Abstract
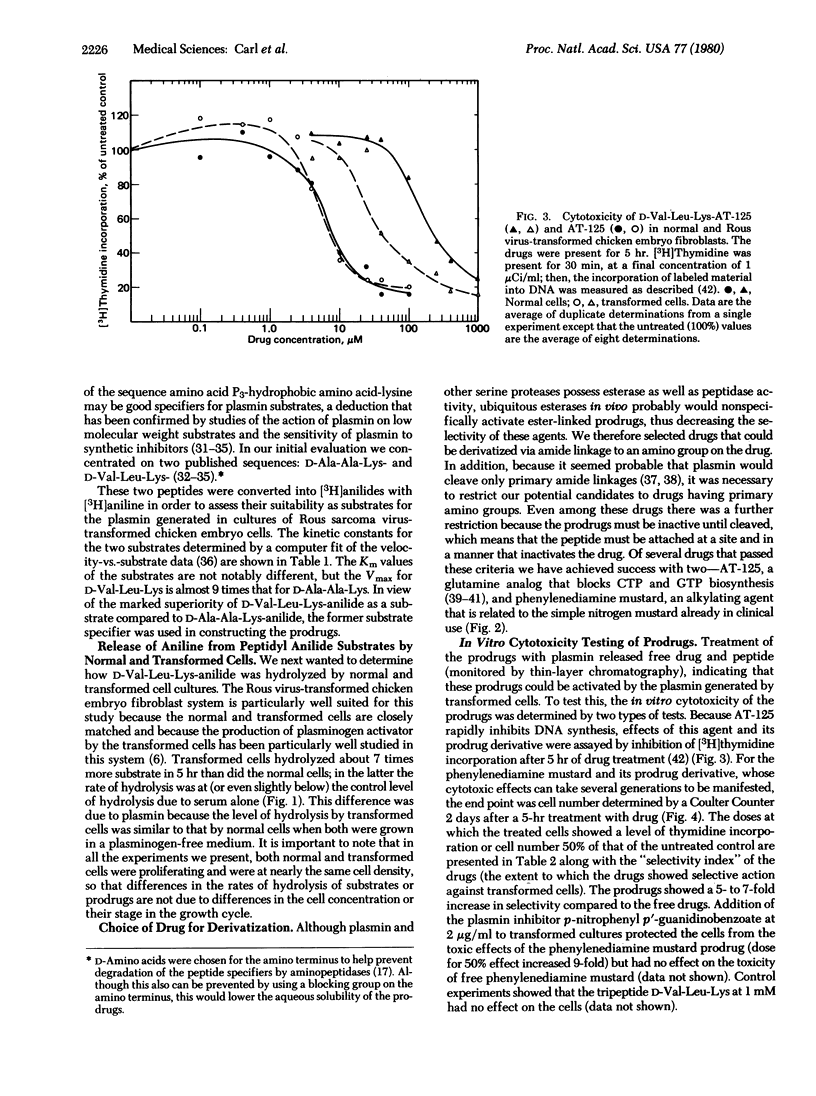
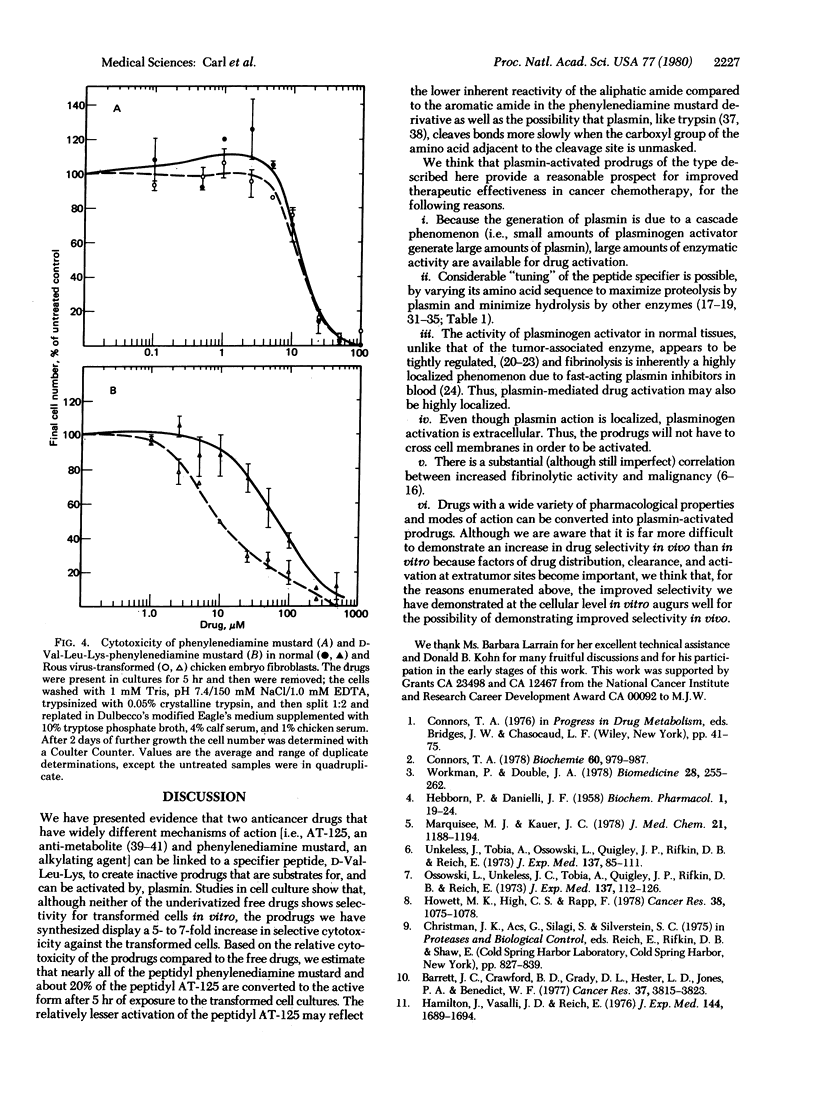
Many types of malignant cells and human tumors display increased concentrations of the protease plasminogen activator that converts plasminogen to the highly active protease, plasmin. Because plasmin rapidly cleaves various low molecular weight compounds coupled to appropriate peptide specifiers, we hypothesized that coupling of such peptide specifiers to anticancer drugs might create “prodrugs” which would be locally activated by tumor-associated plasmin and consequently would be less toxic to normal cells. To provide an initial test of this concept we have synthesized peptidyl prodrugs of the structure D-Val-Leu-Lys-X in which the peptidyl portion has been designed to allow the prodrug to serve as an excellent plasmin substrate and X is an anticancer drug—either the glutamine analog (αS,5S) α-amino-3-chloro-4,5-dihydro-5-isoxazole-acetic acid (AT-125) or the alkylating agent N,N-bis(2-chloroethyl)-p-phenylenediamine (phenylenediamine mustard). Treatment of these prodrugs with plasmin generated the free peptide and the free drug, demonstrating that these prodrugs are plasmin substrates. The prodrugs and free drugs were tested in an in vitro system against either normal chicken embryo fibroblasts, which display a low level of plasminogen activator, or their virally transformed counterparts, which produce high levels of plasminogen activator. In each case the peptidyl prodrugs displayed at least a 5-fold increase in selectivity for the transformed cells compared to the free drug. The greater selectivity of action of the peptidyl prodrugs against transformed cell cultures suggests that these or similar prodrugs that are substrates for tumor-associated proteases may show increased therapeutic effectiveness in the treatment of tumors that produce sufficiently increased amounts of plasminogen activator.
