Abstract
We investigated the molecular and physiological processes of sugar uptake and metabolism during pollen tube growth and plant fertilization. In vitro germination assays showed that petunia (Petunia hybrida) pollen can germinate and grow not only in medium containing sucrose (Suc) as a carbon source, but also in medium containing the monosaccharides glucose (Glc) or fructose (Fru). Furthermore, high-performance liquid chromatography analysis demonstrated a rapid and complete conversion of Suc into equimolar amounts of Glc and Fru when pollen was cultured in a medium containing 2% Suc. This indicates the presence of wall-bound invertase activity and uptake of sugars in the form of monosaccharides by the growing pollen tube. A cDNA designated pmt1 (petunia monosaccharide transporter 1), which is highly homologous to plant monosaccharide transporters, was isolated from petunia. Pmt1 belongs to a small gene family and is expressed specifically in the male gametophyte, but not in any other vegetative or floral tissues. Pmt1 is activated after the first pollen mitosis, and high levels of mRNA accumulate in mature and germinating pollen. A model describing the transport of sugars to the style, the conversion of Suc into Glc and Fru, and the active uptake by a monosaccharide transporter into the pollen tube is presented.
The meiotic division of a pollen mother cell early in the development of the anther generates four immature male gametophytes. The gametophyte undergoes one mitotic division to generate one vegetative and one generative cell, after which the generative cell further divides to form two sperm cells (for review, see McCormick, 1993). The sperm cells are delivered to the female reproductive cells by unidirectional growth of the vegetative cell. This pollen tube grows through the stigma and style toward the ovules in the pistil. Much of the recent molecular research on the physiology of pollen tube growth focuses on specialized processes such as the incompatibility reaction, or on substances such as kinases, pectinases, polygalacturonases, and flavonols (Mascarenhas, 1993; McCormick, 1993). In contrast to this, one of the most striking phenomena of plant fertilization, the extreme speed and long-range capacity of pollen tube growth, has been poorly investigated on a molecular level. To enable the fast growth of the pollen tube, a rapid synthesis of wall material (Derksen et al., 1995) and a high energy supply is necessary. Therefore, a high level of sugar import is required (Schlüpmann et al., 1994).
During maturation in the anther, pollen accumulates high levels of carbohydrates that represent the major part of the mature grain's dry weight (Stanley and Linskens, 1974a; Pacini, 1996). After germination on a compatible stigma, the fast growth of the pollen tube is supported by the pistil. In the stylar fluids of petunia (Petunia hybrida) pistils the free sugars Suc, Glc, and Fru are available to the pollen tube (Konar and Linskens, 1966). After absorption by the pollen, sugars are utilized as an energy source and are converted to wall material as pectins, cellulose, and callose (Mascarenhas, 1993; Derksen et al., 1995). Constant de novo synthesis of cell wall material is essential because many of the carbohydrates used for wall synthesis are dissipated for participation in further pollen tube formation.
The primary source of carbohydrates in the pistil and pollen lies in the photosynthesizing mature leaves, where assimilation takes place. After assimilation sugars are transported through the phloem to sink tissues such as the anther and stylar apoplast, mostly in the form of Suc (Bush, 1993). Nonetheless, it should be noted that the transmitting tract cells of the petunia pistils contain chloroplasts (B. Ylstra, personal observations) and might therefore also contribute directly to sugars in the stylar apoplast (Jansen et al., 1992). The final destination of the sugars, however, is the pollen, which requires translocation from the anther, stigma, and stylar apoplast over the pollen (tube) membrane.
Several sugar transporters were identified in plants (Sauer and Tanner, 1989, 1993; Bush, 1993) and their genes were cloned and characterized by transgenic expression in yeast (Sauer and Tanner, 1993; Sauer and Stolz, 1994). The gene products were designated mono- and dissaccharide transmembrane symporters, and actively translocate sugars across plasma membranes driven by a proton-electrochemical potential (Stadler et al., 1995). The dissaccharide-symporter genes isolated are especially transcribed in mature leaves. The monosaccharide transporters reported so far are primarily transcribed in sink tissues such as young leaves and in storage and floral organs (Sauer and Tanner, 1993). In terms of sink-source relations pollen should be regarded as a strong sink, since it is not able to assimilate but requires high levels of starch and carbohydrates during maturation, germination, and growth.
In vitro germination assays are helpful in the study of the growth requirements of pollen tubes. Different chemical constituents, pH, and viscosity could be related to the quality of pollen development and quantity of tube growth (Stanley and Linskens, 1974a, 1974b; Jahnen et al., 1989; Derksen et al., 1995). Suc was most commonly included in the in vitro germination medium as a carbon source. However, sugar import into germinating and growing pollen has only been studied to a limited extent in lily (Deshusses et al., 1981). We present physiological and biochemical experiments that enable us to describe the uptake of carbohydrates by pollen tubes in molecular terms.
