Abstract
Background:
Osteoporosis and cardiovascular disease are interconnected entities with pathophysiological similarities. Bisphosphonates are therapeutic options available for resorptive bone diseases; however, experimental evidence has demonstrated a role for bisphosphonates in the inhibition of atherogenesis.
Methods:
A systematic review of the vascular effects of bisphosphonates on atherosclerosis was performed. Vascular effects were evaluated by the thickening of the intima-media of carotid arteries and calcification of the coronary and aorta arteries. Electronic databases PubMed, The Cochrane Library, and Embase from January 1980 to May 2011 were searched.
Results:
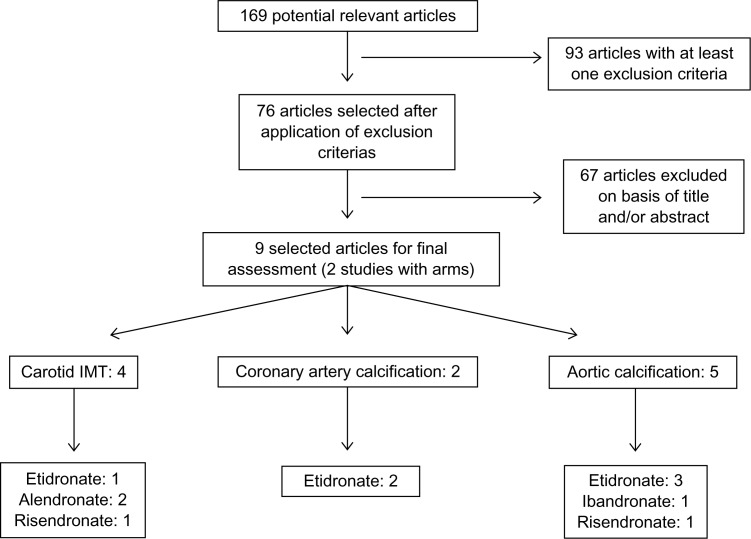
Of 169 potentially relevant articles, 9 clinical trials were selected. Two articles showed the benefit of the use of etidronate (−0.038 mm, P < 0.005) and alendronate (−0.025 mm, P < 0.05) on carotid artery intima-media thickening (CIMT) after one year. One article found no changes associated with the use of alendronate. The use of risedronate was associated with a reduction of plaque score on the carotid arteries (decrease of 1% at 1 year, P = 0.015). Of those studies that evaluated the effect on coronary artery calcification (CAC), the results are conflicting: one study showed no changes with use of etidronate and in another, etidronate resulted in inhibition of the process of CAC after 1 year of follow-up (−372 mm3 in CAC score, P < 0.01). Three studies showed positive effects of etidronate on the aortic calcificaton (AC) score, showing no effect with use of ibandronate, and another showed a inhibition in the progression of the abdominal AC score with use of risendronate (P = 0.043).
Conclusion:
Bisphosphonates seem to have an inhibitory effect on the atherosclerotic process; however, larger placebo-controlled studies are needed to better clarify this issue.
Keywords: Bisphosphonates, osteoporosis, atherosclerosis, carotid IMT, aortic calcification
Background
A new picture had emerged regarding two major public health problems: osteoporosis and cardiovascular disease (CVD). In the United States, CVD is the leading cause of mortality; it is estimated that approximately 58 million americans were affected by CVD in the period 1995 to 1996.1 One study has shown that the number of calcified atherosclerotic plaques correlates with high risk of coronary events.2 Similarly with respect to public interest, 30 million people in the United States have established osteoporosis or low bone mineral density (BMD).3 These entities share similarities in the pathophysiological process, and many studies have shown that osteoporosis is associated with CVD and influences mortality.4,5
Benivieni and Fallopio in the 1500s were the first to document a description of atherosclerosis as a degeneration of “arteries into bone,” which was called at that time ossification of the arteries. In 1863, Virchow stated that the vascular changes were ossification, not calcification, since they occurred by the same mechanism by which an osteophyte forms calcium on the surface of bone, and, in 1906, Bunting demonstrated the presence of bone marrow within a sclerotic aorta.6 Vascular calcifications are proposed to develop at early stages by a process of matrix vesicle formation in the intima of atheromatous plaques. The vesicles are then partially replaced with osteoid synthesized by osteoblast-like cells of mesenchymal origin and subsequent mineralization dependent on neovascularization/angiogenesis from the adventitial vasa vasorum, leading to formation of mature bone tissue in atherosclerotic plaques.7
The similarity of the molecular mechanisms in osteogenesis and vascular calcification led to the knowledge that, in fact, atherosclerotic calcification is an actively regulated process, not a passive mineralization.7 The mineral matrix of the plaque (hydroxyapatite) is identical to that found in bone.8,9 Atheroma plaque calcification is regulated by the process of calcification and plaque resorption.10 The elucidation of the role of various cells and numerous inflammatory mediators involved in the process of calcification/atherosclerosis is the goal of extensive research to identify potential agents for intervention.
