Abstract
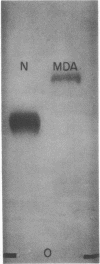
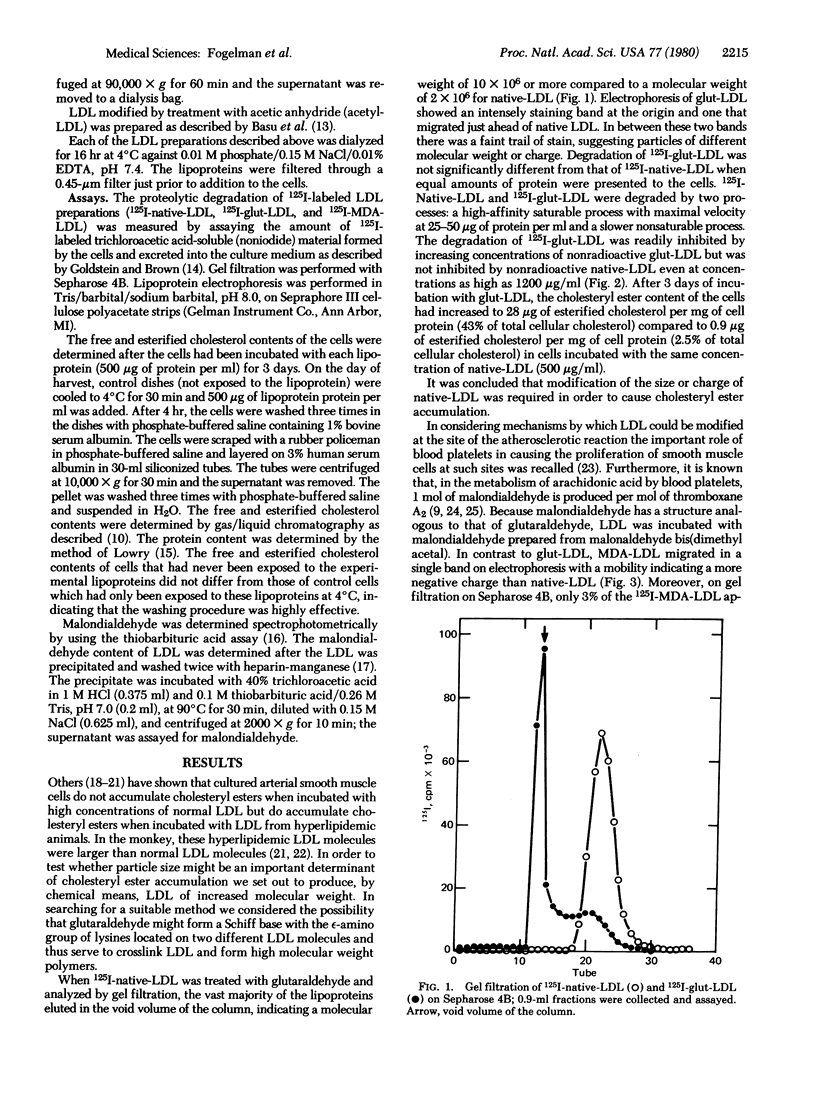
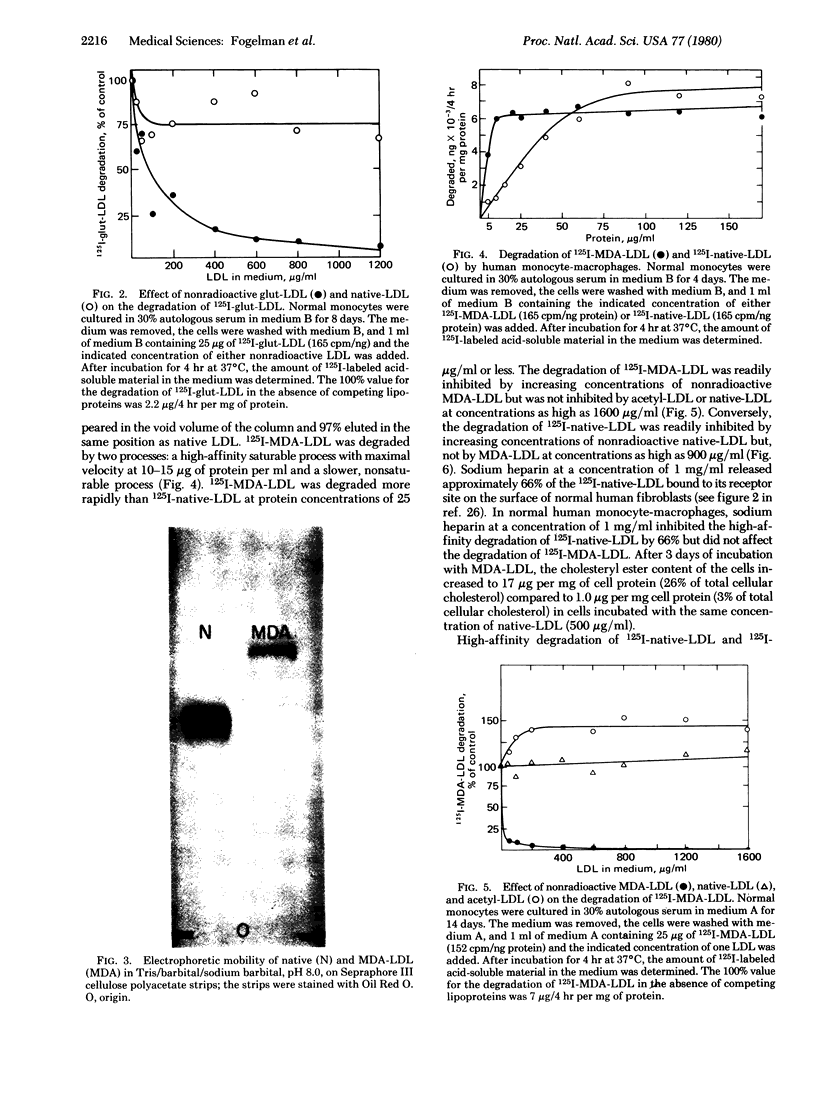
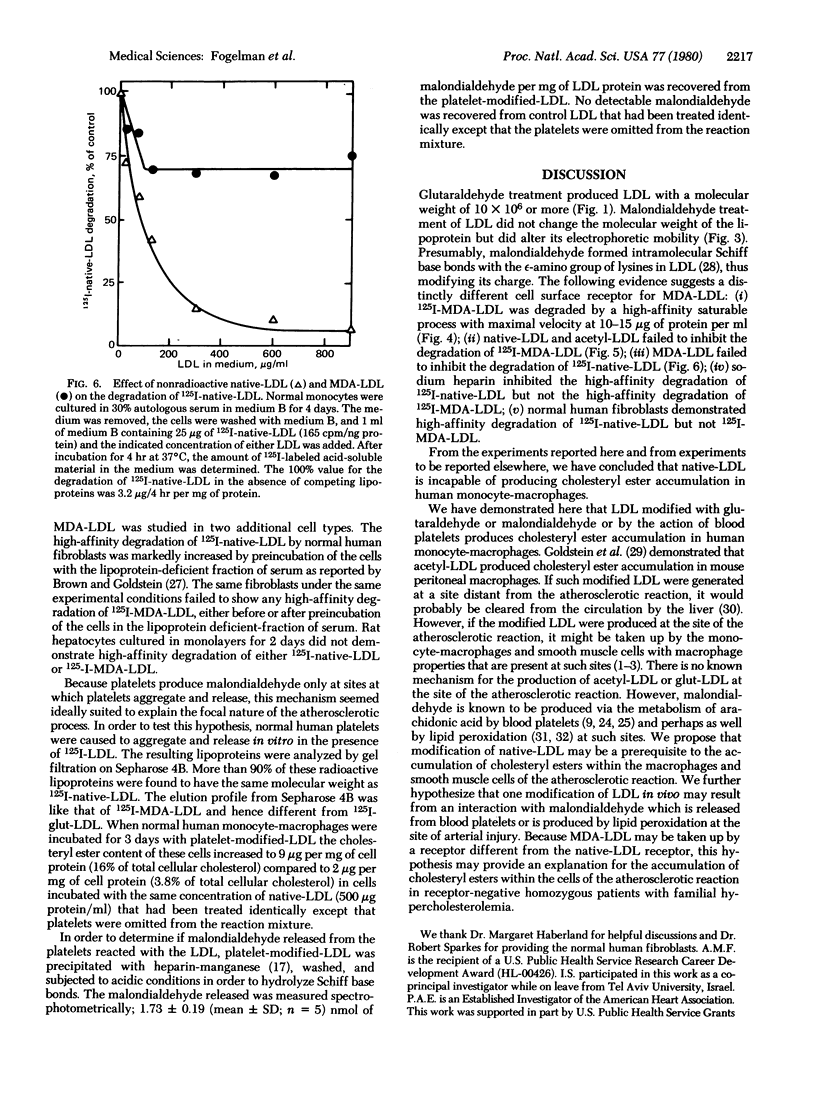
Glutaraldehyde treatment of 125I-labeled low density lipoprotein (125I-native-LDL) produced a modified LDL (125I-glut-LDL) with a molecular weight of 10 × 106 or more. Malondialdehyde treatment of 125I-native-LDL produced a product (125I-MDA-LDL) with a molecular weight not appreciably different from that of the original lipoprotein. However, the electrophoretic mobility of MDA-LDL indicated a more negative charge than native-LDL. 125I-MDA-LDL was degraded by two processes: a high-affinity saturable process with maximal velocity at 10-15 μg of protein per ml and a slower, nonsaturable process. The degradation of 125I-MDA-LDL was readily inhibited by increasing concentrations of nonradioactive MDA-LDL but was not inhibited by acetylated LDL or native-LDL even at concentrations as high as 1600 μg of protein per ml. After exposure of native-LDL to blood platelet aggregation and release in vitro, 1.73 ± 0.19 nmol of malondialdehyde per mg of LDL protein was bound to the platelet-modified-LDL. No detectable malondialdehyde was recovered from native-LDL that had been treated identically except that the platelets were omitted from the reaction mixture.