MATERIALS AND METHODS
Plant Material
Petunia (Petunia hybrida var R27) was grown under standard greenhouse conditions.
Carbohydrates during in Vitro Pollen Tube Growth
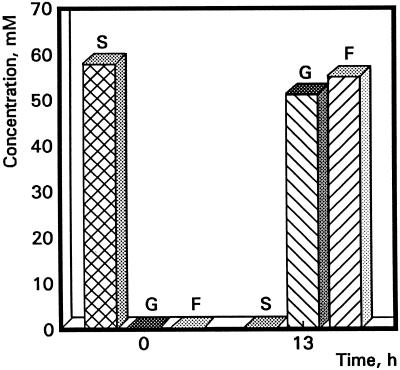
Pollen was collected from flowers at anthesis and germinated in medium according to the method of Jahnen et al. (1989). After 13 h of in vitro pollen tube growth, 1 mL of culture was centrifuged and filtered (0.22 μm, Optex, Millipore) to exclude pollen tubes and remnants. Sugars in the germination medium were quantified by HPLC analysis on a 6.5- × 300-mm column (Shodex SC-1011, Waters Chromatography Division, Millipore) run at 85°C with ultrapure water at 0.75 cm3 min−1. Suc, Glc, and Fru were detected with a 2142 refractor index detector (Pharmacia). One representative experiment out of three is presented in Figure 2.
Figure 2.
Histogram representing HPLC analysis data of the sugar content in the germination medium before and after pollen tube growth. Samples were taken at two different time points, 0 and 13 h after in vitro pollen tube growth. S, Suc (cross-hatch); G, Glc (left hatch); F, Fru (right hatch).
To monitor the carbohydrates that can promote in vitro tube elongation, Suc in the germination medium was substituted by D(+)-Glc monohydrate, D(−)-Fru extra pure (Merck, Darmstadt, Germany), or D(+)-mannitol (Janssen Chimica, Beerse, Belgium) in a concentration of 2% (w/w). Photographs of the cultures were made after 8 and 24 h.
Isolation of the Full-Size Pmt1 cDNA
Total RNA from pollen was purified for the mRNA fraction by an oligo-dT column according to the instructions of the manufacturer (Pharmacia). Subsequently, first-strand cDNA was synthesized using the oligonucleotide primer PR1, 5′-CCGGATCCTCTAGAGCGGCCGC(T)17-3′ and rav-2 reverse transcriptase, according to the instructions of the manufacturer (Amersham). Together with a second oligonucleotide primer PR2, 5′-ATGGTCGACT (G/T)(G/T/C)GCIAA(A/G)(A/G/C)(G/C)(I/C)(I/C)T(I/C)CC(A/T/C)GG-3′, a first PCR was performed (annealing sites of primers are underlined in Fig. 3). Amplification involved 30 PCR thermal cycles with 1 μg of degenerated primer, 200 ng of undegenerated primer, 10 mm of each deoxynucleotide triphosphate, and 5 IU of Taq DNA polymerase (Boehringer Mannheim) in 50 μL of the manufacturer's PCR buffer using a thermal DNA cycler (model 480, Perkin Elmer). The thermal PCR cycle involved denaturating for 30 s at 94°C, a transition of 30 s, annealing for 60 s at 46°C, another transition of 60 s, and synthesis for 60 s at 72°C. Amplified cDNA was fractionated on a 1% agarose gel. A clear fragment 0.6 kb in length was cloned into pEMBL derivates, using the restriction sites BamHI and SalI present in the primers, and sequenced (see below).
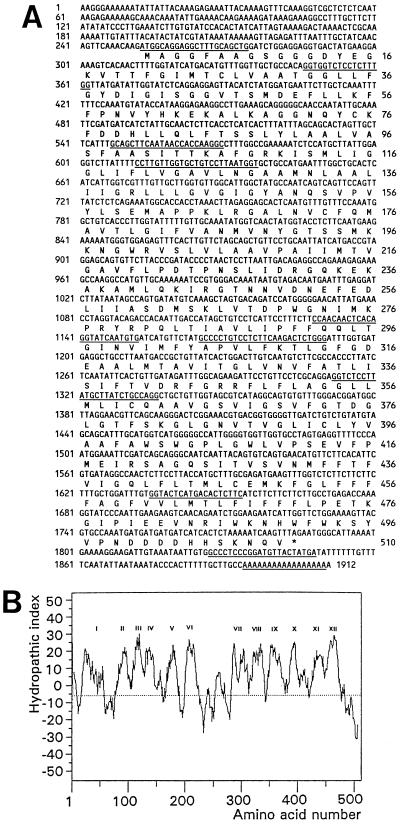
Figure 3.
Pmt1 cDNA, amino acid sequence, and hydropathic character of the protein. A, Pmt1 cDNA sequence (numbered left) and deduced amino acid sequence (numbered right). The annealing sites of the primers used are underlined, successively PR9, PR3, PR8, PR7, PR6, PR5, PR2, PR4, PR10, and PR1. B, Hydrophobicity plot of the deduced pmt1 amino acid sequence with the 12 hydrophobic regions indicated by Roman numerals.