Epidemiological studies have demonstrated that after adjusting for age, aortic calcified plaques or the speed of the arterial pulse wave are inversely related to BMD of the lumbar spine or distal radius.11–13 As well, decreased bone mass in the hip can indicate advanced atherosclerosis and vascular disease.14–17 In addition, low BMD at the distal radius has been found to be associated with increased risk of stroke and CVD mortality.5
The concept of common underlying mechanisms reinforces the idea that pharmacological agents that inhibit bone loss could also provide benefits in terms of slowing the progression of atherosclerosis. In this context, bisphosphonates appear to be a possible therapeutic option for atherosclerotic disease.18,19
Bisphosphonates are effective in treating and preventing osteoporosis by acting on the body’s main source of hydroxyapatite, the skeleton.20 Bisphosphonates are also effective for treating diseases associated with osteoclast-mediated bone resorption such as Paget’s disease, multiple myeloma, and osteolytic tumor metastasis. After the deposition of bone matrix, bisphosphonates are ingested by osteoclasts, inducing apoptosis, and leading to decreased bone resorption.21
These drugs may also be located in the calcified matrix outside the bone and have been found in calcified atherosclerotic plaques in animals and humans.21–24 The accumulation of bisphosphonates in atherosclerotic arteries is probably due to bisphosphonates connection with calcified atheromatous lesions, as it is well known that these drugs have high affinity for calcium and hydroxyapatite. However, the bisphosphonates etidronate and pamidronate were found in high concentrations in aortas without atherosclerotic lesions in mice and in healthy human mammary arteries.25,26 Accumulation of bisphosphonates in healthy aortas may be related to the inhibition of atherosclerosis or anti-cytotoxic effect observed in many studies.27–31
Some studies have demonstrated that bisphosphonates have a positive effect on atherosclerosis,32,33 but these studies are generally limited to animals or in vitro, in which the doses of bisphosphonates are much larger than those used in humans.30,32,34
Etidronate was the first bisphosphonate to demonstrate the suppression of the formation of atherosclerotic lesions in rat arteries, reducing calcification of the aorta and decreasing lipid plaques in medium caliber arteries as well as the mortality rate although there was no reduction in serum calcium, cholesterol, and triglycerides.27 Clodronate in high doses, when applied to rabbits, also significantly reduced the area of atherosclerotic lesions in the aorta, total cholesterol and fraction levels, and total calcium concentration in the aorta.30
More recently, alendronate and ibandronate demonstrated that they inhibit the calcification of all arteries and cardiac valves without effects on serum calcium and phosphorus in rats treated with arterial media calcification.35 Treatment with pamidronate for 2 years in monkeys inhibited the development of diet-induced atherosclerosis in the aorta and carotid arteries without significant change in serum cholesterol, calcium, glucose, blood pressure, cardiac output, and coagulation time.29
Various experimental evidence shows that bisphosphonates have a role in the inhibition of atherogenesis. However, in humans, initial studies have shown favorable or neutral results, and, therefore, this study aims to elucidate the role of bisphosphonates in human atherosclerosis.
Methods
A systematic literature review was performed to assess the association between the use of bisphosphonates and their effects assessed by the CIMT and calcification of the coronary and aorta arteries in humans.
A literature review was conducted using PubMed, The Cochrane Library, and Embase databases from January 1980 to May 2011 by means of the following keywords: “bisphosphonates AND endothelial dysfunction” and “bisphosphonates AND atherosclerosis.”
Articles such as clinical trials published in English and describing the association of bisphosphonates and the parameters of atherosclerosis were included in the review.
