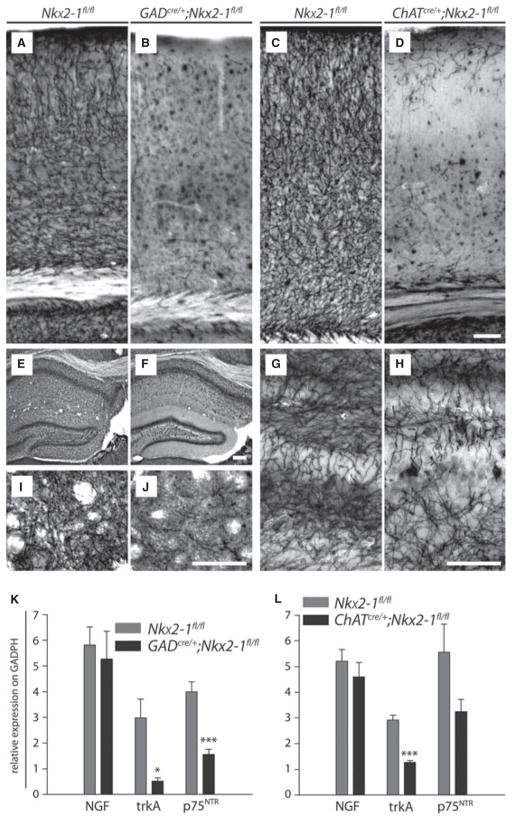
By using histochemistry for AChE (Eckenstein & Sofroniew, 1983; Mesulam et al., 1983), we next investigated whether the loss of cholinergic neurons in the two mouse lines is accompanied by corresponding impairment of their axonal projections. At 3 months of age, a nearly complete absence of AChE-positive fibres was observed throughout the cortical mantle (Fig. 5A and B) including the hippocampal formation (Fig. 5E and F), and within the CPu (Fig. 5I and J) of GADcre/+;Nkx2-1fl/fl mutants. Notably, the AChE labelling of fibres was similarly impaired in the mutants at 2 and 4 weeks of age (data not shown). Only in more latero-ventral parts of the cortex (e.g. in the piriform cortex and the amygdaloid complex) did the AChE staining pattern appear to be unchanged (data not shown).
Fig. 5.
Loss of ventral forebrain cholinergic neurons leads to extended target denervation and impaired expression of the neurotrophin receptors trkA and p75NTR. (A–J) AChE histochemistry performed for control (A, E, I, C, G, Nkx2-1fl/fl) and mutant (B, F, J, GADcre/+;Nkx2-1fl/fl; D, H, ChATcre/+;Nkx2-1fl/fl) mice. As shown for the somatosensory cortex (A, C), the hippocampal formation (E, G) and the CPu (I), an intense labelling of fibres was observed in both control strains whereas this staining pattern was largely missing in the neocortical mantle of the two mutants (B, D). In the GADcre/+;Nkx2-1fl/fl mice, similar observations were made for the dentate gyrus, the hippocampal subfields CA3 and CA1 (F), and the CPu (J). The extent of fibre loss in all parts of the hippocampus of ChATcre/+; Nkx2-1fl/fl mice was less pronounced. The ‘patch-like’ defects are shown for the dentate gyrus at higher magnification (H). Scale bars – 100 μm (A–D), 50 μm (E–J). (K, L) Quantification of NGF, trkA and p75NTR expression levels by using quantitative real-time PCR. No significant differences were detected between GADcre/+;Nkx2-1fl/fl mutants and Nkx2-1fl/fl controls as well as ChATcre/+;Nkx2-1fl/fl mutants and their corresponding Nkx2-1fl/fl controls in the levels of NGF. A strong reduction was observed for trkA transcripts in both GADcre/+;Nkx2-1fl/fl and ChATcre/+;Nkx2-1fl/fl mutant mice compared with their controls, whereas the decrease for p75NTR was found to be significant only for GADcre/+;Nkx2-1fl/fl mice. Histograms show mean values + SEM. Significance is indicated by asterisks –*P < 0.01; ***P < 0.001.
In ChATcre/+;Nkx2-1fl/fl mice similar alterations of the AChE staining were observed in the neocortex at 3 months of age. Only a few aberrant fibres, mostly located in superficial and deep parts of the neocortex, were detected (Fig. 5C and D). Surprisingly, the AChE-staining pattern was better preserved in the CPu and in the hippocampus proper. As shown for the granule cell layer of the dentate gyrus, the AChE pattern exhibited defects in a ‘patch-like’ manner (cf. Fig. 5G and H). Alternative labelling of cholinergic projections by using antibodies against ChAT or p75NTR, the latter known to be a specific marker for the cholinergic magnocellular projections (Sobreviela et al., 1994), resulted in an identical staining pattern (data not shown), which confirms our results obtained from the AChE stainings.
In conclusion, following prenatal mutation of Nkx2-1, an extensive loss of cholinergic fibres was observed in all regions studied. On the other hand, postnatal ablation of the transcription factor is also accompanied by an extensive loss of cholinergic terminals in large parts of the neocortex, but was found to be less pronounced in other regions including the basal ganglia and the allocortical hippocampal formation.