To obtain an additional 5′ cDNA sequence a second PCR cycle was performed according to the same PCR procedure with the degenerated primer PR3, 5′-CGGGATCCGGGTGG(T/C)(T/C)T(C/G)AT(T/C)TT(T/C)GG-3′ homologous 5′ sequences of earlier published monosaccharide transporters (Sauer and Tanner, 1993) and PR4, 5′-CGGAATTCGAAGAGTGTCATGAGTACC-3′, with inverse homology to the 5′ region of the 0.6-kb PCR fragment. The restriction enzymes BamHI and EcoRI were used to clone the new fragment in pEMBL18, however, only a 300-bp additional sequence was obtained by this approach. To obtain the complete cDNA sequence, RACE-PCR was performed in two subsequent experiments according to the manufacturer's protocol (5′ AmpliFINDER RACE kit; Clontech Labs, Palo Alto, CA). The oligonucleotide primer PR5, 5′-GCTCTAGACCAGAGTCTTGAAGAGGACAGGGGC-3′, was used for first-strand cDNA synthesis. A nested primer PR6, 5′-GCTCTAGAGCACATTGATACCTGTGAGTTGTTGG-3′, together with the AmpliFINDER RACE anchor primer was used for amplification by PCR.
In the second round the oligonucleotide primer PR7, 5′-GCTCTAGACCATTAAGGACAGCACCAACAAGG-3′, was used for first-strand cDNA synthesis. Subsequently, the nested primer PR8, 5′-GCTCTAGAGCCTTGGTGGTTATTGAAGCTGC-3′ together with the anchor primer were used for PCR amplification. 5′ RACE-amplification PCR involved 30 thermal cycles with 200 ng of both primers, 10 mm of each deoxynucleotide triphosphate, and 0.5 IU of Taq polymerase (HT-Biotechnology, Cambridge, UK) in 50 μL of the manufacturer's PCR buffer. Synthesis time in the thermocycler was elongated to 120 s, after gel electrophoresis fragments of 700 or 600 kb, respectively, were cloned into pEMBL18 using the restriction sites XbaI and EcoRI present in the 5′ regions of the primers for the first RACE or blunt end (SmaI) for the second RACE.
To obtain the complete monosaccharide transporter cDNA a PCR amplification on pollen mRNA was made with the primers PR9, 5′-GCTCTAGACCATGGCAGGAGGCTTTGCAGCTG-3′, and PR10, 5′-CGGGATCCAGAATCTGAGGTCATAGTAACATCCGGGAGGGC-3′, complementary to, respectively, the 5′ and 3′ of the pmt1-coding region, which yielded a fragment with the expected size of approximately 1.6 kb. This fragment was ligated in pEMBL19 and the pmt1-coding region was verified by sequence analysis. This full-size cDNA served as a probe for further analysis.
DNA and RNA Gel-Blot Analysis
For DNA-blot analysis 10 μg of total plant DNA was isolated from young leaves, digested with EcoRI, HindIII, or XbaI, and electrophoresed (Koes et al., 1986). Total RNA was isolated from different plant parts and organs according to the method of Verwoerd et al. (1989). The developmental stage of the microspores in the anthers was determined using propion carmine staining. For germination, pollen from anthers at anthesis were germinated at room temperature for 13 or 24 h in germination medium. An aliquot was counterstained with 4′,6-diamidino-2-phenylindole and ethidium bromide to determine the amount of nuclei in the tubes. Total RNA was denatured by glyoxal/DMSO and electrophoresed on a 1.2% agarose gel in 15 mm sodium phosphate buffer, pH 6.5, according to the method of Angenent et al. (1992). Gels were blotted onto Hybond N+ membranes and hybridized at 65°C in 1 m NaCl, 1% SDS, and 10% dextrane sulfate. The full-size cDNA-coding region (nucleotides 253–1848) served as a probe (see Fig. 3 and previous section). After overnight hybridization, the membranes were washed in 2× SSC or 0.1× SSC with 0.1% SDS at 50°C or 65°C, and an autoradiogram was made.
DNA Sequencing and Homology Comparison
The full-size pmt1 cDNA was sequenced in both directions. Therefore, dideoxy chain termination was used for sequencing either single- or double-stranded DNA pEMBL plasmid derivates. Reactions were performed with either dyedeoxy or dye primer ready-reaction sequencing kits (PRISM, Applied Biosystems) according to the manufacturer's protocol.
Protein structure was predicted by the program SOAP of the PC gene software, calculated with a window size of nine amino acids (Kyte and Doolittle, 1982). Homology comparison with the EMBL databank was performed using the program BLAST according to the method of Altschul et al. (1990). Homologies presented in Table I represent the percentage of identity from the start codon or first Met residue to the stop codon or the last amino acid, respectively.
