Abstract
Background:
Chronic myeloid leukemia is a neoplasm characterized by clonal expansion of hematopoietic progenitor cells resulting from the (9:22)(q34,11) translocation. The tyrosine kinase abl fusion protein,the initial leukemogenic event in chronic myeloid leukemia, is constitutively activated thus inducing the production of reactive oxygen species. Of particular relevance is the fact that an increase in reactive oxygen species can facilitate genomic instability and may contribute to disease progression.
Objetive:
To evaluate oxidative stress by determining the levels of malondialdehyde and nitrite in chronic myeloid leukemia patients under treatment with 1st and 2nd generation tyrosine kinase inhibitors monitored at a referral hospital in Fortaleza, Ceará.
Methods:
A cross-sectional study was performed of 64 male and female adults. Patients were stratified according to treatment. The levels of malondialdehyde and nitrite were determined by spectrophotometry. Statistical differences between groups were observed using the Student t-test and Fisher's exact test. The results are expressed as mean ± standard error of mean. The significance level was set for a p-value < 0.05 in all analyses.
Results:
The results show significantly higher mean concentrations of nitrite and malondialdehyde in chronic myeloid leukemia patients using second-generation tyrosine kinase inhibitors compared to patients on imatinib. Conclusion: It follows that chronic myeloid leukemia patients present higher oxidative activity and that the increases in oxidative damage markers can indicate resistance to 1st generation tyrosine kinase inhibitors.
Keywords: Leukemia, myelogenous, chronic, BCR-ABL positive; Oxidative stress; Protein-tyrosine kinases; Malondialdehyde
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder resulting from neoplastic transformation of primitive hematopoietic cells. It is characterized by a balanced translocation between the long arms of chromosome 9 and 22, which induces formation of the Philadelphia chromosome, resulting from the binding of the BCR gene on chromosome 22 to the ABL gene on chromosome 9, generating the hybrid BCR-ABL gene on chromosome 22q. The product of this oncogene is the bcr-abl protein that has a higher tyrosine kinase activity and is responsible for the pathogenesis of CML(1-3).
Advances in understanding the pathophysiology of the disease are essential for the development of any molecular therapy for CML. Tyrosine kinase inhibitors (TKIs) competitively bind to the receptor for BCR-ABL, ATP-dependent, and inhibit the phosphorylation of tyrosine kinase, thereby avoiding a change in conformation to the active form and normalizing the mechanisms that regulate cell proliferation with the inhibition of cell proliferation and induction of leukemic apoptose(3-6). Imatinib,a TKI, has revolutionized the treatment of CML. Despite the enduring responses of imatinib, some chronic phase patients and a higher proportion of advanced phase patients exhibit intolerance or resistance to this medication(7). Primary resistance occurs when there is failure to achieve a significant hematologic or cytogenetic response by the patient from the beginning of treatment. Secondary resistance is the resurgence of the leukemic clone after an initial response to the drug(7,8). To treat patients who are resistant or intolerant to imatinib, a new generation of BCR-ABL TKIs has been developed, including dasatinib and nilotinib(9,10).
The mechanism whereby the (9:22) translocation occurs is unknown, although some studies suggest that oxidative stress may be involved in the genesis of CML(11-13). Moreover, BCR-ABL positive cells are a source of reactive oxygen species (ROS), which cause oxidative DNA damage and may contribute to the formation of additional abnormalities leading to disease progression to the more advanced stages (accelerated and blast crisis phases) or resistance to TKIs such as imatinib(14-16).
The present study aimed to investigate markers of oxidative stress in CML patients treated with TKIs.
Methods
This is a cross-sectional study of 64 adult patients diagnosed with CML according to the criteria of the World Health Organization (WHO) classification(17), treated in the hematology service of a referral hospital in Fortaleza, Ceará.
The group of patients consisted of 34 women (53.1%) and 30 men (46.9%), with ages that ranged from 20 to 80 years and with a median age of 44 years. The patients were stratified into two groups according to treatment: imatinib (n = 31) and second-generation TKIs (dasatinib and nilotinib - n = 33). Patients were excluded if they were smokers, drank alcohol, were drug addicts or took vitamins with antioxidant action.