Neurotrophins and their receptors are known to be of considerable importance for the differentiation, maturation and maintenance of cholinergic basal forebrain neurons (Li et al., 1995; Thoenen, 1995; Yoon et al., 1998; Bibel & Barde, 2000; Sofroniew et al., 2001). We therefore investigated whether the cellular changes observed in the mutants at 3 months of age are accompanied by impaired synthesis of these molecules. However, no significant differences were detected for the level of nerve growth factor (NGF) between the mutants and their corresponding controls (n = 5 each, two-tailed unpaired Student’s t-test, d.f. = 8; GADcre/+;Nkx2-1fl/fl vs. Nkx2-1fl/fl P = 0.6872, t = 0.4176; ChATcre/+;Nkx2-1fl/fl vs. Nkx2-1fl/fl P = 0.4262, t = 0.8384; Fig. 5K and L). In contrast, significant decreases in the expression of trkA (−82%, P = 0.01, t = 3.353) and p75NTR (−60%, P = 0.0006, t = 5.477) transcripts were detected for GADcre/+;Nkx2-1fl/fl mice (Fig. 5K), whereas in ChATcre/+;Nkx2-1fl/fl mutants a significant reduction was only observed for trkA (−56%, P < 0.0001, t = 5.497; p75NTR: −41% P = 0.0887, t = 1.937; Fig. 5L). We also tested whether trkB, known to be involved in the brain-derived neurotrophic factor-mediated effects on GABAergic striatal neurons (e.g. Mizuno et al., 1994; Reichardt, 2006), was regulated after targeting of Nkx2-1. Again, no significant differences could be detected between the animal groups regarding the levels of this neurotrophin receptor (data not shown). These results additionally confirm that in both mutations the cholinergic basal forebrain neurons are heavily impaired despite relevant synthesis of NGF (cf. Rocamora et al., 1996; McAllister et al., 1999).
Behavioural impairments following ablation of Nkx2-1
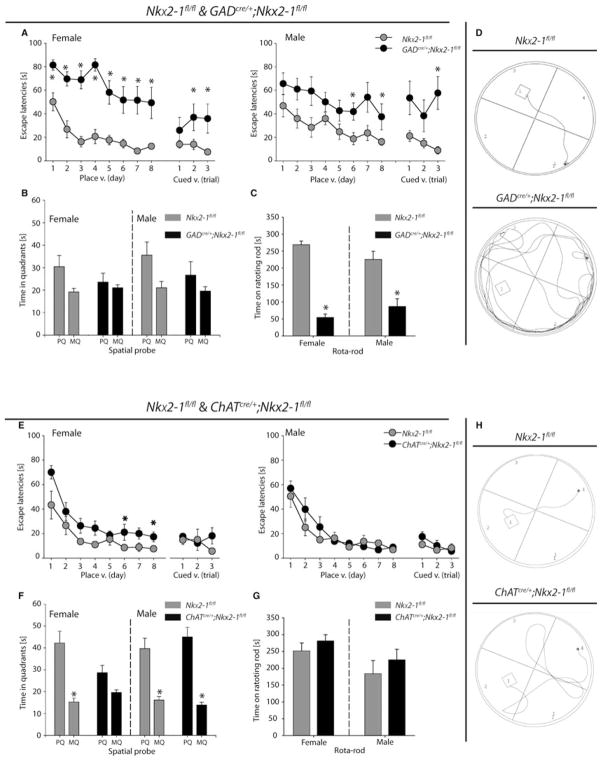
Any substantial loss of cholinergic and GABAergic neurons of the ventral forebrain might be accompanied by impairments of the motor/cognitive behaviour (e.g. Arters et al., 1998; Berger-Sweeney, 2003; Bonsi et al., 2011). Accordingly, GADcre/+;Nkx2-1fl/fl mice of both sexes showed significant deficits in the place version of the MWM task (Fig. 6A). Female mutant mice failed to learn the location of the platform [ (n = 7), P = 0.137], whereas their corresponding controls learned the task, as shown by progressively decreasing escape latencies from day 3 onwards [ (n = 7), P < 0.001; followed by Dunnett’s method; Fig. 6A, left]. Detailed analysis by Mann–Whitney rank sum-tests of each day revealed that, during all 8 days of the test, female GADcre/+;Nkx2-1fl/fl mice showed significantly higher escape latencies than female control mice (see Fig. 6A, left). Additionally, the swimming paths of female mutants were significantly longer (Fig. 6D) and average speed slightly lower compared with female control mice (data not shown). The learning deficit was reflected by the spatial probe after removing the platform. Female GADcre/+;Nkx2-1fl/fl mice spent similar swimming times in all quadrants (P = 0.938), but female control mice also displayed equal swimming times in all quadrants (P = 0.156; Fig. 6B). In the cued version, female mutant mice displayed in two trials longer escape latencies than female control mice, pointing to disturbed visual-motor integrity or reduced overall learning capacity (Fig. 6A, left).
Fig. 6.
Impaired spatial memory and motor deficits in GADcre/+;Nkx2-1fl/fl mice and learning deficits in female ChATcre/+;Nkx2-1fl/fl mice. (A) Escape latencies during the place and cued version (v.) of the Morris water maze (MWM) are shown for female and male GADcre/+;Nkx2-1fl/fl and Nkx2-1fl/fl mice. Female and male mutant mice failed to learn the location of the platform. Data are presented as means ± SEM; *P < 0.05 vs. Nkx2-1fl/fl. (B) The time (spatial probe of the MWM) spent in the platform quadrant (PQ) and the mean time spent in the three other quadrants (MQ) are presented for female and male GADcre/+;Nkx2-1fl/fl and Nkx2-1fl/fl mice. Female and male mutant mice failed to remember the former location of the platform, but control mice also showed slight deficits in spatial memory. Data are presented as means + SEM. (C) Rota-rod test for female and male Nkx2-1fl/fl and GADcre/+;Nkx2-1fl/fl mice. The time spent on the rotating rod is shown for all mutants in comparison with control mice. Data are presented as mean + SEM; *P < 0.05 vs. Nkx2-1fl/fl. (D) Representative paths followed by a female Nkx2-1fl/fl control and a female GADcre/+;Nkx2-1fl/fl mutant mouse on the third day of the place version. (E) Escape latencies during the place and cued version (v.) of the MWM for female and male Nkx2-1fl/fl and ChATcre/+;Nkx2-1fl/fl mice are shown. Female ChATcre/+;Nkx2-1fl/fl mice needed more time to find the platform than female control mice on days 6 and 8. Data are presented as mean ± SEM; *P < 0.05 vs. Nkx2-1fl/fl. (F) The time (spatial probe of the MWM) spent in the platform quadrant (PQ) and the mean time spent in the three other quadrants (MQ) is presented for female and male Nkx2-1fl/fl and ChATcre/+;Nkx2-1fl/fl mice. Only female mutant mice failed to remember the former location of the platform. No difference in the performance of male ChATcre/+;Nkx2-1fl/fl and Nkx2-1fl/fl mice was detected. Data are presented as mean + SEM; *P < 0.05 vs. PQ. (G) The time spent on the rotating rod is plotted on the diagram for female and male Nkx2-1fl/fl and ChATcre/+;Nkx2-1fl/fl mice. No differences between mutants and control mice were detected. Data are presented as mean + SEM. (H) Representative paths followed by a female Nkx2-1fl/fl control and a female ChATcre/+;Nkx2-1fl/fl mutant mouse on day 3 of the place version.