Table I.
Percentage identity between monosaccharide transporters from a variety of species
| Organism | Gene | pmt1 | stp4 | mst1 | stp1 | hup1 | gtr1 |
|---|---|---|---|---|---|---|---|
| Petunia | pmt1 | 65 | 63 | 60 | 54 | 47 | |
| Arabidopsis | stp4 | 62 | 63 | 63 | 53 | 48 | |
| Tobacco | mst1 | 60 | 63 | 70 | 52 | 46 | |
| Arabidopsis | stp1 | 59 | 62 | 79 | 54 | 47 | |
| C. kessleri | hup1 | 44 | 45 | 45 | 46 | 49 | |
| Rat | gtr1 | 30 | 28 | 30 | 30 | 29 |
Nucleotide sequence homologies (top right, coding region) and deduced amino acid sequence homologies (bottom left) are shown.
RESULTS
Pollen Use Monosaccharides as a Carbon Source for Tube Growth
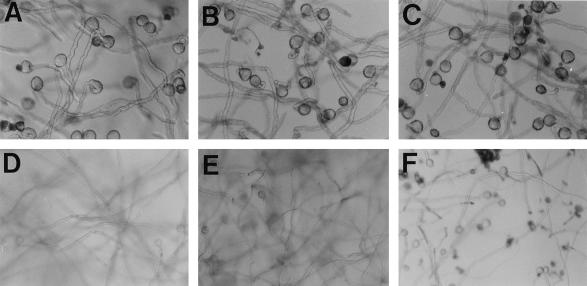
To identify which sugars are required and at what point during in vitro pollen tube growth, Suc in the germination medium was substituted with the monosaccharides Glc, Fru, or mannitol. In Figure 1 pollen tube growth in these media is shown. After 8 h of incubation no differences were observed with regard to the quality and the growth rates of pollen in the four different media (Fig. 1, A–C). Within the following 16 h, tube growth in Suc, Fru, and Glc continued and long tubes developed. These long tubes entangled to wadding-like structures that floated in the media, so the tubes were out of focus when photographed (Fig. 1, D and E). In the mannitol-containing medium, however, tube growth was severely retarded after 24 h of culturing. Mannitol-cultured pollen tubes remained at the bottom of the wells and were often dead, with relatively short tubes (Fig. 1F). Mannitol is not well utilized by plants, and the initial development in mannitol probably reflects autonomous growth on internally stored sugars (Fig. 1C). Taken together, these experiments show that an external carbon source is essential to maintaining pollen tube growth. However, this assay could not discriminate whether monosaccharides, disaccharides, or both were actually imported into growing petunia pollen tubes.
Figure 1.
Light-microscopic photographs of pollen tubes grown in vitro with different carbon sources in 2% Suc after 8 h (A) and 24 h (D); in 2% Fru after 8 h (B) and 24 h (E) (pollen tubes grown in 2% Glc revealed an identical picture (not shown); and in 2% mannitol after 8 h (C) and 24 h (F). Pollen tubes in D and E are out of focus because they float in the medium due to their length. A through C, Magnifications ×100; D through F, magnifications ×64.
In a subsequent experiment the question was addressed whether the composition of the sugars in the 2% Suc medium was altered during in vitro tube growth. An HPLC approach was chosen to determine the amount of free sugars at the start of the germination assay and after 13 h of culturing. In Figure 2 it can be seen that at the onset of germination the medium contained 58.4 mm Suc (2%). After 13 h of growth the Suc was completely hydrolyzed into nearly equimolar amounts of Glc and Fru. The fact that disaccharides were converted into monosaccharides strongly suggests uptake of carbohydrates by pollen tubes as monosaccharides.
Sequence Analysis of the Petunia Pmt1 cDNA from Pollen
In a research program aimed at the isolation of genes specifically active in pollen and homologous to the human androgen receptor, a PCR experiment was performed using mRNA isolated from mid-binucleate pollen, the poly-dT primer PR1, and the degenerated primer PR2. A number of PCR products unrelated to human steroid receptors were isolated and sequenced. One of the cDNA fragments was highly homologous to the sequence of monosaccharide transporters STP1 and MST1. Since our physiological data suggested that pollen tubes import monosaccharides rather than disaccharides (previous section), this previously isolated cDNA clone was selected for further analysis in this study.
The first cDNA fragment obtained contained a 500-bp open reading frame and an approximately 100-bp 3′-untranslated tail. To obtain additional 5′ cDNA sequences a second PCR was performed with the degenerated primer PR3, which is homologous to earlier published monosaccharide transporters (Sauer and Tanner, 1993), combined with the specific primer PR5. This approach resulted in the isolation of a cDNA with only a 300-bp additional sequence. To obtain the complete sequence two subsequent RACE-PCR reactions were performed, which revealed a total cDNA sequence of 1912 bp. Sequence analysis indicated an open reading frame of 1533 bp, a tail of 127 bp, and a 252-bp untranslated leader, encoding a full-size protein designated PMT1 (Fig. 3A). Reverse-transcriptase PCR with the two primers PR9 and PR10, 5′ and 3′ of the pmt1 open reading frame, confirmed the presence of this mRNA in pollen. Analysis of the deduced amino acid sequence displayed 12 hydrophobic regions (Fig. 3B). This strongly suggests that PMT1 is a membrane-spanning protein with 12 transmembrane regions.
