Abstract
Background:
The high frequency of hemoglobinopathies in Brazil constitutes a public health problem and thus educational and preventive measures are necessary to reduce the incidence. Genetic guidance, a modality of genetic counseling, and family screening are measures that can assist in reproductive decisions and mitigate clinical, psychological and social problems of families with these disorders.
Objetive:
The objective of the current study was to evaluate the effectiveness of educational and preventive measures for hemoglobinopathies using genetic guidance and laboratory screening of families.
Methods:
The diagnoses of patients with hemoglobinopathies were confirmed and then the level of knowledge about their disease was evaluated and genetic guidance was provided. Three months later, the level of assimilated information of these patients was evaluated. In addition, laboratory diagnosis of family members was carried out.
Results:
Diagnosis of sickle cell anemia was confirmed for most patients. Moreover, the majority of the patients who had a low level of knowledge before genetic guidance (68.8%) demonstrated a higher level of assimilated information after the process (81.8%). Almost 70% of the family members had hemoglobin changes and some had hemoglobinopathies(2.6%). They were duly informed about the results of the examinations, which made it possible to investigate further.
Conclusion:
Genetic guidance and family screening were effective preventive and educational measures that improved the quality of life of patients, preventing complications and sequels and allowed the referral of those who may transmit altered genes for clinical diagnosis and to genetic counseling services.
Keywords: Genetic counseling, Hemoglobinopathies/diagnosis, Primary prevention, Quality of life
Introduction
Currently 270 million people have some kind of hemoglobinopathy(1), the most common genetic diseases in the world(2), with clinical profiles that range from asymptomatic to lethal(3). Of the most common hemoglobinopathies in Brazil, it is estimated that one child with sickle cell anemia is born in every thousand live births(4) and that the prevalence of thalassemias is from 0.5% to 1.5% of the Brazilian population(5). There is an additional two to ten million people with the sickle cell trait(6), asymptomatic individuals who need to be identified because their children could have the disease(7). In Goiás, according to the Ministry of Health, for every 100 births, four children are diagnosed as having the sickle cell trait(8).
This high prevalence of genetic hemoglobin disorders justifies the need for free early diagnosis programs and medical, social and psychological guidance with educational and preventive measures for patients and laboratory screening of the family(1,9).
The Brazilian government recognized the importance of adopting educational and preventive measures for hemoglobinopathies when it made the Guthrie test for diagnosing the disorder(10,11) mandatory in 2002. This test identifies a number of congenital or infectious diseases in the neonatal period which in turn allows health professionals to select prophylactic measures(12). Consequently, SUS, the government healthcare system, established guidelines for a "National Policy of Comprehensive Care for People with Sickle Cell Disease and other Hemoglobinopathies" which includes the promotion of lifelong learning and access to information and genetic counseling for people with the disease or sickle cell trait and their families(13).
Genetic counseling is an educational and preventive communication process for patients and family members that, among other things, explains the occurrence or risk of a genetic disease(14). Its purpose is to help individuals understand the medical facts related to prognosis, diagnosis and treatment,the role of heredity in the occurrence of the disorder, the likely impact on other family members, family planning options and the most appropriate means of managing hematological changes(15,16).
Genetic guidance is a type of genetic counseling which targets people at potential risk of having children with genetic alterations such as hemoglobinopathies(17). In this approach, even when patients or family members are directly involved with the disorder, they do not need to make any immediate reproductive decision(18). They are given educational and reproductive counseling by a multidisciplinary team of professionals suitably trained to transmit information relevant to the management of genetic diseases(19).