Male mutant mice showed no significant decreases in escape latencies during the place version of the MWM [ (n = 7), P = 0.089], whereas male control mice learned to locate the platform [ (n = 7), P = 0.010; Fig. 6A, right]. Day-by-day comparison of the performances by Mann–Whitney rank sum-tests revealed significant differences between mutant and controls for days 6 and 8 (see Fig. 6A, right). The swimming speed was only slightly lower in male mutant mice than in male control mice, whereas no differences in the swim path length were observed between both genotypes (data not shown). The spatial probe trial revealed that both male control (P = 0.078) and GADcre/+;Nkx2-1fl/fl mice (P = 0.813) were not able to remember the former platform location (Fig. 6B). It is possible that the learning and memory impairment of male mutant mice is due either to general learning deficits or deficits in vision/motor activity as suggested by the results of the cued version (Fig. 6A, right).
The deficits in visual-motor integrity of GADcre/+;Nkx2-1fl/fl mice of both sexes observed during the cued version of the MWM are reflected in the rota-rod test, a frequently applied test for detecting disturbances of subcortical organization of motor functions (Bert et al., 2002). Male (t6 = 30.00, P = 0.002) and female (t6 = 28.00, P < 0.001) mutant mice spent significantly less time on the rotating rod than the corresponding Nkx2-1fl/fl control mice (Fig. 6C).
Postnatal Nkx2-1 deletion in ChAT-expressing cells caused sex-specific deficits in spatial learning (Berger-Sweeney, 2003). Female ChATcre/+;Nkx2-1fl/fl [ (n = 7), P < 0.001] and control mice learned to locate the platform during the place version [ (n = 7), P < 0.001]. However, direct comparison of the two performances using Mann–Whitney rank sum-tests revealed that on days 6 and 8 female mutant mice needed significantly more time to locate the platform (Fig. 6E, left). The slightly impaired performance of female mutant mice in the place version was reflected by the spatial probe. These animals showed no significant difference in the time spent in the platform quadrant compared with the mean of the three remaining quadrants (P = 0.098; Fig. 6F). In contrast, female control mice spent significantly more time in the former platform quadrant (P = 0.016). This impairment of the female mutant mice is not related to visual-motor deficits as they showed a comparable performance to female control mice in the cued version (Fig. 6E). It should be noted here that female mutant mice display longer swimming paths (Fig. 6H) at higher swim speed than female control mice (data not shown).
Both mutant [ (n = 7), P < 0.001] and control [ (n = 7), P < 0.001] male mice showed good performances in the place version of the MWM (Fig. 6E, right). This was also reflected by the spatial probe, in which mice of both genotypes remembered the former location of the platform (Fig. 6F). There were also no differences in the cued version of the MWM (see Fig. 6E, right).
Finally, the rota-rod test showed no motor deficit for female (t6 = 39.00, P = 0.491) and male (t6 = 49.00, P = 0.463) ChATcre/+;Nkx2-1fl/fl mice and their corresponding control mice (Fig. 6G).
Expression of NKX2-1 in the human basal ganglia
As mentioned in the Introduction, human haploinsufficiency for NKX2-1 is accompanied by severe impairments of coordinated movements. Consistently, we have recently shown that Nkx2-1 expression is maintained throughout life by many cholinergic and GABAergic neurons of the mouse ventral forebrain, including the CPu and LGP (Magno et al., 2009). To determine whether NKX2-1 is also expressed by the same neurons in humans, we analysed tissue samples obtained from the basal ganglia. Overall, IHC for NKX2-1 resulted in a more intense darkening of the external part of the globus pallidus (GPe) compared with its internal part (GPi; Fig. 7A). Accordingly, many more NKX2-1-immunoreactive cell profiles could be detected in the GPe (inset in Fig. 7A). Double-IF showed that most NKX2-1-positive cells expressed NeuN and GAD67 (Fig. 7B–G). A smaller proportion of these cells co-expressed PV (in the GPe; Fig. 7H–J) or ChAT (Fig. 7K–M).
Fig. 7.