Studies were excluded if they were not available, on children, animals, in vitro or experimental, case reports, case series, letters to the editor, comments, review articles published in languages other than English and articles that did not fulfill the objectives of this review.
After using the keywords, 169 articles were identified of which 93 were excluded due to meeting at least one exclusion criteria, and 67 articles were excluded on the basis of title and/or abstract (Fig. 1).
Figure 1.
Search strategy for articles.
Results
Nine articles were included in the review, two of which had two arms. Carotid artery intima-media thickening was evaluated in 4 studies using alendronate, etidronate, and risedronate. The calcification of the coronary arteries was investigated in 2 studies with etidronate, while in 5 studies the authors observed the calcification of the aorta artery after administration of etidronate, ibandronate, and risendronato (Table 1).
Table 1.
Studies showing the effects of bisphosphonates on atherosclerosis.
| Study | Treatment | Duration (m) | Subjects (n) | Study design | Controlled study (n) | Objetives evaluated | Results |
|---|---|---|---|---|---|---|---|
| Koshiyama et al36 | ETD 200 mg/d during 14 d and every 3 m | 12 | Type 2 DM patients with osteopenia (57) | Clinical trial | Yes (57) | Carotid artery IMT |
↓ 0.038 mm (P < 0.005) |
| Celiloglu et al37 | Alendronate 70 mg/w + CaCO3 600 mg/d and VitD 400 UI/d | 12 | Women with osteoporosis (39) | Clinical trial | Yes (33) | Carotid artery IMT |
↓ 0.025 mm (P < 0.005) |
| Delibasi et al38 | Alendronate 70 mg/w | 12 | Postmenopausal women with osteoporosis (71) | Open clinical trial | No | Carotid artery IMT |
↓ 0.022 mm (P > 0.05) |
| Kanazawa et al39 | Risedronate 2.5 mg/d + alfacalcidol 1 μg/d | 12 | Postmenopausal women with type 2 diabetes and osteoporosis (13) | Clinical trial | Yes (33) | Plaque score AC |
↓ 1% (P = 0.015) ↓ 0.1% (P = 0.043) |
| Nitta et al40 | Etidronate 200 mg/d during 14 d and every 3 m | 9 | Dialysis patients (35) | Clinical trial | Yes (21) | CAC | ↓ 372 mm3 (P < 0.01) |
| Odate et al41 | Etidronate 400 mg/d | 6 | Patients on hemodialysis (8) | Clinical trial | Yes (6) | CAC AC |
↑ 880 mm3 (P = 0.069) ↓ 650 mm3 (P = 0.009) |
| Kawahara et al42 | Etidronate 400 mg/d + atorvastatine 20 mg/d during 14 d and every 3 m | 12 | Patients with hypercholesterolemia (45) | Clinical trial | Yes (42) | Thoracic and abdominal AC | ↓ 15% toracic AC (P < 0.001) ↓ 14% abdominal AC (P < 0.001) |
| Hashiba et al43 | Etidronate 200 mg/d | 23 | Dialysis patients (12) | Open clinical trial | Yes (9) | AC | ↑ 10.5% ETD × 115% placebo (P < 0.005) |
| Tankó et al44 | Ibandronate oral or IV* | 36 | Elderly post-menopausal osteoporotic women (263) | Clinical trial | Yes (128) | AC | No changes |
Note:
Oral: 2.5 mg/d or 20 mg every 2 days during 24 days every 3 months/IV: 0,5 mg or 1 mg every 3 months.
Abbreviations: D, day; M, months; W, week; N, number of subjects or control; ETD, etidronate; ALD, alendronate; RIS, risendronate; IBD, ibandronate; CaCO3, calcium carbonate; VitD, vitamine D; IMT, intima-media thickening; CAC, coronary artery calcification; AC, aortic calcification.