After incubation with glut-LDL, MDA-LDL, or platelet-modified-LDL for 3 days, human monocyte-macrophages showed a dramatic increase in cholesteryl ester content whereas the cholesteryl ester content of cells incubated with the same concentration of native-LDL did not. Based on these experiments we propose that modification of native-LDL may be a prerequisite to the accumulation of cholesteryl esters within the cells of the atherosclerotic reaction. We further hypothesize that one modification of LDL in vivo may result from malondialdehyde which is released from blood platelets or is produced by lipid peroxidation at the site of arterial injury.
Keywords: cell surface receptors, blood platelets, atherosclerosis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton D. R., Golde D. W. Differentiation of macrophages from normal human bone marrow in liquid culture. Electron microscopy and cytochemistry. J Clin Invest. 1978 Jun;61(6):1555–1569. doi: 10.1172/JCI109076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates S. R., Wissler R. W. Effect of hyperlipemic serum on cholesterol accumulation in monkey aortic medial cells. Biochim Biophys Acta. 1976 Oct 21;450(1):78–88. doi: 10.1016/0005-2760(76)90300-3. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Familial hypercholesterolemia: defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc Natl Acad Sci U S A. 1974 Mar;71(3):788–792. doi: 10.1073/pnas.71.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. M., Fischer-Dzoga K. Effect of hyperlipemic serum lipoproteins on the lipid accumulation and cholesterol flux of rabbit aortic medial cells. Atherosclerosis. 1977 Nov;28(3):339–353. doi: 10.1016/0021-9150(77)90181-2. [DOI] [PubMed] [Google Scholar]
- Chio K. S., Tappel A. L. Inactivation of ribonuclease and other enzymes by peroxidizing lipids and by malonaldehyde. Biochemistry. 1969 Jul;8(7):2827–2832. doi: 10.1021/bi00835a020. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Seager J., Edwards P. A., Popják G. Mechanism of induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase in human leukocytes. J Biol Chem. 1977 Jan 25;252(2):644–651. [PubMed] [Google Scholar]
- Fogelman A. M., Seager J., Hokom M., Edwards P. A. Separation of and cholesterol synthesis by human lymphocytes and monocytes. J Lipid Res. 1979 Mar;20(3):379–388. [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K., Richardson M., Schwartz C. J. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol. 1979 Jun;95(3):775–792. [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brunschede G. Y., Brown M. S. Release of low density lipoprotein from its cell surface receptor by sulfated glycosaminoglycans. Cell. 1976 Jan;7(1):85–95. doi: 10.1016/0092-8674(76)90258-0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Wakabayashi T., Samuelsson B. Isolation and structure of two prostaglandin endoperoxides that cause platelet aggregation. Proc Natl Acad Sci U S A. 1974 Feb;71(2):345–349. doi: 10.1073/pnas.71.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström S., Falardeau P. Resolution of prostaglandin endoperoxide synthase and thromboxane synthase of human platelets. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3691–3695. doi: 10.1073/pnas.74.9.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T. L., Windmueller H. G. Accelerated clearance of low-density and high-density lipoproteins and retarded clearance of E apoprotein-containing lipoproteins from the plasma of rats after modification of lysine residues. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1746–1750. doi: 10.1073/pnas.76.4.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Rudel L. L., Pitts L. L., 2nd, Nelson C. A. Characterization of plasma low density lipoproteins on nonhuman primates fed dietary cholesterol. J Lipid Res. 1977 Mar;18(2):211–222. [PubMed] [Google Scholar]
- Small D. M. Cellular mechanisms for lipid deposition in atherosclerosis (first of two parts). N Engl J Med. 1977 Oct 20;297(16):873–877. doi: 10.1056/NEJM197710202971608. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Ingerman C. M., Silver M. J. Malondialdehyde formation as an indicator of prostaglandin production by human platelets. J Lab Clin Med. 1976 Jul;88(1):167–172. [PubMed] [Google Scholar]
- St Clair R. W., Leight M. A. Differential effects of isolated lipoproteins from normal and hypercholesterolemic rhesus monkeys on cholesterol esterification and accumulation in arterial smooth muscle cells in culture. Biochim Biophys Acta. 1978 Aug 25;530(2):279–291. doi: 10.1016/0005-2760(78)90013-9. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Smith A. L. Lipid peroxidation by human blood phagocytes. J Clin Invest. 1974 Sep;54(3):638–645. doi: 10.1172/JCI107801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnick G. R., Albers J. J. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978 Jan;19(1):65–76. [PubMed] [Google Scholar]
- Wurster N. B., Zilversmit D. B. The role of phagocytosis in the development of atherosclerotic lesions in the rabbit. Atherosclerosis. 1971 Nov-Dec;14(3):309–322. doi: 10.1016/0021-9150(71)90060-8. [DOI] [PubMed] [Google Scholar]