The level of malondialdehyde (MDA) in heparinized plasma was calculated by determining the quantity of reactive substances using thiobarbituric acid (TBARS) at a temperature of 100ºC. The absorbance of the colored MDA-TBARS complex was measured by spectrophotometry at a wavelength of 560 nm(18). Nitrite levels in heparinized plasma were determined using Green's method where nitrite in an acid medium reacts with sulfanilamide. The resulting diazo compound reacts with N-naphthyl-ethylenediamine (NEED), thereby generating a compound with an intense red color. Absorbance was measured by spectrophotometry at a wavelength of 540 nm(19).
All subjects signed written consent forms and the study was approved by the Research Ethics Committee of the Universidade Federal do Ceará, under protocol number 042.05.10 which is in accordance with Resolution 196/96 of the National Health Council.
The GraphPrism (version 5.01) program was used for statistical analysis. The Kolmogorov-Smirnov test was used to check for normal distribution of the data. Statistical differences between groups were identified using the Student t-test and Fisher's exact test with the significance level being set for a p-value < 0.05 in all analyses. The results are expressed as means ± standard error of mean (SEM).
Results
The clinical characteristics of patients in the study are shown in Table 1. There were no significant differences for gender, current age, age at diagnosis and time since diagnosis between the groups (p-value > 0.05).
Table 1.
Analysis of measurements of Imatinib and 2nd-generation tyrosine kinase inhibitors Groups on day D-1
| Parameter | CML (n = 64) | Imatinib (n = 31) | 2nd-generation (n = 33) | p-value |
| Gender (male/female) | 34/30 | 16/15 | 18/15 | 0.5062 |
| Age (years) - mean ± SE | 45.30 ± 2.08 | 43.92 ± 2.99 | 46.63 ± 2.93 | 0.2609a |
| Age at diagnosis (years) - mean ± SE | 44.22 ± 2.01 | 41.64 ± 2.78 | 46.17 ± 2.80 | 0.1345a |
| Time since diagnosis (years) - mean ± SE | 7.755 ± 0.54 | 7.200 ± 0.99 | 8.778 ± 0.86 | 0.1296a |
| High Sokal risk - n (%) | 20 (31.2) | 5 (16.1) | 15 (44.5) | 0.0215b |
| Accelerated phase + blast crisis - n (%) | 27 (42.2) | 6 (19.5) | 21 (63.6) | 0.0014b |
| Nitrite (µM) - mean ± SE | 2.638 ± 0.327 | 2.010 ± 0.449 | 3.730 ± 0.429 | < 0.0001a |
| MDA (µM) - mean ± SE | 3.582 ± 0.306 | 2.818 ± 0.349 | 4.197 ± 0.445 | < 0.01a |
CML: chronic myeloid leukemia; MDA: malondialdehyde
a Student t-test; b Fisher's exact Test; statistically significance < 0.05
Of the 33 patients treated with second-generation TKIs, 15 (44.5%) had high Sokal risk at diagnosis, whereas this finding was observed in five patients (16.1%) in the group treated with imatinib (p-value < 0.05). In relation to disease progression, 21 patients (63.6%) treated with second-generation TKIs were in the accelerated or blast crisis phases, while this was observed in six patients (19.5%) treated with imatinib (p-value < 0.05).
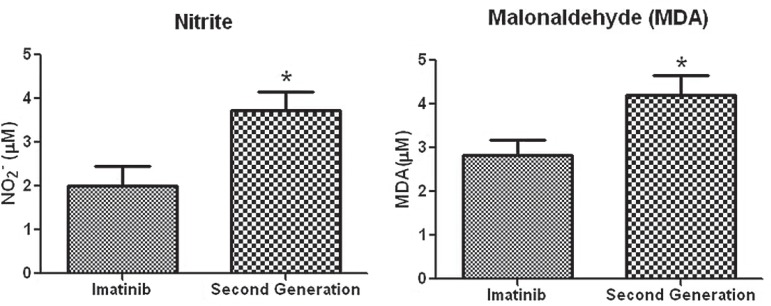
The mean level of nitrite in the plasma of CML patients was 2.638 ± 0.327 mM and the mean level of MDA was 3.582 ± 0.306 mM. On stratifying the CML patients according to treatment, it was observed that serum levels of nitrite and MDA were significantly higher in patients treated with second-generation TKIs than those treated with imatinib (Figure 1 and Table 1).
Figure 1.