The objective of the present study was thus to evaluate the effectiveness of genetic guidance and laboratory screening of family members as educational and preventive measures in respect to hemoglobinopathies in Goiás
Methods
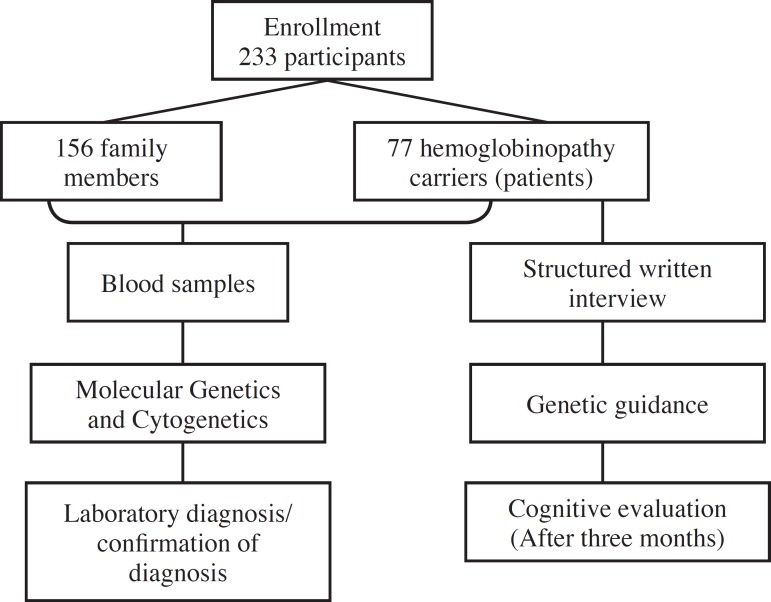
After approval by the Human and Animal Research Ethics Committee (CEPMHA) of the Hospital das Clínicas of the Universidade Federal de Goiás (HC-UFG), 77 patients with hemoglobinopathies and156 family members were enrolled at the UFG Hemoglobinopathy Outpatient Clinic between June 2010 and June 2011 (Figure 1).
Figure 1.
Methodological flowchart of the application of educational and preventive measures
Venous blood samples (4 mL) were collected from all 233 subjects in specific tubes containing the anticoagulant ethylenediaminetetraacetic acid (EDTA). The biological material was sent to the UFG Molecular Genetics and Cytogenetics Laboratory (LGMC-UFG) to confirm the patients' diagnosis and to test other family members.
The laboratory investigation was performed using selective and diagnostic tests such as the filter paper solubility test(20), osmotic resistance test in 0.36% saline(21), erythrocyte morphology(22) and alkaline electrophoresis in cellulose acetate at pH 8.6(23). Positive results were confirmed by acid electrophoresis in agarose gel(24); hemoglobin (Hb) A2 (23) was measured when the beta thalassemia trait was suspected and intra-erythrocyte Hb H testing was performed when electrophoresis was positive(25).
The 77 patients with hemoglobinopathies (41 adults, 12 patientsbetween 12 and 17 who had their parents' permission to participate and the legal guardians of 24 patients under 12 years old) answered a written interview divided into two parts (Appendix 1). The first part included data about sociodemographic variables such as age, nationality, place of residence, education, professional activity, marital status and ethnic background. The second part consisted of questions directly related to the concept and cause of the disease under consideration, genetic inheritance, the difference between the carrier of sickle cell trait and sickle cell anemia and genetic guidance thereby assessing the respondent's level of knowledge. It is important to point out that the "education" variable may have influenced the answers given in the second part of the interview. Therefore, the educational level of the parents of children under 12 was considered as they participated in the research.
After the interview, the carriers of hemoglobinopathies received individualized genetic guidance in a suitable place to safeguard the privacy of those involved. Using simple language, a trained professional transmitted scientific information about the disease, its course, its main clinical manifestations, the risk of recurrence and measures used to prevent transmission. As teaching materials in this educational process, small wooden cubes with letters representing different hemoglobins on each side were used as well as objects that simulated sickle-shaped red blood cells, small and pale red blood cells, red blood cells with hemoglobin C and unchanged red blood cells. In addition, illustrative figures were presented to reinforce the concepts.
At the end of the guidance session, patients received written material containing information about the aspects covered.
Three months after the genetic guidance session, patients with hemoglobinopathies returned to HC-UFG for a cognitive assessment, that is, their level of assimilated information was assessed (Appendix 2). For this purpose patients answered hypothetical questions regarding the likelihood of children being born with the disease according to the parents' genotype. The written cognitive evaluation was scored and the result was compared with the level of knowledge demonstrated before the guidance session.
The data obtained from the experimental part of the study were entered into Microsoft Excel 2007 and analyzed using the nonparametric Descartes' Rule of Signs(26).
Results
Table 1 shows the lab test confirmation of the patients' diagnosis and the laboratory diagnosis of the family members.
Table 1.