Carotid artery intima-media thickening
In the study of Koshiyama et al, the use of cyclical etidronate at a dose of 200 mg/day for 14 days in 57 Japanese patients with type 2 diabetes and osteopenia showed significant decrease in CIMT (−0.038 ± 0.011 mm) in relation to the control group (0.023 ± 0.015 mm, P < 0.005) after 1 year.36 Regarding alendronate, in a study of 39 women with post-menopausal osteoporosis, the use of alendronate (70 mg/week) along with calcium and vitamin D for a period of one year was correlated with a significant difference in CIMT. The measurements of CIMT at the beginning of the study and at 6 and 12 months were respectively 0.599, 0.611 and 0.620 mm in the placebo group and 0.622, 0.616 and 0.597 mm in the alendronate group (P < 0.05).37 However, Delibase et al in a study of 71 women with postmenopausal osteoporosis observed that the use of alendronate at a dose of 70 mg/week for 1 year showed no significant difference in CIMT: at the beginning of the study, the average CIMT was 0.734 ± 0.121 mm, and after treatment, 0.712 ± 0.111 mm (P > 0.05).38 The use of risedronate at 2.5 mg/day along with alfacalcidol 1 μg/day for 1 year in 13 Japanese patients with type 2 diabetes and osteoporosis was associated with inhibition of the progression of carotid calcification, identified by the plaque score of the carotid arteries (11.85 × 4.9 at baseline, 12.3 × 6.5 at 6 months, and 10.6 × 8.37 at 1 year) and significant differences in percent changes at each time point were found between the 2 groups (1.8 ± 12.9 × 69.6 ± 84.8, P = 0.016 at 6 months and −1.0 ± 14.2 × 101.2 ± 125.9, P = 0.015 at 1 year).39
Coronary artery calcification
Etidronate in a dose of 200 mg/day for 14 days (3 cycles repeated every 90 days) was used in 35 hemodialysis patients who experienced a reduction of the CAC score after 1 year of treatment. In the treatment group, the initial CAC was 1.489 mm3 (168 to 8768 mm3) vs. 1.303 mm3 (231 to 3133 mm3) in the placebo group, and after 1 year of follow-up, CAC was 1.117 mm3 (213 to 7348 mm3) in the etidronate group vs. 1.462 mm3 (220 to 3450 mm3) in the control group (P < 0.01).40 On the other hand, Odate et al, in a study with 8 patients on dialysis and prior ischemic artery disease, used etidronate at a dose of 400 mg/day for 24 weeks. There was no significant diference in the CAC score. There were increases in both the etidronate group (4100 ± 2440 mm3 at baseline to 4520 ± 2350 mm3 at 6 months and 4980 ± 2830 mm3 after 1 year, P = 0.069) and in the placebo group (8990 ± 15 180 mm3 at baseline to 9620 ± 15650 mm3 at 6 months and 11 210 ± 18 270 mm3 after one year, P = 0.51).41
Aortic calcification
The effect of etidronate (at a dose of 400 mg/day for 24 weeks) on the AC was evaluated in 8 patients with chronic renal failure on dialysis and with a prior history of ischemic arterial disease. A decrease was observed in AC over time (1000 ± 460 mm3 at baseline, 970 ± 580 at 6 months, and 350 ± 180 mm3 at 1 year, P = 0.009) corresponding to a mean percentage reduction of −64.1% in 1 year.41 Kawahara et al evaluated the effect of etidronate associated with atorvastatin on plaque regression of the thoracic and abdominal aorta in 45 patients. Etidronate was used at a dose of 400 mg/day and atorvastatin at 20 mg/day for 14 consecutive days every 3 months for a period of 12 months. It was observed a reduction in the maximum thickness of the vessel wall of thoracic and abdominal lesions (−15% and −14%, respectively, P < 0.001) and wall area of thoracic and abdominal aorta (−17% and −14%, respectively, P < 0.001).42 A third study with etidronate was conducted by Hashiba et al in 21 dialysis patients using a 200 mg dose on days of dialysis for 23 months. It was observed that the average aortic calcification area (ACA) showed no progression after treatment (352 ± 48.1 at baseline vs. 401 ± 63.6 after 23 months) although there was a significant increase in the control group (383 ± 138 at 0 month vs. 512 ± 134 after 23 months, P < 0.005).43