Nitrite and malondialdehyde levels in chronic myeloid leukemia patients according to treatment
Student t-test * p-value < 0.05
Discussion
CML accounts for approximately 14% of all leukemias with an annual incidence of one to two cases per 100,000 inhabitants and a slight predominance of men(20,21). It affects all ages, but the incidence rises with age(21). The average age at diagnosis is 55 to 60 years, with less than 10% of patients being younger than 20 years old(21,22). In the present study, no significant difference in the incidence of the disease between genders was found, however there was a slight predominance of males, which agrees with published data. The mean age at diagnosis was 44 years in the study patients. In recent years, there has been a decrease in mean age at diagnosis of CML. Studies in India, Pakistan and Brazil showed mean ages of 38, 35 and 41 years, respectively. This fact may be attributed to the occurrence of widespread routine screening, as well as genetic factors, environmental factors and public health(23,24).
The prognosis of CML may vary significantly even in patients who are at the same stage of the disease. Strategies for risk stratification are important as a guide for the prognosis and treatment of patients. The Sokal score is the most widely used and has prognostic significance for factors such as age over 60 years, spleen size, platelet count above 700 x109/L and the number of basophils and blasts in peripheral blood and bone marrow(25,26). In the present study, most patients treated with second-generation TKIs had high Sokal risk at diagnosis and were in advanced stages of the disease at the time of the study. This finding suggests that the Sokal score, although it was created for patients on drugs that are no longer used to treat this disease, retains its predictive value after the advent of TKIs to treat CML as has been reported in recent studies(27).
Markers of cell damage are being studied in order to elucidate the role of oxidative stress in CML(28). These include the free radical, nitrite (NO2-), which is associated to direct damage of cellular components and MDA, which reflects the extent of lipid peroxidation and modulates the expression of genes related to promoting tumor progression. Previous studies have shown severe oxidative stress in CML and other hematological cancers(29-35).
Imatinib has been used as first-line therapy for CML providing lasting responses in most patients, especially those in the chronic phase. Even so some in the chronic phase and a higher proportion of patients in the more advanced stages of CML are resistant or intolerant to imatinib(36). Mechanisms of resistance to imatinib can be classified as BCR-ABL independent or dependent. The first group includes the binding of imatinib-α 1-acid glycoprotein, increased expression of drug efflux pumps and reduced expression of drug influx transporters. The BCR-ABL dependent mechanisms are increased expression of the oncoprotein and point mutations in the tyrosine kinase domain. These are the most common occurrences in imatinib resistance(8,37,38).
Second-generation TKIs (dasatinib and nilotinib) are inhibitors of multiple targets, able to inhibit the active and inactive forms of the bcr-abl tyrosine kinase protein as well as Src family kinases. In addition, second-generation TKIs have been shown to be active against all the mutations of the Bcr-Abl oncoprotein resistant to imatinib except T315I(8,37-39).
In the present study, patients treated with second-generation TKIs had significantly higher levels of nitrite and MDA when compared to patients treated with imatinib. Such results suggest that oxidative stress parameters can be used to predict lack of response to 1st generation TKIs. Patients who are refractory to imatinib had higher levels of oxidative damage markers, possibly due to a high rate of additional mutations, the leading causes of resistance to first-line therapy. Studies show that the occurrence of mutations is closely associated with oxidative stress and disease progression(39). Therefore, it is likely that reactive oxygen species (ROS) participate in combination with the BCR-ABL gene by introducing new mutations in the fusion protein, including those that cause resistance to imatinib(40,41).