Laboratory confirmation of the diagnosis of hemoglobinopathy carriers and laboratory diagnosis of family members
| Patients (n = 77) | n (%) |
| No changes (Hb AA) | 1 (1.3) |
| Increase in Hb F (Hb AA/↑F) | 1 (1.3) |
| Sickle cell trait (Hb AS) | 1 (1.3) |
| Hb CC disease | 2 (2.6) |
| Hb SC disease | 22 (28.6) |
| Sickle cell anemia (Hb SS) | 42 (54.5) |
| Sickle cell anemia and increase in Hb F (Hb SS/↑F) | 5 (6.5) |
| Sickle cell anemia and presence of Hb H (Hb SS/H) | 3 (3.9) |
| Family members (n = 156) | n (%) |
| Sickle cell trait (HB AS) | 79 (50.6) |
| Sickle cell trait and Hb H (Hb AS/H) | 2 (1.3) |
| Hb C trait (Hb AC) | 24 (15.4) |
| Presence of Hb H | 1 (0.6) |
| Increase in Hb A2 | 1 (0.6) |
| Hb SC disease | 3 (2.0) |
| Hb CC disease | 1 (0.6) |
| No changes (Hb AA) | 45 (28.9) |
Hb: hemoglobin; Hb F: fetal hemoglobin; ↑: increase
The laboratory diagnoses of 67 (87.0%) of the 77 patients with hemoglobinopathies were confirmed, demonstrating that most patients in this study had sickle cell anemia. Of the 10 (13%) remaining patients, one individual was diagnosed with sickle cell trait and one did not have laboratory abnormalities, although both reported the presence of clinical manifestations. Moreover, the association of sickle cell anemia and thalassemia, represented by an increase in fetal hemoglobin andhemoglobin H, had not been detected previously.
Of the 156 family members, 111 (71.2%) presented some kind of hemoglobin change; four (2.6%) family members had hemoglobinopathies and 107 (68.6%) had heterozygous forms of diseases. All family members with hemoglobin alterations received information about the test results and, specifically, the four family members diagnosed with the disease were advised to seek medical help because they did not know about their clinical conditions.
Besides confirmation of the diagnosis, 77 patients provided data in the interview about sociodemographic characteristics and their knowledge on the subject. The majority of cases were male, over 18 years old, of mixed race and had not completed primary school (Table 2).
Table 2.
Sociodemographic characteristics of individuals with hemoglobinopathies (n = 77)
| Characteristic | n (%) |
| Gender | |
| Male | 39 (50.6) |
| Female | 38 (49.4) |
| Age | |
| 0 to 11 | 24 (31.2) |
| 12 to17 | 13 (16.9) |
| ≥18 | 40 (51.9) |
| Ethnical background | |
| White | 14 (18.2) |
| Mixed race | 38 (49.4) |
| Black | 25 (32.5) |
| Place of Birth | |
| Goiás | 48 (62.3) |
| Tocantins | 9 (11.7) |
| Other | 20 (26.0) |
| Place of Residence | |
| Goiás | 74 (96.1) |
| Mato Grosso | 3 (3.9) |
| Occupation | |
| Student | 41 (53.2) |
| Homemaker | 9 (11.7) |
| Others | 27(35.1) |
| Marital Status | |
| Single | 36 (46.8) |
| Married | 17 (22.1) |
| Under 12 years old* | 24 (31.2) |
| Educational Level** | |
| Primary school incomplete | 29 (37.7) |
| Primary school complete | 14 (18.2) |
| Secondary school incomplete | 12 (15.6) |
| Secondary school complete | 22 (28.6) |
* Marital status of minors under twelve was not included
** The educational level of the parents of children under 12 was included
Fifty-three (68.8%) patients had little knowledge at the time of the interview (Table 3), that is, they were unable to demonstrate the knowledge about terminology, concepts, causes and heredity needed to answer simple questions about the disease.
Table 3.
Distribution of hemoglobinopathy patients by knowledge level before and after genetic guidance (n = 77)
| Level of knowledge | Pre test | Post test |
| n (%) | n (%) | |
| Low | 53 (68.8) | 3 (3.9) |
| Medium | 22 (28.6) | 11 (14.3) |
| High | 2 (2.6) | 63 (81.8) |
p-value < 0.001 - Descartes' Rule of Signs
Patients with hemoglobinopathies received genetic guidance and after 3 months returned to HC-UFG for their level of assimilated knowledge to be assessed. On the basis of this cognitive assessment, 63 respondents (81.8%) had a high level of assimilated knowledge after genetic guidance (Table 3).