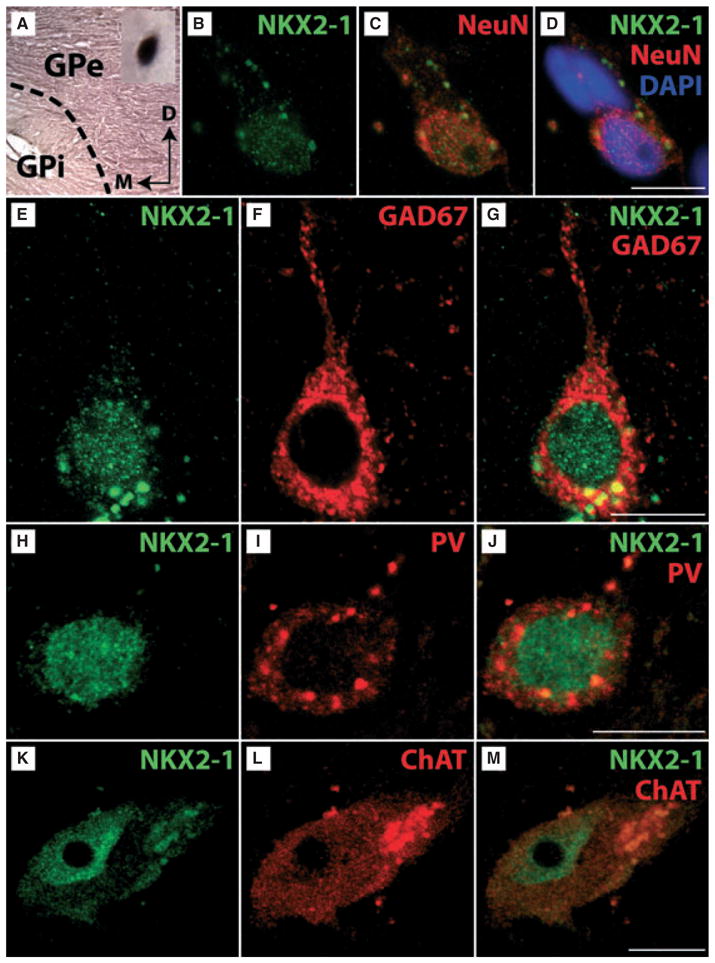
Expression of NKX2-1 in the human basal ganglia. (A) IHC for NKX2-1 for a tissue sample of the human globus pallidus is shown (D, dorsal; M, medial) at low magnification. Note that the external part of the globus pallidus (GPe) is more intensively labelled than the internal part (GPi). The dashed line indicates the border between the two compartments of the GP. At high magnification (see inset) a NKX2-1-positive cell profile is shown. (B–D) Triple-staining for Nkx2-1 (B), the neuronal marker NeuN (C), and the nuclear marker DAPI (D) confirmed nuclear expression of NKX2-1 by the neurons. (E–G) Double-labelling for NKX2-1 (E) and GAD67 (F) revealed that NKX2-1 is expressed by GABAergic GPe-neurons (merged, G). Additional double-labellings for NKX2-1 (H) and PV (I) revealed that at least part of these neurons are also immunoreactive for parvalbumin (merged, J). (K–M) Double IHC for NKX2-1 (K) and ChAT (I) revealed that NKX2-1 is expressed by cholinergic neurons mostly located in the putamen bordering the GPe (merged, M). Note intense fluorescence of lipofuscin particles which are frequently seen in human neurons (see Buhl & Schlote, 1987). Scale bar (for B–M) – 10 μm.
Discussion
A recent study has shown that a certain degree of gene expression established at prenatal stages is maintained into adulthood. It was suggested that this embryonic ‘imprint’ could be important for the maintenance of the phenotype and connectivity of already established neuronal populations (Zapala et al., 2005). It is well described (see Introduction) that expression of the transcription factor Nkx2-1 is of fundamental importance for the specification of several neuronal populations. Here we investigated whether prenatal and postnatal expression of the transcription factor is necessary for the integrity of the cholinergic neurons of the basal forebrain.
Targeting of Nkx2-1 in GAD67- and ChAT-expressing cells in the ventral telencephalon
Nkx2-1 becomes detectable around E9 in the neuroepithelium of ventro-medial parts of the telencephalic vesicle, which subsequently differentiates into the medial ganglionic eminence and the preoptic area (Sussel et al., 1999; Puelles et al., 2000). Notably, transcripts for GAD67 have also been found as early as E10 in the differentiating zones of these regions (Katarova et al., 2000). Several reports (Sussel et al., 1999; Marin et al., 2000; Magno et al., 2009; Nobrega-Pereira et al., 2010), including a recent fate-mapping study (Xu et al., 2008), have shown that Nkx2-1-lineage cells of the subpallium mostly differentiate into cholinergic and GABAergic neurons. Thus, the nearly complete loss of cholinergic and PV-positive neurons observed after early inactivation of the Nkx2-1 gene in GADcre/+;Nkx2-1fl/fl mice corresponds to the findings of these earlier studies. In contrast, precursors of cortical GABAergic interneurons (Butt et al., 2005, 2008), which rapidly downregulate Nkx2-1 expression prior to their migration to the cortical mantle (Nobrega-Pereira et al., 2008), were most likely not affected, as the number of cortical cholinergic or PV-positive GABAergic interneurons, including synthesis of NGF by the latter (present study; cf. Rocamora et al., 1996; McAllister et al., 1999), appeared unchanged. Similarly, no obvious cell loss was observed for striatal SOM-expressing GABAergic neurons, which are also known to downregulate the expression of Nkx2-1 as they exit the neuroepithelium (Marin et al., 2000). As Nkx2-1 protein and mRNA was not detectable in the differentiating nuclei of the ventral telencephalon of GADcre/+;Nkx2-1fl/fl mice prior to birth, the persistence of a small number of neurons immunoreactive for ChAT or PV in the regions studied could be explained by their origin in Nkx2-1-negative domains (Flames et al., 2007; Garcia-Lopez et al., 2008).
To determine a possible postnatal role of the transcription factor, we targeted Nkx2-1 in ChAT-expressing cells. Several studies performed in rodents have shown that ChAT synthesis reaches mature levels around P15 (Korsching, 1993; Bender et al., 1996; Naumann et al., 2002). In line with this, ChAT-driven Cre activity was first observed between P4 and P6 (present study). This observation probably explains why targeting of Nkx2-1 in ChATcre/+;Nkx2-1fl/fl mice had no effect on the number of ChAT-ir neurons until P15.
The fate of Nkx2-1-deficient cholinergic and PV-expressing GABAergic neurons
Recent studies have revealed that Nkx2-1 plays an important role in the cell-type specification by suppressing the cell fate programmes of the surrounding caudal and lateral ganglionic eminences (Sussel et al., 1999; Butt et al., 2008). Thus, it would be conceivable that the effects of prenatal Nkx2-1 ablation resulted in enhanced generation of other, non-Nkx2-1-dependent neurons. Moreover, Nkx2-1 ablation could lead to migration of such cells to different target regions (Nobrega-Pereira et al., 2008). However, the results of our fine-structural analysis do not support these possibilities. The presence of cells in the MGE showing severe signs of degeneration leads to the conclusion that prenatal deletion of Nkx2-1 causes substantial local cell loss rather than changes in cell fate and/or migratory behaviour. Accordingly, the LGP, known to contain a very high number of neurons expressing Nkx2-1 at postnatal stages, is much smaller in GAD-cre/+;Nkx2-1fl/fl mice due to a strong reduction of the total cell number. This also shows that prenatal deletion of Nkx2-1 is unlikely to cause the generation of functionally different neurons for this heavily affected region (cf. Butt et al., 2008).