The results of this study suggest that high levels of oxidative stress may be associated with mutations that cause resistance to first-line treatment. Moreover, high levels of oxidative stress markers are associated with the group of patients with high Sokal scores suggesting that this score may also be useful as a prognostic tool to identify patient's response to imatinib. However, detailed studies are needed to identify the origin of oxidative stress and the ROS produced by cells in BCR-ABLto better understand the role and prognostic impact of elevated levels of ROS in CML.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interest
References
- 1.Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D.The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene Sciences 1986233 (4760) 212–214. [DOI] [PubMed] [Google Scholar]
- 2.Daley GQ, Van Etten RA, Baltimore D.Induction of chronic myelogenous leukemia in mice by the P210 gene of the Philadelphia chromosome Science 1990247 (4944) 824–830. [DOI] [PubMed] [Google Scholar]
- 3.Cortes J.Natural history and staging of chronic myelogenous leukemia Hematol Oncol Clin North Am 200418 (3) 569–584. [DOI] [PubMed] [Google Scholar]
- 4.Buchdunger E, Zimmermann J, Mett H, Meyer T, Müller M, Druker BJ, et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative Cancer Research 199656 (1) 100–104. [PubMed] [Google Scholar]
- 5.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia N Engl J Med 2001344 (14) 1031–1037.Comment in: N Engl J Med. 2001; 344(14):1084-6; N Engl J Med. 2001;345(8):618-9. [DOI] [PubMed] [Google Scholar]
- 6.Funke VA, Setubal DC, Ruiz J, Azambuja AP, Lima DH, Kojo TK, et al. O tratamento da Leucemia Mielóide Crônica com mesilato de imatinibe. Rev Bras Hematol Hemoter. 2008;30(suppl. 1):27–31. [Google Scholar]
- 7.An X, Tiwaria AK, Suna Y, Dinga PR, Ashby-Jr CR, Chena ZS.BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review Leuk Res 201034 (10) 1255–1268. [DOI] [PubMed] [Google Scholar]
- 8.Deininger WN.Resistance to imatinib: mechanisms and management J Natl Compr Canc Netw 20053 (6) 757–768. [DOI] [PubMed] [Google Scholar]
- 9.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, Cervantes F, Deininger M, Gratwohl A, Guilhot F, Hochhaus A, Horowitz M, Hughes T, Kantarjian H, Larson R, Radich J, Simonsson B, Silver RT, Goldman J, Hehlmann R, European LeukemiaNet Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet J Clin Oncol 200927 (35) 6041–6051.Comment in: J Clin Oncol. 2010;28(18):e310; author reply e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4- amino) thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays J Med Chem 200447 (27) 6658–6661. [DOI] [PubMed] [Google Scholar]
- 11.O'Hare T, Walters DK, Stoffregen EP, Jia T, Manley PW, Mestan J, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants Cancer Res 200565 (11) 4500–4505. [DOI] [PubMed] [Google Scholar]
- 12.Penserga ET, Skorski T.Fusion tyrosine kinases: a result and cause of genomic instability Oncogene 200726 (1) 11–20. [DOI] [PubMed] [Google Scholar]
- 13.Cooke MS, Evans MD, Dizdaroglu M, Lunec J.Oxidative DNA damage: mechanisms, mutation, and disease FASEB J 200317 (10) 1195–1214. [DOI] [PubMed] [Google Scholar]
- 14.Hole PS, Darley RL, Tonks A.Do reactive oxygen species play a role in myeloid leukemias? Blood 2011117 (22) 5816–5826. [DOI] [PubMed] [Google Scholar]
- 15.Hochhaus A, Kreil S, Corbin AS, La Rosée P, Muller MC, Lahaye T, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy Leukemia 200216 (11) 2190–2196. [DOI] [PubMed] [Google Scholar]
- 16.Burke BA, Carroll M.BCR-ABL: a multi-faceted promoter of DNA mutation in chronic myelogenous leukemia Leukemia 201024 (6) 1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The International Agency for Research on Cancer Classification of tumours of haematopoietic and lymphoid tissues 4nd ed. Lyon: WHO Press; 2008439 p (IARC WHO Classification of Tumours) [Google Scholar]
- 18.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 19.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR.Analysis of nitrate, nitrite and [15N] nitrate in biological fluids Anal Biochem 1982126 (1) 131–138. [DOI] [PubMed] [Google Scholar]
- 20.Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian H.The biology of chronic myeloid leukemia N Engl J Med 1999341 (3) 164–172. [DOI] [PubMed] [Google Scholar]