Pmt1 mRNA Is Highly and Specifically Transcribed during Pollen Maturation and Tube Growth
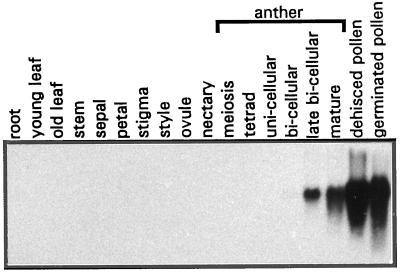
Pmt1 cDNA was isolated using RNA derived from maturing bicellular pollen grains. To monitor the expression pattern of the pmt1 gene, RNA isolated from many different plant tissues, including young seed pods (not shown), root, leaf, stem, and a variety of reproductive floral tissues, was subjected to northern-blot analysis. Special attention was given to tissues and organs that are known to serve an important function in carbohydrate metabolism. Young and old leaves act as, respectively, carbohydrate sink or source, and therefore their RNAs were isolated separately. The nectaries in the petunia flower are specialized organs that contain and excrete high levels of Suc (A.J. Koops and A.J. van Tunen, personal observations). Furthermore, RNAs from stigmas and styles were separately extracted because these tissues supply sugars to the growing pollen tubes. Moreover, RNA was isolated from anthers at different developmental stages. Finally, RNA was derived from mature and germinated pollen. Northern analysis revealed transcription of the pmt1 gene exclusively in mature anthers and pollen (Fig. 4). No transcripts were detected in any of the other tissues tested. Pmt1 transcription starts to be detectable in anthers directly after the first pollen mitosis and rapidly increases to very high levels in mature pollen. After 13 h of in vitro germination high levels of pmt1 transcripts were still detected (Fig. 4) and did not decrease after 24 h of germination, when the second pollen mitosis occurs (data not shown).
Figure 4.
Northern-blot analysis of mRNA derived from vegetative and reproductive tissues, anthers, and pollen. Vegetative tissues include: root, young leaf (sink leaf), old leaf (source leaf), and stem. Reproductive tissues (open flowers) include: sepal, petal, stigma, style, ovule, and nectary. Also shown are anthers with male generative gametophytes at successive developmental stages: meiosis; tetrad; unicellular; bicellular, late bicellular, and mature (immediately before anther dehiscence); and mature dehisced and germinated pollen (after 13 h of in vitro germination).
The signal on the northern blot can be regarded as highly specific for the pmt1 gene, since the hybridization pattern was similar under less stringent washing conditions (2× SSC at 65°C, data not shown) and cross-hybridization to other members of the monosaccharide transporter family in petunia was not observed. This is in line with the results of the Southern-blot analysis (see below), which showed that other family members are less than 70% identical to pmt1.
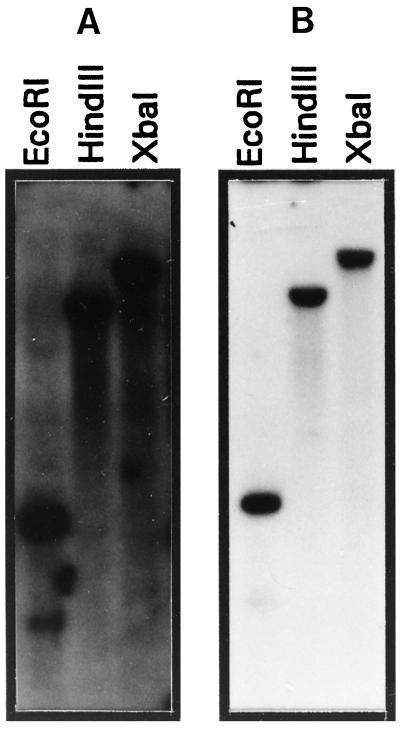
Pmt1-Related Sequences in the Petunia Genome and Other Species
Southern-blot analysis was performed to determine the presence of related genes in the genome of petunia. Under low-stringency conditions (2× SSC at 50°C) four to five bands hybridized to the pmt1 probe in separate digestions (Fig. 5A). This indicates a family of four to five pmt1-related members in the petunia genome. The same probe also detected multiple bands with DNA digests from tobacco and Arabidopsis (data not shown). At high-stringency conditions (0.1× SSC at 65°C) only a single band was observed for each digestion of the petunia DNA (Fig. 5B). After washing at moderate stringency (2× SSC at 65°C) one single band was detected (data not shown). Based on this hybridization pattern, the homology of pmt1 to the other genes of the family is less than 70% based on DNA level, and indicates the presence of a single pmt1 copy in the petunia genome.