At the end of the study the knowledge of none of the individuals was less. On the contrary, the knowledge of 70 patients (90.9%) was higher (Table 4).
Table 4.
Changes in knowledge of patients with hemoglobinopathies (n = 77)
| Difference in knowledge | n (%) |
| Worse | - (0.0) |
| Remained the same | 7 (9.1) |
| Better | 70 (90.9) |
p < 0.001 - Descartes' Rule of Signs
Discussion
As a result of the genetic guidance provided by the researchers of this study, the majority of patients with hemoglobinopathies who entered the study with a low level of knowledge demonstrated a high level of assimilated knowledge after the procedure. The low level of information at the beginning of the research can be explained by the fact that most interviewees had a low educational level and had not participated in guidance programs about this genetic condition.
This educational process increased the knowledge of more than 80% of patients about key aspects of the disease such as the process of gene transmission to offspring and the differences between asymptomatic carriers and homozygous individuals, concepts essential for the adoption of preventive measures. Moreover, genetic guidance may also have helped reinforce and retain the knowledge of the 11 participants (14.3%) who did not show any improvement at the end of the study but remained aware of what was presented after participating in this educational process.
The importance of this type of intervention in preventing hemoglobinopathies is confirmed by Guimarães & Coelho(18), who state that genetic counseling can guide individuals and families to make balanced decisions about procreation given the limitations ofgene therapy and the incurability of the disorder. Viana-Baracioliet al.(9) also claim that proper guidance can minimize clinical, psychosocial and financial problems related to the disease.
Moreover, Giordano(27) affirms that the key elements to prevent hemoglobinopathies are information, carrier diagnosis and genetic counseling. He makes it clear that it is necessary to investigate the family of carriers in an attempt to find other members with the gene and to allow those with the disease a free and well-informed reproductive choice.
The effectiveness of genetic guidance depends on accurate diagnosis since it is not possible to base the procedure on hypotheses. In this study, confirmation of the patients' diagnosis was indispensable, since different patterns were found in the hemoglobin electrophoresis of ten patients. Previous laboratory tests had not detected the presence of hemoglobin H or the increase infetal hemoglobin, findings characteristic of thalassemia. Moreover, the two patients with clinical symptoms of disease but without the corresponding laboratory abnormalities should be carefully evaluated to see if in fact they have other hemoglobin alterations that were not detected by the techniques used here, if another hematologic disorder may be involved or if there really are clinical manifestations, even if they are manifestations of sickle cell trait.
The laboratory screening of family members also brought positive results. More than 70% of these individuals were identified as having hemoglobin changes and all were informed about their laboratory diagnosis and instructed to seek clinical follow-up and genetic counseling.
The identification of a large number of asymptomatic carriers underlines the preventive importance of laboratory screening because each heterozygous carrier has a 50% chance of passing the altered gene on to each child that he or she may have. Indeed, the identification of four family members (2.6%) who had diseases but had no clinical manifestations was fundamental in advising them of the need of quick clinical care.
Few studies on family screening were found in Brazil. A work of Bandeira et al.(1), that screened family members of patients with hemoglobin S at a referral center in Pernambuco,is worth mentioning. These researchers found only 26.1% of heterozygotes for sickle cell disease.
Even with the few family screening studies in Brazil, most health policies for hemoglobinopathies direct their educational measures toward potential parents of sickle cell children in order to identify them and then advise them about genetic risk.
The effectiveness of these diagnostic and guidance programs is not unique to Brazil. In Italy and the Netherlands, for example, prevention programs have opted family screening and counseling for patients and family members(28). Fucharoen(29) reports that prevention programs for hemoglobinopathies in Asia need adequate educational support and investigations need to be expanded to the patient's family in order for individuals to make informed reproductive decisions. In Africa, Fattoum(30) says that even with the difficulties in finding sufficient epidemiological data for hemoglobinopathies, prevention is possible by means of a solid program that provides information and population screening.
Overall, genetic guidance and laboratory screening of the family turned out to be effective educational and preventive measures as the majority of patients improved their level of knowledge and a large number of family members who were carriers of hemoglobin changes were identified. Such measures can reduce the morbidity and mortality due to the disease, improve patients' quality of life, prevent complications and sequelae, detect changes in hemoglobin, refer patients for genetic counseling and help with reproductive decisions.