Proper development of the forebrain nuclei depends on the expression of several transcription factors (Sussel et al., 1999; Marin et al., 2000; Fragkouli et al., 2005; Flandin et al., 2010; Nobrega-Pereira et al., 2010). Nkx2-1 has been shown to regulate the expression of downstream targets such as members of the LIM home domain family, Lhx6 and Lhx7 (Sussel et al., 1999; Fragkouli et al., 2005). Additional analysis of the expression pattern of Lhx6 and Lhx7 transcripts further confirmed that cholinergic and PV-positive neurons were almost completely absent in the four regions investigated. These data are also in line with the nearly complete lack of cholinergic fibres in the target structures of the cholinergic basal forebrain neurons. Finally, the lack of fine-structural hallmarks of ongoing degeneration in young adult and adult GADcre/+;Nkx2-1fl/fl animals (e.g. long-lasting activation of microglia, cell debris; cf. Hollerbach et al., 1998) supports the idea that the affected neurons died rapidly and were removed locally within a very short time.
In contrast, the loss of cholinergic neurons in the ChATcre/+; Nkx2-1fl/fl mice is probably a result of prolonged degeneration. As was seen for septal neurons following axotomy or excitotoxic lesions performed in early postnatal and adult rodents (cf. Naumann et al., 1992b; Plaschke et al., 1997), numerous cells exhibited a very electron-dense cytoplasm, and intact organelles or membranes could barely be detected. The presence of such cell profiles was most frequently observed in tissue probes obtained from 4- to 8-week-old mutant mice. It should also be mentioned here that additional IHC for detection of apoptotic cell death (e.g. by using antibodies against caspase 3) never resulted in substantial labelling of cells in the various subcortical nuclei. Moreover, in ChATcre/+;Nkx2-1fl/fl mice degenerating neurons were observed in close proximity to intact ChAT-ir neurons (cf. Naumann et al., 1992a). This indicates the heterogeneity of cholinergic basal forebrain neurons. As shown from cell counts at 6 months of age, as well as the results gained from our crossings of the mutants with the ROSA26LacZ indicator strain, the remaining large set of cholinergic neurons is not affected by the mutation because they have most likely down-regulated Nkx2-1 synthesis prior to upregulation of ChAT and Cre. A third, and very small, subset of these neurons could derive from Nkx2-1-negative domains (see above) and is, with the methods applied, indistinguishable from the former population. In line with this, the decrease in the expression levels of the NGF-receptors trkA and p75NTR (for reviews see Chao & Hempstead, 1995; Thoenen, 1995) and the loss of cholinergic fibres in the target structures of the cholinergic neurons were found to be attenuated in ChATcre/+;Nkx2-1fl/fl mice compared with those of GADcre/+;Nkx2-1fl/fl mice.
Functional implications for pre- and postnatal mutations of Nkx2-1 transcription factor
In Nkx2-1 knockout mice, a substantial loss of cortical interneurons was observed together with severe morphological alterations of the basal ganglia and various nuclei of the diencephalon (Kimura et al., 1996). Recently, it was shown that a postnatal synapsin I-driven deletion of the Nkx2-1 gene led to various vegetative dysfunctions (for reviews see Son et al., 2003; Kim et al., 2006) due to alterations restricted to the hypothalamus (Mastronardi et al., 2006). On the other hand, humans suffering from NKX2-1-haploinsufficiency show severe deficit of coordinated movement, but notable impairments of higher cognitive functions or those related to the function of the diencephalon have not yet been described (reviewed by Kleiner-Fisman & Lang, 2007). In the present study, we show that GADcre/+;Nkx2-1fl/fl mice have lost nearly all striatal cholinergic and PV-positive interneurons. In addition, a severe loss of the projection neurons in the LGP targeting the subthalamic nucleus or the substantia nigra (reviewed by Gerfen, 2004) was observed. Thus, the pronounced impairment of coordinated movement in these animals points to severe deficits within the loops of the motorcortex–CPu–LGP axis. Although the prenatal Nkx2-1 mutation in mice cannot be directly considered as a model for the human NKX2-1 haploinsufficiency, the neurological symptoms of the patients can be partially explained by the results obtained from these mice. In fact, expression of the transcription factor by cholinergic and PV-positive neurons was detected in the telencephalic parts of the basal ganglia of humans (present study) and mice (Magno et al., 2009). Little is known about the function of these neuronal subpopulations (e.g. Calabresi et al., 2000) although in ChATcre/+;Nkx2-1fl/fl mice the remaining cholinergic interneurons are obviously sufficient to compensate for the cell loss, at least until 3 months of age.