Figure 5.
Genomic organization of the pmt1 gene family. A, Southern-blot analysis under low-stringency conditions (2× SSC at 50°C). B, Southern-blot analysis under high-stringency conditions (0.1× SSC at 65°C). Restriction enzymes used were EcoRI, HindIII, or XbaI.
A homology search was performed to determine the homology of pmt1 with other sequences in the databanks. This search revealed significant levels of nucleotide and amino acid homology to several monosaccharide transporters from various unicellular, mammalian, and plant species (Table I). The highest homology was found with the monosaccharide-H+ symporter STP4 from Arabidopsis, which was 62% at the amino acid level (Truernit et al., 1996). A high level of homology was also found to the solanaceous monosaccharide transporter MST1 and a second Arabidopsis monosaccharide transporter STP1, with, respectively, 60% or 59% identity to the PMT1 protein (Sauer et al., 1990; Sauer and Stadler, 1993). Furthermore, moderate but significant homology was found between pmt1 and the hup1 cDNA from Chlorella kessleri and the Glc transporter isolated from rat brain (Birnbaum et al., 1986; Sauer and Tanner, 1989; Table I).
DISCUSSION
Pollen tubes require high and rapid sugar uptake to support their growth. The physiological data presented in this article suggest that pollen tubes import carbohydrates in the form of monosaccharides rather than disaccharides. This observation was supported by the isolation of the cDNA clone pmt1, isolated from petunia pollen. Pmt1 shared high and significant homology to earlier reported monosaccharide-proton symporters and its mRNA transcripts were exclusively detected in mature anthers, isolated pollen, and growing tubes.
In conventional media used for germination assays, the disaccharide Suc is included as a carbon source (Stanley and Linskens, 1974b; Jahnen et al., 1989; O'Driscoll et al., 1993; Schlüpmann et al., 1994; Derksen et al., 1995). The HPLC analysis presented in this article showed that during the in vitro germination of petunia pollen the Suc in the medium was rapidly converted into Glc and Fru (Fig. 2). This rapid and dramatic hydrolysis of Suc during pollen tube growth was most likely the result of wall-bound invertase activity. Pollen invertase activity was also reported for in vitro-germinated pollen of the monocot lily (Singh and Knox, 1984). The fact that pollen exhibits such invertase activity strongly suggests that pollen imports carbohydrates in the form of monosaccharides. Nonetheless, alternative ways of sugar uptake by pollen should not be excluded. O'Driscoll et al. (1993), for example, showed uptake of fluorescein isothiocyanate-labeled dextrans via endocytosis.
The PMT1 protein shared high overall homology to earlier reported monosaccharide transporters (Table I). PMT1 contains all conserved amino acids and motifs: Q175, Q293, Q294, HWFW490-493, (R/K)-GR(R/K)343-347, and (V/L)PETKG472-476 (Sauer and Stadler, 1993; Caspari et al., 1994; Will et al., 1994; Harrison, 1996; Fig. 3A). In addition, an Asp residue essential for the activity of the C. kessleri HUP1 gene in Schizosaccharomyces pombe was conserved at position 39 of PMT1, as well as the residues V433 and N436 of HUP1, which compared to V428 and N431 of PMT1 (Caspari et al., 1994; Will et al., 1994). Analogous to the earlier reported transmembrane sugar transporters, PMT1 contains 12 putative transmembrane regions (Sauer and Tanner, 1993; Fig. 3B). Taken together, the high overall homology, the conservation of specific amino acids, and the presence of 12 membrane-spanning domains strongly suggest that pmt1 encodes a monosaccharide-transporter protein.
Pmt1 transcription is pollen specific, and expression starts after the first pollen mitosis. The mRNA transcripts accumulate to remarkably high levels in mature pollen and remain present during pollen tube growth. PMT1 is more closely related to the cruciferous monosaccharide transporter, STP4 (Truernit et al., 1996), than to the solanaceous monosaccharide transporter, MST1 (Sauer and Stadler, 1993). The homology of pmt1 to stp4 is reflected in their site of transcription. Aside from expression in the anther, stp4 is also expressed in root tips and in response to stress (Truernit et al., 1996). Arabidopsis monosaccharide-transporter stp1 is more closely related to mst1 from tobacco (Sauer et al., 1991; Sauer and Stadler, 1993; Table I). This suggests the presence of an additional monosaccharide-transporter gene in petunia more homologous to MST1/STP1, and could suggest different classes of monosaccharide transporters. Arabidopsis contains a family of at least five functionally active monosaccharide-transporter genes, and seven more homologous genes were isolated (Sauer and Tanner, 1993; Sauer et al., 1994; Treurnit et al., 1996). Southern-blot analysis demonstrated the presence of a small multigene family in petunia with four to five members (Fig. 5).