Since the HC-UFG does not offer a specific registry for patients with hemoglobinopathies, there was difficulty in recruiting patients; the methodology would have been better if the sample were larger and stratified. Different medical conduct with appropriate prophylaxis should be adopted by the hospital team for patients with different diagnoses. Further guidance sessions, preferably performed by a larger number of qualified professionals, should be offered to individuals who were unable to retain the instructions provided and the practice should be extended to family members. More specific tests, preferably based on molecular biology, are needed to establish a definitive diagnosis of thalassemias and identify other anomalous hemoglobins.
Appendix 1
Questionnaire to assess the knowledge of patients with hemoglobinopathies prior to genetic guidance
Questionnaire
Part 2: Evaluation of the level of knowledge of patients with hemoglobinopathies
1) Have you ever heard of hereditary anemia or hemoglobinopathies or sickle cell disease and thalassemia?
A - ( ) Yes, hereditary anemia
B - ( ) Yes, hemoglobinopathies
C - ( ) Yes, sickle cell disease or thalassemias
D - ( ) I do not know
Note: answers A or B: patient has knowledge of broader terms. Answer C: he or she only knows the name of his or her own disease. Answer D: has no knowledge of nomenclature.
2) Can you define or explain what your illness is (what you have?)
A - ( ) anemia that has no cure
B - ( ) anemia that requires continued treatment
C - ( ) sickle cell anemia (name only)
D - ( ) sickle cell disease (name only)
E - ( ) sickle cell trait (name only)
F - ( ) thalassemia (name only)
G - ( ) I do not know
Note: Answer A: knowledge of one of the most relevant aspects of the disease. Answer B: knowledge about the character of an incurable genetic disease. Answers C, D, E and F: knowledge only of the name, but without explanation. Response G: has no knowledge about the type of hereditary anemia.
3) What is the cause of your illness? (Why do you have this disease?)
A - ( ) heredity (came from my parents / ancestors)
B - ( ) problem inherited from my parents / ancestors
C - ( ) red blood cells or hemoglobin are different
D - ( ) blood doesn't have oxygen
E - ( ) I do not know Note: Answers A and B: knowledge about the hereditary nature of the disease. Answers C and D: some pathophysiological knowledge, but does not address heredity. Answer E: has no knowledge of heredity.
4) Do you know if there is a difference between a person who is sick and a person with sickle cell trait?
A - ( ) the person with the trait does not feel anything, or doesn't need treatment
B - ( ) the patient has symptoms and must be treated
C - ( ) the person with the trait only carries the gene and does not get sick
D - ( ) the person with the trait is half affected
E - ( ) the person with the trait is half normal
F - ( ) the trait person is AS and the sick person is SS
G - ( ) the person with the trait is SC
H - ( ) I do not know
Note: Answers A, B and C show knowledge about the condition of asymptomatic carriers. Answers D, E and F: has knowledge about half of the altered genetic load, but does not differentiate between the patient and carrier. Response G: considers SC hemoglobinopathy to be a trait. Response H: does not differentiate between trait and disease.
5) Do you know what genetic guidance is?
A - ( ) Yes, it's when a professional transmits information to us about the disease.
B - ( ) Yes, it's when a professional tells us what the disease is.
C - ( ) attended lectures
D - ( ) No
Note: Answers A and B: knows about the practice of genetic guidance - it raises the level of knowledge. Answer C: has participated in an educational process - raises the level of knowledge. Answer D: has no knowledge about this educational process.
Appendix 2
Cognitive questionnaire for patients with hemoglobinopathies after genetic guidance
Appendix 2 Cognitive questionnaire for patients with hemoglobinopathies after genetic guidance
Cognitive assessment of patients with hemoglobinopathies after genetic guidance
1) When only one parent is a carrier of the trait, can the child have the disease?
A - ( ) No, because in order to have the disease both parents need to be carriers of the trait
B - ( ) No, because only one parent with the trait cannot transmit the disease
C - ( ) No, (did not want to explain verbally, but showed a response using the educational material)
D - ( ) I cannot explain
E - ( ) Yes
Note: Answers A and B: knowledge about the need for alterations in both parents in order to have the disease. Answer C: patients correctly demonstrated the transmission of genes, but not verbally. Answers D and E: no knowledge about genetic transmission
2) When both parents have the trait, can the child have the disease?