In our experiments, the almost complete loss of cholinergic neurons in GADcre/+;Nkx2-1fl/fl mice resulted in severe impairments of learning and spatial memory functions. This correlates with the results of many excellent studies that have highlighted the central role of the cholinergic ventral forebrain systems projecting to nearly all parts of the telencephalon, including the neocortical mantle, the hippocampal formation and the amygdaloid complex (Berger-Sweeney, 2003; McKinney & Jacksonville, 2005). However, our results are only partially comparable with those obtained by other prenatal mutations of the cholinergic basal forebrain system, e.g. following deletion of Lhx7. Although Lhx7 mutation has also been described as causing disturbances in spatial learning and memory, Lhx7-deficient mice displayed normal locomotor activity and motor coordination (Fragkouli et al., 2005, 2009a). This discrepancy could be explained by the fact that we have targeted PV-positive GABAergic neurons in addition to their cholinergic counterparts. In fact, GABAergic neurons of the ventral forebrain contribute significantly to the long projections that originate in the large magnocellular nuclei (Freund, 1989; Kermer et al., 1995; Gritti et al., 1997). It remains unclear to what extent the loss of GABAergic neurons might have contributed to the deficits in higher cognitive functions, as their role has been less intensively investigated than that of the cholinergic neurons (Schwegler et al., 1996). However, previous studies have also shown that deficits in higher cognitive functions, resulting from manipulation of the cholinergic systems, are less pronounced if performed in adult rodents (Berger-Sweeney, 2003). Indeed, in our study only a minor deficit in spatial learning and memory was observed for female ChATcre/+; Nkx2-1fl/fl mice (cf. Arters et al., 1998; Berger-Sweeney, 2003; Fragkouli et al., 2009a). A more detailed analysis performed for the MSvDB and hDB-SI did not provide evidence for a sex-specific effect of the mutations, at least at the level of cell numbers. It is reasonable to assume that more subtle changes contribute to cognitive impairments because prenatal targeting of Nkx2-1 expression was also found to be accompanied by an aberrant development of axonal projections in several regions of the mouse brain (Kawano et al., 2003).
Our study shows that (i) prenatal expression of Nkx2-1 is a prerequisite for the development of cholinergic neurons of the ventral forebrain and (ii) postnatal targeting of this transcription factor is still accompanied by substantial neuronal degeneration, corresponding target denervation, and mild impairments of the spatial memory.
Acknowledgments
We would like to thank H. Schwegler, H-D. Hofmann and J. A. Liebkowsky for critical reading of our manuscript. We also would like to thank G. Thomaschek, C. Guijarro, A. Steuer, J. Schuler and A. Wistel-Wozniak for their technical assistance. This work was supported by the DFG, Sonderforschungsbereich 665/A3 (Berlin) until 2009, and thereafter by B3.
Abbreviations
- AChE
acetylcholine esterase
- CB
calbindin
- ChAT
choline acetyltransferase
- CPu
caudate putamen
- CR
calretinin
- GAD67
glutamate decarboxylase 67
- GPe
globus pallidus - external segment
- GPi
globus pallidus - internal segment
- hDB-SI
horizontal limb of the diagonal band-substantia innominata
- IHC
immunohistochemistry
- ir
immunoreactive
- ISH
in situ hybridization
- LGP
lateral globus pallidus
- LS
lateral septum
- MGE
medial ganglionic eminence
- MS
medial septum
- MSvDB
medial septum-ventral limb of the diagonal band
- MWM
Morris water maze
- PB
phosphate buffer
- PFA
paraformaldehyde
- POA
preoptic area
- PV
parvalbumin
- SOM
somatostatin
- SVZ
subventricular zone
- VZ
ventricular zone
References
- Arters J, Hohmann CF, Mills J, Olaghere O, Berger-Sweeney J. Sexually dimorphic responses to neonatal basal forebrain lesions in mice: I. Behavior and neurochemistry. J Neurobiol. 1998;37:582–594. doi: 10.1002/(sici)1097-4695(199812)37:4<582::aid-neu7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Bayer SA. The development of the septal region in the rat. I Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol. 1979;183:89–106. doi: 10.1002/cne.901830108. [DOI] [PubMed] [Google Scholar]
- Bender R, Plaschke M, Naumann T, Wahle P, Frotscher M. Development of cholinergic and GABAergic neurons in the rat medial septum: different onset of choline acetyltransferase and glutamate decarboxylase mRNA expression. J Comp Neurol. 1996;372:204–214. doi: 10.1002/(SICI)1096-9861(19960819)372:2<204::AID-CNE4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J. The cholinergic basal forebrain system during development and its influence on cognitive processes: important questions and potential answers. Neurosci Biobehav Rev. 2003;27:401–411. doi: 10.1016/s0149-7634(03)00070-8. [DOI] [PubMed] [Google Scholar]
- Bert B, Fink H, Huston JP, Voits M. Fischer 344 and wistar rats differ in anxiety and habituation but not in water maze performance. Neurobiol Learn Mem. 2002;78:11–22. doi: 10.1006/nlme.2001.4040. [DOI] [PubMed] [Google Scholar]
- Bert B, Dere E, Wilhelmi N, Kusserow H, Theuring F, Huston JP, Fink H. Transient overexpression of the 5-HT1A receptor impairs water-maze but not hole-board performance. Neurobiol Learn Mem. 2005;84:57–68. doi: 10.1016/j.nlm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, Martella G, Madeo G, Schirinzi T, Puglisi F, Ponterio G, Pisani A. Centrality of striatal cholinergic transmission in Basal Ganglia function. Front Neuroanat. 2011;5:6. doi: 10.3389/fnana.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedveld GJ, van Dongen JW, Danesino C, Guala A, Percy AK, Dure LS, Harper P, Lazarou LP, van der Linde H, Joosse M, Gruters A, MacDonald ME, de Vries BB, Arts WF, Oostra BA, Krude H, Heutink P. Mutations in TITF-1 are associated with benign hereditary chorea. Hum Mol Genet. 2002;11:971–979. doi: 10.1093/hmg/11.8.971. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Schlote W. Intracellular lucifer yellow staining and electron microscopy of neurones in slices of fixed epitumourous human cortical tissue. Acta Neuropathol. 1987;75:140–146. doi: 10.1007/BF00687074. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- do Carmo Costa M, Costa C, Silva AP, Evangelista P, Santos L, Ferro A, Sequeiros J, Maciel P. Nonsense mutation in TITF1 in a Portuguese family with benign hereditary chorea. Neurogenetics. 2005;6:209–215. doi: 10.1007/s10048-005-0013-1. [DOI] [PubMed] [Google Scholar]
- Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- Du T, Xu Q, Ocbina PJ, Anderson SA. NKX2.1 specifies cortical interneuron fate by activating Lhx6. Development. 2008;135:1559–1567. doi: 10.1242/dev.015123. [DOI] [PubMed] [Google Scholar]
- Eckenstein F, Sofroniew MV. Identification of central cholinergic neurons containing both choline acetyltransferase and acetylcholinesterase and of central neurons containing only acetylcholinesterase. J Neurosci. 1983;3:2286–2291. doi: 10.1523/JNEUROSCI.03-11-02286.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandin P, Kimura S, Rubenstein JL. The progenitor zone of the ventral medial ganglionic eminence requires Nkx2-1 to generate most of the globus pallidus but few neocortical interneurons. J Neurosci. 2010;30:2812–2823. doi: 10.1523/JNEUROSCI.4228-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkouli A, Hearn C, Errington M, Cooke S, Grigoriou M, Bliss T, Stylianopoulou F, Pachnis V. Loss of forebrain cholinergic neurons and impairment in spatial learning and memory in LHX7-deficient mice. Eur J Neurosci. 2005;21:2923–2938. doi: 10.1111/j.1460-9568.2005.04141.x. [DOI] [PubMed] [Google Scholar]
- Fragkouli A, Pachnis V, Stylianopoulou F. Sex differences in water maze performance and cortical neurotrophin levels of LHX7 null mutant mice. Neuroscience. 2009a;158:1224–1233. doi: 10.1016/j.neuroscience.2008.11.026. [DOI] [PubMed] [Google Scholar]
- Fragkouli A, van Wijk NV, Lopes R, Kessaris N, Pachnis V. LIM homeodomain transcription factor-dependent specification of bipotential MGE progenitors into cholinergic and GABAergic striatal interneurons. Development. 2009b;136:3841–3851. doi: 10.1242/dev.038083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. GABAergic septohippocampal neurons contain parvalbumin. Brain Res. 1989;478:375–381. doi: 10.1016/0006-8993(89)91520-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Lopez M, Abellan A, Legaz I, Rubenstein JL, Puelles L, Medina L. Histogenetic compartments of the mouse centromedial and extended amygdala based on gene expression patterns during development. J Comp Neurol. 2008;506:46–74. doi: 10.1002/cne.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR. Basal ganglia. In: Paxinos G, editor. The Rat Nervous System. Elsevier Academic Press; San Diego: 2004. pp. 455–509. [Google Scholar]
- Gnahn H, Hefti F, Heumann R, Schwab ME, Thoenen H. NGF-mediated increase of choline acetyltransferase (ChAT) in the neonatal rat forebrain: evidence for a physiological role of NGF in the brain? Brain Res. 1983;285:45–52. doi: 10.1016/0165-3806(83)90107-4. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol. 1997;383:163–177. [PubMed] [Google Scholar]
- Hollerbach EH, Haas CA, Hildebrandt H, Frotscher M, Naumann T. Region-specific activation of microglial cells in the rat septal complex following fimbria-fornix transection. J Comp Neurol. 1998;390:481–496. [PubMed] [Google Scholar]
- Katarova Z, Sekerkova G, Prodan S, Mugnaini E, Szabo G. Domain-restricted expression of two glutamic acid decarboxylase genes in midgestation mouse embryos. J Comp Neurol. 2000;424:607–627. doi: 10.1002/1096-9861(20000904)424:4<607::aid-cne4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Kawano H, Horie M, Honma S, Kawamura K, Takeuchi K, Kimura S. Aberrant trajectory of ascending dopaminergic pathway in mice lacking Nkx2.1. Exp Neurol. 2003;182:103–112. doi: 10.1016/s0014-4886(03)00030-x. [DOI] [PubMed] [Google Scholar]
- Kermer P, Naumann T, Bender R, Frotscher M. Fate of GABAergic septohippocampal neurons after fimbria-fornix transection as revealed by in situ hybridization for glutamate decarboxylase mRNA and parvalbumin immunocytochemistry. J Comp Neurol. 1995;362:385–399. doi: 10.1002/cne.903620307. [DOI] [PubMed] [Google Scholar]
- Kim JG, Nam-Goong IS, Yun CH, Jeong JK, Kim ES, Park JJ, Lee YC, Kim YI, Lee BJ. TTF-1, a homeodomain-containing transcription factor, regulates feeding behavior in the rat hypothalamus. Biochem Biophys Res Commun. 2006;349:969–975. doi: 10.1016/j.bbrc.2006.08.147. [DOI] [PubMed] [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Kleiner-Fisman G, Lang AE. Benign hereditary chorea revisited: a journey to understanding. Mov Disord. 2007;22:2297–2305. doi: 10.1002/mds.21644. quiz 2452. [DOI] [PubMed] [Google Scholar]
- Korsching S. The neurotrophic factor concept: a reexamination. J Neurosci. 1993;13:2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krude H, Schutz B, Biebermann H, von Moers A, Schnabel D, Neitzel H, Tonnies H, Weise D, Lafferty A, Schwarz S, DeFelice M, von Deimling A, van Landeghem F, DiLauro R, Gruters A. Choreoathetosis, hypothyroidism, and pulmonary alterations due to human NKX2-1 haploinsufficiency. J Clin Invest. 2002;109:475–480. doi: 10.1172/JCI14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakabe T, Kawaguchi A, Hoshi N, Kawaguchi R, Hoshi S, Kimura S. Thyroid-specific enhancer-binding protein/NKX2.1 is required for the maintenance of ordered architecture and function of the differentiated thyroid. Mol Endocrinol. 2006;20:1796–1809. doi: 10.1210/me.2005-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Holtzman DM, Kromer LF, Kaplan DR, Chua-Couzens J, Clary DO, Knusel B, Mobley WC. Regulation of TrkA and ChAT expression in developing rat basal forebrain: evidence that both exogenous and endogenous NGF regulate differentiation of cholinergic neurons. J Neurosci. 1995;15:2888–2905. doi: 10.1523/JNEUROSCI.15-04-02888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magno L, Catanzariti V, Nitsch R, Krude H, Naumann T. Ongoing expression of Nkx2.1 in the postnatal mouse forebrain: potential for understanding NKX2.1 haploinsufficiency in humans? Brain Res. 2009;1304:164–186. doi: 10.1016/j.brainres.2009.09.050. [DOI] [PubMed] [Google Scholar]