In a clinical trial with ibandronate, 263 women with postmenopausal osteoporosis and at least 1 vertebral fracture within the preceding 3 years were assessed at the following doses: orally 2.5 mg/day or 20 mg every two days for 24 days during a 3 month period and 0.5 or 1 mg intravenously (IV) every 3 months. In addition, women in all groups were given supplements of 500 mg of elemental calcium and 400 IU of vitamin D. Aortic calcification at baseline and at each subsequent year was assessed on lateral radiographs. Calcified deposits in the aorta adjacent to the lumbar vertebra (L1–L4) were assessed separately for the anterior and posterior wall using the midpoint of the inter-vertebral space as boundaries. Each wall of each segment was graded for the presence of calcified deposits with a score from 0 to 3 (0, no deposits; 1, calcified deposits on less than one-third of the aortic wall; 2, calcified deposits on one-third to two-thirds of the aortic wall; and 3, more than two-thirds of the aortic wall covered with calcified deposits). The relative increases from baseline (score at follow-up minus score at baseline) were defined as the progression of aortic calcification. The progression rate in the placebo group was 0.205 in the year compared with the progression rates the treatment groups which were rates of 0.205 and 0.199, respectively, in the IV ibandronate groups (0.5 mg and 1 mg) and rates of 0.173 and 0.271, respectively, in the oral ibandronate groups (2.5 and 20 mg).44 In a study of risedronate given at a dose of 2.5 mg/day along with alfacalcidol at 1 μg/day in 13 Japanese women with diabetes and postmenopausal osteoporosis, in a period of 1 year, there was no evidence of progression in the abdominal aortic calcification score (4.0 × 1.0 at baseline and 4.0 × 2.0 in 1 year) with significant differences in percent changes: −0.1 ± 43.6 × 54.4 ± 53.6, P = 0.043.39
Discussion
Many bisphosphonates have been evaluated regarding their vascular effects. Etidronate has been used in most studies and is considered the most potent inhibitor of vascular calcification. Many studies were small, having few patients (most of them with less 50 patients in the sample) and short duration of follow-up, and a few were placebo-controlled. The evaluated subjects, although they were mostly elderly, had independent cardiovascular risk factors such as dyslipidemia, diabetes mellitus, menopause, and dialysis treatment.
Koshiyama et al36 demonstrated that treatment with etidronate significantly reduced the CIMT, suggesting that this medication has an inhibitory effect on the early atherogenic process, although the dose used was much lower than the dose used in animals or in vitro.25,26,28–30,32,34
Alendronate, currently the most used drug for osteoporosis treatment, had conflicting effects on CIMT. In a study of osteoporotic women, Celiloglu et al37 demonstrated a significant decrease in CIMT compared with the significant increase in the control group, suggesting an inhibitory effect of alendronate on atherosclerosis. In contrast, Delibase et al38 showed no therapeutic benefit with alendronate in CIMT, although these values tended to decrease in post-menopausal women.
CVD is the leading cause of death in patients with chronic kidney disease. Studies are conflicting about the positive effect of bisphosphonates on the coronary arteries in this group of patients. The use of etidronate on dialysis patients showed no benefit in the suppression of coronary artery calcification, and this could be explained by the inhomogeneous accumulation of bisphosphonates in the different histological vessel layers.41 However, in one study, etidronate was associated with the suppression of coronary artery calcification in hemodialysis patients, suggesting a direct effect on the vascular wall, inhibiting arterial calcification.40 The administration of etidronate resulted in decreased aortic calcification in patients with end stage renal disease (ESRD) even after stopping treatment.41,43 This phenomenon could be explained by the long half-life of etidronate in bone45 and thus the high likelihood of a similar long half-life in ectopic calcified lesions.41 Likewise, these conflicting results may have been explained by the differences in the number of patients.
In one study, the combination therapy of atorvastatin and etidronate significantly reduced thoracic and abdominal aortic plaques as shown by magnetic ressonance imaging (MRI), whereas the isolated use of statins only reduced thoracic plaques. This suggest an additive vascular effect of bisphosphonates in association with statins.42
Finally, two studies evaluated the effects of newer bisphosphonates (risendronate and ibandronate) on vascular calcifications. In a randomized placebo-controlled study of 263 postmenopausal women, the use of ibandronate was not associated with significant changes in calcification scores during three years of follow-up.44 In contrast, in a small study of 13 post-menopausal women with type 2 diabetes, the use of oral risedronate at a dose of 2.5 mg per day along with alfacalcidol significantly reduced the progression of the aortic calcification score as shown by dual-emission x-ray absorptiometry (DXA) at one-year follow-up.39
Conclusion
Bisphosphonates seem to have an inhibitory effect on the atherosclerotic process; however, larger placebo-controlled studies are needed to better clarify this issue.