A - ( ) Yes, because parents can transmit their altered halves and cause the disease
B - ( ) Yes, the father and mother transmit their altered hemoglobin at the same time
C - ( ) Yes (would not explain orally, but showed a response using the course material)
D - ( ) I cannot explain
E - ( ) No
Note: Answers A and B: knowledge about the need for alterations in both parents in order to have the disease. Answer C: patients correctly demonstrated the transmission of genes, but not verbally. Answers D and E: No knowledge about genetic transmission
3) When neither of the parents has alterations, can the child have the disease?
A - ( ) No, because in order to have the disease, both parents need to be carriers of the trait or disease
B - ( ) No, because there is no way to transmit anything
C - ( ) No (would not explain verbally, but showed a response using the course material)
D - ( ) I cannot explain
E - ( ) Yes
Note: Answers A and B: knowledge of the need for alterations in both parents to have the disease. Answer C: patients correctly demonstrated the transmission of genes, but not verbally. Answers D and E: No knowledge about genetic transmission
4) When both parents have the disease, can the child have the disease?
A - ( ) Yes, the parents will transmit altered halves to the child
B - ( ) Yes, the parents have only alterations to transmit
C - ( ) Yes (would not explain verbally, but showed a response using the course material)
D - ( ) I cannot explain
E - ( ) No
Note: Answers A and B: knowledge about the need for alterations in both parents to have the disease. Answer C: patients correctly demonstrated the transmission of genes, but not verbally. Answers D and E: No knowledge about genetic transmission
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interest
References
- 1.Bandeira FM, Santos MN, Bezerra MA, Gomes YM, Araujo AS, Braga MC, et al. Triagem familiar para o gene HBB*S e detecção de novos casos de traço falciforme em Pernambuco Rev Saude Publica 200842 (2) 234–241.[Article in Portuguese ] [PubMed] [Google Scholar]
- 2.Simões BP, Pieroni F, Barros GM, Machado CL, Cançado RD, Salvino MA, et al. Consenso brasileiro em transplante de células-tronco hematopoéticas: Comitê de hemoglobinopatias. Rev Bras Hematol Hemoter. 2010;32(Supp1):46–53. [Google Scholar]
- 3.Souza RA, Pratesi R, Fonseca SF.Programa de Triagem Neonatal para Hemoglobinopatias em Dourados, MS - uma análise Rev Bras Hematol Hemoter 201032 (2) 126–130. [Google Scholar]
- 4.Cançado RD, Jesus JA.A doença falciforme no Brasil Rev Bras Hematol Hemoter 200729 (3) 203–206. [Google Scholar]
- 5.Naoum PC, Naoum FA. Doença das células falciformes. 1rd ed. São Paulo: Sarvier; 2004. 224p [Google Scholar]
- 6.Moraes KC, Galioti JB.A doença falciforme: um estudo genético populacional a partir de doadores de sangue em São José dos Campos, São Paulo, Brasil Rev Bras Hematol Hemoter 201032 (4) 286–290. [Google Scholar]
- 7.Guedes C, Diniz D.Um caso de discriminação genética: o traço falciforme no Brasil Rev Saude Coletiva 200717 (3) 501–520. [Google Scholar]
- 8.Brasil. Ministério da saúde . Manual de informação e orientação genética em herança falciforme. Brasília: Editora do Ministério da Saúde; 2010. 32p [Google Scholar]
- 9.Viana-Baracioli LM, Bonini-Domingos CR, Pagliusi RA, Naoum PC.Prevenção de hemoglobinopatias a partir do estudo em gestantes Rev Bras Hematol Hemoter 200123 (1) 31–39. [Google Scholar]
- 10.Brasil. Ministério da saúde Portaria nº 822, de 06 de junho de 2001. Institui o Programa Nacional de Triagem Neonatal/PNTN .[Internet]Brasília, DF: 06June2001[ cited 2012 March 10] Available from: http://www.saude.mg.gov.br/atos_normativos/legislacao-sanitaria/estabelecimentos-de-saude/neonatologia/PORTARIA_822.pdf [Google Scholar]
- 11.Ramalho AS, Magna LA, Silva RB.A Portaria MS n.º 822/01 e a triagem neonatal das hemoglobinopatias Rev Bras Hematol Hemoter 200224 (4) 244–250. [Google Scholar]