- Marin O, Anderson SA, Rubenstein JL. Origin and molecular specification of striatal interneurons. J Neurosci. 2000;20:6063–6076. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronardi C, Smiley GG, Raber J, Kusakabe T, Kawaguchi A, Matagne V, Dietzel A, Heger S, Mungenast AE, Cabrera R, Kimura S, Ojeda SR. Deletion of the Ttf1 gene in differentiated neurons disrupts female reproduction without impairing basal ganglia function. J Neurosci. 2006;26:13167–13179. doi: 10.1523/JNEUROSCI.4238-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McKinney M, Jacksonville MC. Brain cholinergic vulnerability: relevance to behavior and disease. Biochem Pharmacol. 2005;70:1115–1124. doi: 10.1016/j.bcp.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Carnahan J, Nawa H. Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol. 1994;165:243–256. doi: 10.1006/dbio.1994.1250. [DOI] [PubMed] [Google Scholar]
- Mobley WC, Rutkowski JL, Tennekoon GI, Gemski J, Buchanan K, Johnston MV. Nerve growth factor increases choline acetyltransferase activity in developing basal forebrain neurons. Brain Res. 1986;387:53–62. doi: 10.1016/0169-328x(86)90020-3. [DOI] [PubMed] [Google Scholar]
- Naumann T, Linke R, Frotscher M. Fine structure of rat septohippocampal neurons: I. Identification of septohippocampal projection neurons by retrograde tracing combined with electron microscopic immunocytochemistry and intracellular staining. J Comp Neurol. 1992a;325:207–218. doi: 10.1002/cne.903250206. [DOI] [PubMed] [Google Scholar]
- Naumann T, Peterson GM, Frotscher M. Fine structure of rat septohippocampal neurons: II. A time course analysis following axotomy. J Comp Neurol. 1992b;325:219–242. doi: 10.1002/cne.903250207. [DOI] [PubMed] [Google Scholar]
- Naumann T, Casademunt E, Hollerbach E, Hofmann J, Dechant G, Frotscher M, Barde YA. Complete deletion of the neurotrophin receptor p75NTR leads to long-lasting increases in the number of basal forebrain cholinergic neurons. J Neurosci. 2002;22:2409–2418. doi: 10.1523/JNEUROSCI.22-07-02409.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega-Pereira S, Kessaris N, Du T, Kimura S, Anderson SA, Marin O. Postmitotic Nkx2-1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron. 2008;59:733–745. doi: 10.1016/j.neuron.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega-Pereira S, Gelman D, Bartolini G, Pla R, Pierani A, Marin O. Origin and molecular specification of globus pallidus neurons. J Neurosci. 2010;30:2824–2834. doi: 10.1523/JNEUROSCI.4023-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos GFK. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Plaschke M, Naumann T, Kasper E, Bender R, Frotscher M. Development of cholinergic and GABAergic neurons in the rat medial septum: effect of target removal in early postnatal development. J Comp Neurol. 1997;379:467–481. doi: 10.1002/(sici)1096-9861(19970324)379:4<467::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Pohlenz J, Dumitrescu A, Zundel D, Martine U, Schonberger W, Koo E, Weiss RE, Cohen RN, Kimura S, Refetoff S. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest. 2002;109:469–473. doi: 10.1172/JCI14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JL. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocamora N, Pascual M, Acsady L, de Lecea L, Freund TF, Soriano E. Expression of NGF and NT3 mRNAs in hippocampal interneurons innervated by the GABAergic septohippocampal pathway. J Neurosci. 1996;16:3991–4004. doi: 10.1523/JNEUROSCI.16-12-03991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol. 2002;12:26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- Schwegler H, Boldyreva M, Linke R, Wu J, Zilles K, Crusio WE. Genetic variation in the morphology of the septo-hippocampal cholinergic and GABAergic systems in mice: II. Morpho-behavioral correlations. Hippocampus. 1996;6:535–545. doi: 10.1002/(SICI)1098-1063(1996)6:5<535::AID-HIPO6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Sobreviela T, Clary DO, Reichardt LF, Brandabur MM, Kordower JH, Mufson EJ. TrkA-immunoreactive profiles in the central nervous system: colocalization with neurons containing p75 nerve growth factor receptor, choline acetyltransferase, and serotonin. J Comp Neurol. 1994;350:587–611. doi: 10.1002/cne.903500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Pearson RC, Powell TP. The cholinergic nuclei of the basal forebrain of the rat: normal structure, development and experimentally induced degeneration. Brain Res. 1987;411:310–331. doi: 10.1016/0006-8993(87)91084-5. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Son YJ, Hur MK, Ryu BJ, Park SK, Damante G, D’Elia AV, Costa ME, Ojeda SR, Lee BJ. TTF-1, a homeodomain-containing transcription factor, participates in the control of body fluid homeostasis by regulating angiotensinogen gene transcription in the rat subfornical organ. J Biol Chem. 2003;278:27043–27052. doi: 10.1074/jbc.M303157200. [DOI] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Eisenstat DD. Homeobox genes in vertebrate forebrain development and disease. Clin Genet. 2008;73:212–226. doi: 10.1111/j.1399-0004.2008.00967.x. [DOI] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Yoon SO, Casaccia-Bonnefil P, Carter B, Chao MV. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapala MA, Hovatta I, Ellison JA, Wodicka L, Del Rio JA, Tennant R, Tynan W, Broide RS, Helton R, Stoveken BS, Winrow C, Lockhart DJ, Reilly JF, Young WG, Bloom FE, Barlow C. Adult mouse brain gene expression patterns bear an embryologic imprint. Proc Natl Acad Sci USA. 2005;102:10357–10362. doi: 10.1073/pnas.0503357102. [DOI] [PMC free article] [PubMed] [Google Scholar]