Footnotes
Author Contributions
Conceived and designed the experiments: LLS, TBC, FAB. Analysed the data: LLS, TBC, FAB. Wrote the first draft of the manuscript: LLS, TBC. Contributed to the writing of the manuscript: LLS, TBC. Agree with manuscript results and conclusions: LLS, TBC, FAB. Jointly developed the structure and arguments for the paper: LLS, TBC, FAB. Made critical revisions and approved final version: LLS, TBC, FAB. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Funding
Author(s) disclose no funding sources.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Desai MM, Zhang P, Hennessy CH. Surveillance for morbidity and mortality among older adults—United States, 1995–1996. MMWR CDC Surveill Summ. 1999;48:7–25. [PubMed] [Google Scholar]
- 2.Wayhs R, Zelinger A, Raggi P. High coronary artery calcium scores pose an extremely elevated risk for hard events. J Am Coll Cardiol. 2002;39:225–30. doi: 10.1016/s0735-1097(01)01737-5. [DOI] [PubMed] [Google Scholar]
- 3.Looker AC, Orwoll ES, Johnston CC, Jr, et al. Prevalence of low femoral bone density in older US adults from NHANES III. J Bone Miner Res. 1997;12:1761–8. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 4.McFarlane SI, Muniyappa R, Shin JJ, et al. Osteoporosis and cardiovascular disease: brittle bones and boned arteries, is there a link? Endocrine. 2004;23:1–10. doi: 10.1385/ENDO:23:1:01. [DOI] [PubMed] [Google Scholar]
- 5.von der Recke P, Hansen MA, Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999;106:273–8. doi: 10.1016/s0002-9343(99)00028-5. [DOI] [PubMed] [Google Scholar]
- 6.Acierno LJ. Atherosclerosis (arteriosclerosis) In: Acierno LJ, editor. The History of Cardiology. New York, NY: Parthenon Publishing Group Inc; 1994. pp. 109–26. [Google Scholar]
- 7.Demer LL, Tintut Y. Mineral exploration: search for the mechanism of vascular calcification and beyond: the 2003 Jeffrey M. Hoeg Award lecture. Arterioscler Thromb Vasc Biol. 2003;23:1739–43. doi: 10.1161/01.ATV.0000093547.63630.0F. [DOI] [PubMed] [Google Scholar]
- 8.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mautner GC, Mautner SL, Froehlich J, et al. Coronary artery calcification: assessment with electron beam CT and histomorphometric correlation. Radiology. 1994;192:619–23. doi: 10.1148/radiology.192.3.8058924. [DOI] [PubMed] [Google Scholar]
- 10.Bostrom K, Demer LL. Regulatory mechanisms in vascular calcification. Crit Rev Eukaryot Gene Expr. 2000;10:151–8. [PubMed] [Google Scholar]
- 11.Vogt MT, San Valentin R, Forrest KY, et al. Bone mineral density and aortic calcification: the Study of Osteoporotic Fractures. J Am Geriatr Soc. 1997;45:140–5. doi: 10.1111/j.1532-5415.1997.tb04498.x. [DOI] [PubMed] [Google Scholar]
- 12.Frye MA, Melton LJ, III, Bryant SC, et al. Osteoporosis and calcification of the aorta. Bone Miner. 1992;19:185–94. doi: 10.1016/0169-6009(92)90925-4. [DOI] [PubMed] [Google Scholar]
- 13.Ouchi Y, Akishita M, de Souza AC, et al. Age-related loss of bone mass and aortic/aortic valve calcification—reevaluation of recommended dietary allowance of calcium in the elderly. Ann N Y Acad Sci. 1993;676:297–307. doi: 10.1111/j.1749-6632.1993.tb38743.x. [DOI] [PubMed] [Google Scholar]
- 14.Van Der Klift M, Pols HA, Hak AE, et al. Bone mineral density and the risk of peripheral arterial disease: the Rotterdam study. Calcif Tissue Int. 2002;70:443–9. doi: 10.1007/s00223-001-2076-9. [DOI] [PubMed] [Google Scholar]