- 12.Brasil. Ministério da saúde manual de normas técnicas e rotinas operacionais do programa nacional de triagem neonatal .[internet]Brasília, DF: Editora do Ministério da Saúde; 200291p[cited 2012 August 23] Available from: http://dtr2001.saude.gov.br/sas/dsra/MANUAL%202002%200456%20Neo%20Natal-%2006.JUN02.pdf [Google Scholar]
- 13.Brasil. Ministério da saúde Portaria nº 1391, de 16 de agosto de 2005. Institui as diretrizes para a Política Nacional de Atenção Integral às Pessoas com Doença Falciforme e outras Hemoglobinopatias .[Internet]Brasília, DF: 16August2005[cited 2012 March 10] Available from: http://www.saude.mg.gov.br/atos_normativos/legislacao-sanitaria/estabelecimentos-de-saude/hemoterapia/por_1391.pdf [Google Scholar]
- 14.Brunoni D.Aconselhamento genético Rev Ciênc Saúde Coletiva 20027 (1) 101–107. [Google Scholar]
- 15.Pina-Neto JM.Aconselhamento genético J Pediatr 200884 (4) 20–26. [Google Scholar]
- 16.Muthuswamy V. Ethical issues in genetic counseling whit special reference to haemoglobinophaties. Indian J Med Res. 2011;134:547–551. [PMC free article] [PubMed] [Google Scholar]
- 17.Ramalho AS, Magna LA.Aconselhamento genético do paciente com doença falciforme Rev Bras Hematol Hemoter 200729 (3) 229–232. [Google Scholar]
- 18.Guimarães CT, Coelho GO. A importância do aconselhamento genético na anemia falciforme. Cien Saude Colet. 2010;15(Suppl 1):1733–1740. doi: 10.1590/s1413-81232010000700085. [DOI] [PubMed] [Google Scholar]
- 19.Guedes C. O campo da anemia falciforme e a informação genética: um estudo dobre aconselhamento genético. Brasília: Instituto de Ciências Sociais e Departamento de Sociologia - Universidade de Brasília; 2006. [dissertação] [Google Scholar]
- 20.Oliveira RA, Neto AP. Anemias e Leucemias. Conceitos básicos e diagnóstico por técnicas laboratoriais. 1rd. ed. São Paulo: Roca; 2004. 436p [Google Scholar]
- 21.Silvestroni E, Bianco I.Screening for microcytemia in Italy: analysis ofdata collected in the past 30 years Am J Hum Genet 197527 (2) 198–212. [PMC free article] [PubMed] [Google Scholar]
- 22.Bonini-Domingos CR. Hemoglobinopatias no Brasil - variabilidade genética e metodologia laboratorial. São Paulo: Instituto de Biociências, Letras e Ciências Exatas, Universidade Estadual Paulista; 1993. 231 p [thesis ] [Google Scholar]
- 23.Marengo-Rowe AJ.Rapid electrophoresis and quantitation of haemoglobin on cellulose acetate J Clin Pathol 196518 (6) 790–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vella F.Acid-agar gel electrophoresis of human hemoglobin Am J Clin Pathol 196849 (3) 440–442. [DOI] [PubMed] [Google Scholar]
- 25.Papayannopoulos R, Stamatonyannopoulos G. Standartization of laboratory reagents and methodos for detection of haemoglobinopathies. Atlanta: Hew Publications; 1974. Stains for inclusions bodies. [Google Scholar]
- 26.Monteiro G., Filho . Segredos da estatística em pesquisa científica. 1ª edição. Goiânia: Vieira; 2004. 188 p [Google Scholar]
- 27.Giordano PC.Prospective and retrospective primary prevention of hemoglobinopathies Clin Biochem 200942 (18) 1757–1766. [DOI] [PubMed] [Google Scholar]
- 28.Amato A, Giordano PC.Screening and genetic diagnosis of hemoglobinopathies in Southern and Northern Europe: Two examples Mediterr. J. Hematol. Infect. Dis 2009[cited 2012 March 2] Available from: http://www.mjhid.org/article/view/4658/e2009007Acesso em 02 de março de 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fucharoen S, Winichagoon PraneePrevention and control of thalassemia in Asia Asian Biomed 20071 (1) 1–6. [Google Scholar]
- 30.Fattoum S.Evolution of Hemoglobinopathy Prevention in Africa: Results, Problems and Prospect 2009[cited 2012 March 4] Available from: http://www.mjhid.org/article/view/5012/e2009005 [DOI] [PMC free article] [PubMed]