- 21.Bortolheiro TC, Chiattone CS.Leucemia mielóide crônica: história natural e classificação Rev Bras Hematol. Hemoter. 200830 (1) 3–7. [Google Scholar]
- 22.Rohrbacher M, Hasford J.Epidemiology of chronic myeloid leukaemia (CML) Best Pract Res Clin Haematol 200922 (3) 295–302. [DOI] [PubMed] [Google Scholar]
- 23.Hehlmann R, Hochhaus A, Baccarani M, European LeukemiaNet Chronic myeloid leukemia Lancet 2007370 (9584) 342–350.Comment in: Lancet. 2007;370(9593):1127. [DOI] [PubMed] [Google Scholar]
- 24.Deshmukh C, Saikia T, Baksi A, Amare-Kadam P, Baisane C, Parikh P. Imatinib mesylate in chronic myeloid leukemia: a prospective single arm. Non-randomized study. J Assoc Physicians India. 2005;53:291–295. [PubMed] [Google Scholar]
- 25.Syed NN, Usman M, Khaliq G, Adil SN, Khurshid M.Clinico-pathologic features of chronic myeloid leukemia and risk stratification according to Sokal score J. Coll Physicians Surg Pak 200616 (5) 336–339. [PubMed] [Google Scholar]
- 26.Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in "good-risk" chronic granulocytic leukemia Blood 198463 (4) 789–799. [PubMed] [Google Scholar]
- 27.Campos MG, Arantes AM, Oliveira JSR, Chauffaille ML.Chronic myeloid leukemia: a disease of youth in Brazil Leuk Res 201034 (4) 542–544. [DOI] [PubMed] [Google Scholar]
- 28.Cerutti PA.Pro-oxidant status and tumor promotion Science 1985227 (4685) 375–381. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad R, Anil KT, Payal T, Ranjana S, Sushma S, Raj KS.Oxidative stress and antioxidant status in patients with chronic myeloid leukemia Ind J Clin Biochem 200823 (4) 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad R, Tripathi AK, Tripathi P, Singh S, Singh R, Singh RK.Malondialdehyde and protein carbonyl as biomarkers for oxidative stress and disease progression in patients with chronic myeloid leukemia In Vivo [ Internet] 2008[cited 2010 Jun 21] 22 (4) 525–528. Available from: http://iv.iiarjournals.org/content/22/4/525.long [PubMed] [Google Scholar]
- 31.Ciarcia R, D'angelo D, Pacilio C, Pagnini D, Galdiero M, Fiorito F, et al. Dysregulated calcium homeostasis and oxidative stress in chronic myeloid leukemia (CML) cells J Cell Physiol 2010224 (2) 443–453. [DOI] [PubMed] [Google Scholar]
- 32.Devi GS, Prasad MH, Saraswathi I, Raghu D, Rao DN, Reddy PP.Free radicals antioxidant enzymes and lipid peroxidation in different types of leukemias Clin Chim Acta 2000293 (1-2) 53–62. [DOI] [PubMed] [Google Scholar]
- 33.Oltra AM, Carbonell F, Tormos C, Iradi A, Guillermo T, Saez GT.Antioxidant enzyme activities and the production of MDA and 8-oxo-dg in chronic lymphocytic leukemia Free Radic Biol Med 200130 (11) 1286–1292. [DOI] [PubMed] [Google Scholar]
- 34.Battisti V, Madersa LD, Bagatinia MD, Santos KF, Spanevello RM, Maldonado PA, et al. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients Clin Biochem 200841 (7-8) 511–518. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, Hileman EO, Plunkett W, Keating MJ, Huang P.Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents Blood [ Internet] 2003[cited 2011 Jan 23] 101 (10) 4098–4104.http://bloodjournal.hematologylibrary.org/cgi/pmidlookup?view=long&pmid=12531810 [DOI] [PubMed] [Google Scholar]
- 36.Goldman JM.Treatment strategies for CML Best Pract Res Clin Haematol 200922 (3) 303–313. [DOI] [PubMed] [Google Scholar]
- 37.Natoli C, Perrucci B, Perrotti F, Falchi L, Iacobelli S, Consorzio Interuniversitario Nazionale per Bio-Oncologia (CINBO) Tyrosine kinase inhibitors Curr Cancer Drug Targets 201010 (5) 462–483. [DOI] [PubMed] [Google Scholar]
- 38.La Rosée P, Deininger MW.Resistance to imatinib: mutations and beyond Semin Hematol 201047 (4) 335–343. [DOI] [PubMed] [Google Scholar]
- 39.Delamain MT, Conchon M. Os inibidores de tirosino quinase de segunda geração. Rev Bras Hematol Hemoter. 2008;30(suppl. 1):37–40. [Google Scholar]
- 40.Slupphaug G, Kavli B, Krokan HE.The interacting pathways for prevention and repair of oxidative DNA damage Mutat Res 2003531 (1-2) 231–251. [DOI] [PubMed] [Google Scholar]
- 41.Koptyra M, Falinski R, Nowicki MO, Stoklosa T, Majsterek I, Nieborowska-Skorska M, et al. BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance Blood [ Internet] 2006. [cited 2011 Jan 21 ] 108 (1) 319–327. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1895841 [DOI] [PMC free article] [PubMed] [Google Scholar]