- 15.Vogt MT, Cauley JA, Kuller LH, et al. Bone mineral density and blood flow to the lower extremities: the study of osteoporotic fractures. J Bone Miner Res. 1997;12:283–9. doi: 10.1359/jbmr.1997.12.2.283. [DOI] [PubMed] [Google Scholar]
- 16.Tankó LB, Bagger YZ, Christiansen C. Low bone mineral density in the hip as a marker of advanced atherosclerosis in elderly women. Calcif Tissue Int. 2003;73:15–20. doi: 10.1007/s00223-002-2070-x. [DOI] [PubMed] [Google Scholar]
- 17.Montalcini T, Emanuele V, Ceravolo R, et al. Relation of low bone mineral density and carotid atherosclerosis in postmenopausal women. Am J Cardiol. 2004;94:266–9. doi: 10.1016/j.amjcard.2004.03.083. [DOI] [PubMed] [Google Scholar]
- 18.Watts NB. Bisphosphonate treatment of osteoporosis. Clin Geriatr Med. 2003;19:395–414. doi: 10.1016/s0749-0690(02)00069-1. [DOI] [PubMed] [Google Scholar]
- 19.Papapoulos SE. Ibandronate: a potent new bisphosphonate in the management of postmenopausal osteoporosis. Int J Clin Pract. 2003;57:417–22. [PubMed] [Google Scholar]
- 20.Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- 21.Izutani H, Miyagawa S, Shirakura R, et al. Recipient macrophage deletion reduces the severity of graft coronary arteriosclerosis in the rat transplantation model. Transplant Proc. 1997;29:861–2. doi: 10.1016/s0041-1345(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 22.Peller PJ, Ho VB, Kransdorf MJ. Extraosseous Tc-99m MDP uptake: a pathophysiologic approach. Radiographics. 1993;13:715–34. doi: 10.1148/radiographics.13.4.8356264. [DOI] [PubMed] [Google Scholar]
- 23.Myers DT, Karvelis KC. Incidental detection of calcified dialysis graft on Tc-99m MDP bone scan. Clin Nucl Med. 1998;23:173–4. doi: 10.1097/00003072-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Kim P, Turoglu T, Akisik MF, et al. Tc-99m MDP uptake in a calcified untreated non-Hodgkin’s lymphoma of spleen. Clin Nucl Med. 1997;22:343–4. doi: 10.1097/00003072-199705000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Ylitalo R, Monkkonen J, Urtti A, et al. Accumulation of bisphosphonates in the aorta and some other tissues of healthy and atherosclerotic rabbits. J Lab Clin Med. 1996;127:200–6. doi: 10.1016/s0022-2143(96)90079-7. [DOI] [PubMed] [Google Scholar]
- 26.Ylitalo R, Kalliovalkama J, Wu X, et al. Accumulation of bisphosphonates in human artery and their effects on human and rat arterial function in vitro. Pharmacol Toxicol. 1998;83:125–31. doi: 10.1111/j.1600-0773.1998.tb01455.x. [DOI] [PubMed] [Google Scholar]
- 27.Rosenblum IY, Flora L, Eisenstein R. The effect of disodium ethane-1-hydroxy-1,1-diphosphonate (EHDP) on a rabbit model of atheroarteriosclerosis. Atherosclerosis. 1975;22:411–24. doi: 10.1016/0021-9150(75)90021-0. [DOI] [PubMed] [Google Scholar]
- 28.Kramsch DM, Chan CT. The effect of agent interfering with soft tissue calcification and cell proliferation on calcific fibrous-fatty plaques in rabbits. Circ Res. 1978;42:562–71. doi: 10.1161/01.res.42.4.562. [DOI] [PubMed] [Google Scholar]
- 29.Kramsch DM, Aspen AJ, Rozler LJ. Atherosclerosis: prevention by agents not affecting abnormal levels of blood lipids. Science. 1981;213:1511–2. doi: 10.1126/science.6792706. [DOI] [PubMed] [Google Scholar]
- 30.Ylitalo R, Oksala O, Yla-Herttuala S, et al. Effects of clodronate (dichloro-methylene bisphosphonate) on the development of experimental atherosclerosis in rabbits. J Lab Clin Med. 1994;123:769–76. [PubMed] [Google Scholar]
- 31.Zhu B-Q, Sun Y-P, Sievers RE, et al. Effects of etidronate and lovastatin on the regression of atherosclerosis in cholesterol-fed rabbits. Cardiology. 1994;85:370–7. doi: 10.1159/000176738. [DOI] [PubMed] [Google Scholar]