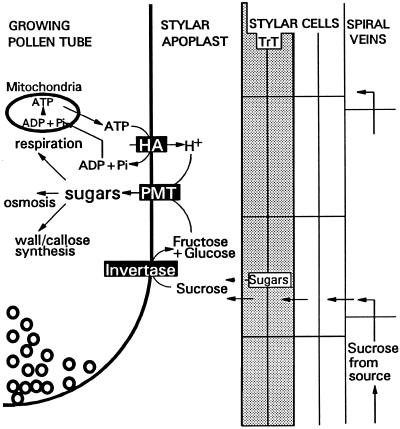
Based on the data presented in this article and the current knowledge of carbohydrate transport, we propose a model for carbohydrate supply, metabolism, and translocation into growing pollen tubes (Fig. 6). Suc is transported from the source tissues, especially mature leaves, through the phloem toward the pistils (Bush, 1993). To reach the pollen tube, Suc has first to be unloaded from the spiral veins into the outer layers of the style, and is subsequently transported to the stylar apoplast of the transmitting tract. A low amount of sugars might also be produced by the green transmitting tract cells (Jansen et al., 1992). In the apoplast, the sugars encounter the growing pollen tubes that have invertase activity converting Suc into Fru and Glc. Subsequently, these monosaccharides are translocated over the pollen tube membrane by the PMT1 protein. In their studies on H+ and [U-14C] uptake of labeled Suc, Deshusses et al. (1981) proposed a proton-sugar cotransport in lily pollen tubes. Like transport in lily and like all other monosaccharide transporters, PMT1 would act as a symporter, transferring monosaccharides together with a proton. This requires proton export driven by ATP. Houlné and Boutry (1994) isolated a plasma membrane-bound H+-ATPase, aha9, from Arabidopsis. Aha9 proved to be anther specific, which could mean that for carbon loading in pollen, in addition to a specific monosaccharide transporter present in the plasma membrane, there is also a specific H+-ATPase gene. After transport over the pollen tube membrane the monosaccharides are used for callose plug formation, (callose) tube wall synthesis, and respiration required for successful fertilization.
Figure 6.
Schematic representation of a model for carbohydrate loading of pollen tubes growing through the transmitting tract, with PMT1, H+-ATPase (HA), and wall-bound invertase (inverse highlighted). Left to right, One-half of a tip of a growing pollen tube, the stylar apoplast with transmitting tract cells (TrT, shaded), flanked by nongreen stylar cells with phloem cells (spiral veins). “Sugars” in the TrT cells represent additional sugars synthesized and eventually exported (dotted arrow) from green stylar cells. “Sugars” in the pollen tube represent carbohydrates after transport as monosaccharides over the membrane by PMT1. Small circles at the tube apex represent membrane vesicles and exocytosis (Derksen et al., 1995).
ACKNOWLEDGMENTS
The authors thank Dr. A.J. Koops for assistance with HPLC analysis. Dr. A.J. Koops, Prof. Dr. J.N.M. Mol, and Dr. H. Dons are thanked for helpful suggestions and critical reading of the manuscript.
Abbreviation:
- RACE
rapid amplification of cDNA ends
Footnotes
This work was supported in part by a grant from the Spanish Ministry of Science and Education to D.G.
The accession number for the sequence reported in this work is AF061106.
LITERATURE CITED
- Altschul SF, Gish W, Miller W, Meyers WM, Lippmann DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Angenent GC, Busscher M, Franken J, Mol JNM, van Tunen AJ. Differential expression of two MADS box genes in wild-type and mutant petunia flowers. Plant Cell. 1992;4:983–993. doi: 10.1105/tpc.4.8.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum MJ, Haspel HC, Rosen OM. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci USA. 1986;83:5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DR. Proton-coupled sugar and amino acid transporters in plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:513–542. [Google Scholar]
- Caspari T, Stadler R, Sauer N, Tanner W. Structure/function relationship of the Chlorella glucose/H+ symporter. J Biol Chem. 1994;269:3498–3502. [PubMed] [Google Scholar]
- Derksen J, Rutten T, van Amstel T, de Win A, Doris F, Steer M. Regulation of pollen tube growth. Acta Bot Neerl. 1995;44:93–119. [Google Scholar]
- Deshusses J, Gumber SC, Loewes FA. Sugar uptake in lily pollen. A proton symport. Plant Physiol. 1981;67:793–796. doi: 10.1104/pp.67.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MJ. A sugar transporter from Medicago truncatula: altered expression patterns in roots during vascular-arbuscular (VA) mycorrhizal associations. Plant J. 1996;9:491–503. doi: 10.1046/j.1365-313x.1996.09040491.x. [DOI] [PubMed] [Google Scholar]
- Houlné G, Boutry M. Identification of an Arabidopsis thaliana gene encoding a plasma membrane H+-ATPase whose expression is restricted to anther tissues. Plant J. 1994;5:311–317. doi: 10.1111/j.1365-313x.1994.00311.x. [DOI] [PubMed] [Google Scholar]