- 32.Daoud AS, Frank AS, Jarmolych J, et al. The effect of ethane-1-hydroxy-1,1-d iphosphonate (EHDP) on necrosis of atherosclerotic lesions. Atherosclerosis. 1987;67:41–8. doi: 10.1016/0021-9150(87)90263-2. [DOI] [PubMed] [Google Scholar]
- 33.Price PA, Roublick AM, Williamson MK. Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int. 2006;70:1577–83. doi: 10.1038/sj.ki.5001841. [DOI] [PubMed] [Google Scholar]
- 34.Shioi A, Nishizawa Y, Jono S, et al. b-Glycerophosphonate accelerates calcification in cultured bovine vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1995;15:2003–9. doi: 10.1161/01.atv.15.11.2003. [DOI] [PubMed] [Google Scholar]
- 35.Price PA, Faus SA, Williamson MK. Bisphosphonates alendronate and ibandronate inhibitartery calcification at doses comparable to those that inhibit bone resorption. Arterioscler Thromb Vasc Biol. 2001;21:817–24. doi: 10.1161/01.atv.21.5.817. [DOI] [PubMed] [Google Scholar]
- 36.Koshiyama H, Nakamura Y, Tanaka S, et al. Decrease in carotid intima-media thickness after 1-year therapy with etidronate for osteopenia associated with type 2 diabetes. J Clin Endocrinol Metab. 2000;85:2793–6. doi: 10.1210/jcem.85.8.6748. [DOI] [PubMed] [Google Scholar]
- 37.Celiloglu M, Aydin Y, Balci P, et al. The effect of alendronate sodium on carotid artery intima-media thickness and lipid profile in women with post-menopausal osteoporosis. Menopause. 2009;16(4):689–93. doi: 10.1097/gme.0b013e318194cafd. [DOI] [PubMed] [Google Scholar]
- 38.Delibasi T, Emral R, Erdogan MF, et al. Effects of alendronate sodium therapy on carotid intima media thickness in postmenopausal women with osteoporosis. Adv Ther. 2007;24(2):319–25. doi: 10.1007/BF02849900. [DOI] [PubMed] [Google Scholar]
- 39.Kanazawa I, Yamaguchi T, Hayashi K, et al. Effects of treatment With risedronate and alfacalcidol on progression of atherosclerosis in postmenopausal women with type 2 diabetes mellitus accompanied with osteoporosis. Am J Med Sci. 2010;339(6):519–24. doi: 10.1097/MAJ.0b013e3181db6dfe. [DOI] [PubMed] [Google Scholar]
- 40.Nitta K, Akiba T, Suzuki K, et al. Effects of cyclic intermittent etidronate therapy on coronary artery calcification in patients receiving long-term hemodialysis. Am J Kidney Dis. 2004;44(4):680–8. [PubMed] [Google Scholar]
- 41.Odate T, Ariyoshi T, Eishi K, et al. Effect of etidronic acid on arterial calcification in dialysis patients. Clin Drug Invest. 2006;26(4):215–22. doi: 10.2165/00044011-200626040-00006. [DOI] [PubMed] [Google Scholar]
- 42.Kawahara T, Nishikawa M, Furusawa T, et al. Effect of atorvastatin and etidronate combination therapy on regression of aortic atherosclerotic plaques evaluated by magnetic resonance imaging. J Atheroscler Thromb. 2011;18(5):384–95. doi: 10.5551/jat.7104. [DOI] [PubMed] [Google Scholar]
- 43.Hashiba H, Aizawa S, Tamura K, et al. Inhibition of the progression of aortic calcification by etidronate treatment in hemodialysis patients: long-term effects. Ther Apher Dial. 2006;10(1):59–64. doi: 10.1111/j.1744-9987.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 44.Tankó LB, Qin G, Alexandersen P, et al. Effective doses of ibandronate do not influence the 3-year progression of aortic calcification in elderly osteoporotic women. Osteoporos Int. 2005;16:184–90. doi: 10.1007/s00198-004-1662-x. [DOI] [PubMed] [Google Scholar]
- 45.Ylitalo R. Bisphosphonates and atherosclerosis. Gen Pharmacol. 2000;35:287–96. doi: 10.1016/s0306-3623(01)00121-5. [DOI] [PubMed] [Google Scholar]