- Jahnen W, Lush WM, Clarke AE. Inhibition of in vitro pollen tube growth by isolated S-glycoproteins of Nicotiana alata. Plant Cell. 1989;1:501–510. doi: 10.1105/tpc.1.5.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen MAK, Sessa G, Malkin S, Fluhr R. PEPC-mediated carbon fixation in transmitting tract cells reflects style-pollen tube interactions. Plant J. 1992;2:507–515. [Google Scholar]
- Koes RE, Spelt CE, Reif HJ, van den Elzen PJM, Veltkamp E, Mol JNM. Floral tissue of Petunia hybrida (V30) expressed only one member of the chalcone synthase multi-gene family. Nucleic Acids Res. 1986;14:379–392. doi: 10.1093/nar/14.13.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konar RN, Linskens HF. Physiology and biochemistry of the stigmatic fluid of Petunia hybrida. Planta. 1966;71:372–387. doi: 10.1007/BF00396322. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Mascarenhas JP. Molecular mechanisms of pollen tube growth and differentiation. Plant Cell. 1993;5:1303–1314. doi: 10.1105/tpc.5.10.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S. Male gametophyte development. Plant Cell. 1993;5:1265–1275. doi: 10.1105/tpc.5.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Driscoll D, Hann C, Read SM, Steer MW. Endocytotic uptake of fluorescent dextrans by pollen tubes grown in vitro. Protoplasma. 1993;175:126–130. [Google Scholar]
- Pacini E. Types and meaning of pollen carbohydrate reserves. Sex Plant Reprod. 1996;9:362–366. [Google Scholar]
- Sauer N, Baier K, Gahrtz M, Stadler R, Stolz J, Truernitt E. Sugar transport across the plasma membranes of higher plants. Plant Mol Biol. 1994;64:1671–1679. doi: 10.1007/BF00016496. [DOI] [PubMed] [Google Scholar]
- Sauer N, Friedländer K, Gräml-Wicke U. Primary structure, genomic organization and heterologous expression of a glucose transporter from Arabidopsis thaliana. EMBO J. 1990;9:3045–3050. doi: 10.1002/j.1460-2075.1990.tb07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer N, Stadler R. A sink-specific H+/monosaccharide co-transporter from Nicotiana tabacum, cloning and heterologous expression in baker's yeast. Plant J. 1993;4:601–610. doi: 10.1046/j.1365-313x.1993.04040601.x. [DOI] [PubMed] [Google Scholar]
- Sauer N, Stolz J. SUC1 and SUC2: Two sucrose transporters from Arabidopsis thaliana: expression and characterization in baker's yeast and identification of the histidine-tagged protein. Plant J. 1994;6:67–77. doi: 10.1046/j.1365-313x.1994.6010067.x. [DOI] [PubMed] [Google Scholar]
- Sauer N, Tanner W. The hexose carrier from Chlorella. FEBS Lett. 1989;259:43–46. doi: 10.1016/0014-5793(89)81489-9. [DOI] [PubMed] [Google Scholar]
- Sauer N, Tanner W. Molecular biology of sugar transporters in plants. Bot Acta. 1993;106:277–286. [Google Scholar]
- Schlüpmann H, Bacic A, Read SM. Uridine diphosphate glucose metabolism and callose synthesis in cultured pollen tubes of Nicotiana alata link et otto. Plant Physiol. 1994;105:659–670. doi: 10.1104/pp.105.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MB, Knox RB. Invertases of Lilium pollen: characterization and activity during in vitro germination. Plant Physiol. 1984;74:510–515. doi: 10.1104/pp.74.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Wolf K, Hilgarth C, Tanner W, Sauer N. Subcellular localization of the inducible chlorella HUP1 monosaccharide-H+ symporter and cloning of a co-induced galactose-H+ symporter. Plant Physiol. 1995;107:33–41. doi: 10.1104/pp.107.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley RG, Linskens HF (1974a) Carbohydrates and cell walls. In Pollen Biology, Biochemistry and Management. Springer-Verlag, Berlin, pp 129–144
- Stanley RG, Linskens HF (1974b) Viability tests. In Pollen Biology, Biochemistry and Management. Springer-Verlag, Berlin, pp 67–86
- Truernit E, Schmid J, Epple P, Illig J, Sauer N. The sink specific and stress-regulated Arabidopsis STP4 gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell. 1996;8:2169–2182. doi: 10.1105/tpc.8.12.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd TC, Dekker BMM, Hoekema A. A small scale procedure for the rapid isolation of RNAs. Nucleic Acids Res. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will A, Caspari T, Tanner W. Km mutants of the Chlorella monosaccharide/H+ cotransporter randomly generated by PCR. Proc Natl Acad Sci USA. 1994;91:10163–10167. doi: 10.1073/pnas.91.21.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]