Keywords: plasminogen activator, plasmin, fibrinolysis, antineoplastic agents, peptides
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bang N. U., Mattler L. E. Sensitivity and specificity of plasma serine protease chromogenic substrates. Haemostasis. 1978;7(2-3):98–104. doi: 10.1159/000214244. [DOI] [PubMed] [Google Scholar]
- Barrett J. C., Crawford B. D., Grady D. L., Hester L. D., Jones P. A., Benedict W. F., Ts'o P. O. Temporal acquistion of enhanced fibrinolytic activity by syrian hamster embryo cells following treatment with benzo(a)pyrene. Cancer Res. 1977 Oct;37(10):3815–3823. [PubMed] [Google Scholar]
- Clavin S. A., Bobbitt J. L., Shuman R. T., Smithwick E. L., Jr Use of peptidyl-4-methoxy-2-naphthylamides to assay plasmin. Anal Biochem. 1977 Jun;80(2):355–365. doi: 10.1016/0003-2697(77)90656-x. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Friberger P., Axelsson G., Korsan-Bengtsen K. Proceedings: Determination of plasminogen by means of a chromogenic peptide substrate. Thromb Diath Haemorrh. 1975 Sep 30;34(1):321–321. [PubMed] [Google Scholar]
- Hamilton J., Vassalli J. D., Reich E. Macrophage plasminogen activator: induction by asbestos is blocked by anti-inflammatory steroids. J Exp Med. 1976 Dec 1;144(6):1689–1694. doi: 10.1084/jem.144.6.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchens D. P., Ovejera A. A., Sheridan M. A., Johnson R. K., Bogden A. E., Neil G. L. Therapy for mouse tumors and human tumor xenografts with the antitumor antibiotic AT-125. Cancer Treat Rep. 1979 Mar;63(3):473–476. [PubMed] [Google Scholar]
- Howett M. K., High C. S., Rapp F. Production of plasminogen activator by cells transformed by herpesviruses. Cancer Res. 1978 Apr;38(4):1075–1078. [PubMed] [Google Scholar]
- Kettner C., Shaw E. Synthesis of peptides of arginine chloromethyl ketone. Selective inactivation of human plasma kallikrein. Biochemistry. 1978 Oct 31;17(22):4778–4784. doi: 10.1021/bi00615a027. [DOI] [PubMed] [Google Scholar]
- Kohn D. B., Weber M. J., Carl P. L., Katzenellenbogen J. A., Chakravarty P. K. A peptidyl derivative of [3H]aniline as a sensitive, stable, protease substrate. Anal Biochem. 1979 Sep 1;97(2):269–276. doi: 10.1016/0003-2697(79)90071-x. [DOI] [PubMed] [Google Scholar]
- Laug W. E., Jones P. A., Benedict W. F. Relationship between fibrinolysis of cultured cells and malignancy. J Natl Cancer Inst. 1975 Jan;54(1):173–179. doi: 10.1093/jnci/54.1.173. [DOI] [PubMed] [Google Scholar]
- Marquisee M. J., Kauer J. C. Collagenase-sensitive peptidyl-nitrogen mustards as potential antitumor agents. J Med Chem. 1978 Dec;21(12):1188–1194. doi: 10.1021/jm00210a003. [DOI] [PubMed] [Google Scholar]
- Mattler L. E., Bang N. U. Serine protease specificity for peptide chromogenic substrates. Thromb Haemost. 1977 Dec 15;38(4):776–792. [PubMed] [Google Scholar]
- Nagy B., Ban J., Brdar B. Fibrinolysis associated with human neoplasia: production of plasminogen activator by human tumours. Int J Cancer. 1977 May 15;19(5):614–620. doi: 10.1002/ijc.2910190504. [DOI] [PubMed] [Google Scholar]
- Neil G. L., Berger A. E., McPartland R. P., Grindey G. B., Bloch A. Biochemical and pharmacological effects of the fermentation-derived antitumor agent, (alphaS,5S)-alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid (AT-125). Cancer Res. 1979 Mar;39(3):852–856. [PubMed] [Google Scholar]
- Nieuwenhuizen W., Wijngaards G., Groeneveld E. Flourogenic peptide amide substrates for the estimation of plasminogen activators and plasmin. Anal Biochem. 1977 Nov;83(1):143–148. doi: 10.1016/0003-2697(77)90519-x. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Unkeless J. C., Tobia A., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):112–126. doi: 10.1084/jem.137.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin D. B., Loeb J. N., Moore G., Reich E. Properties of plasminogen activators formed by neoplastic human cell cultures. J Exp Med. 1974 May 1;139(5):1317–1328. doi: 10.1084/jem.139.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Seifert S. C., Gelehrter T. D. Mechanism of dexamethasone inhibition of plasminogen activator in rat hepatoma cells. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6130–6133. doi: 10.1073/pnas.75.12.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker W. S., Kirsch W. M., Martinez-Hernandez A., Fink L. M. In vitro plasminogen activator activity in human brain tumors. Cancer Res. 1978 Feb;38(2):297–302. [PubMed] [Google Scholar]
- Unkeless J. C., Tobia A., Ossowski L., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. I. Chick embryo fibroblast cultures transformed by avian RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):85–111. doi: 10.1084/jem.137.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]
- Weber M. J., Rubin H. Uridine transport and RNA synthesis in growing and in density-inhibited animal cells. J Cell Physiol. 1971 Apr;77(2):157–168. doi: 10.1002/jcp.1040770205. [DOI] [PubMed] [Google Scholar]
- Workman P., Double J. A. Drug latentiation in cancer chemotherapy. Biomedicine. 1978 Oct;28(5):255–262. [PubMed] [Google Scholar]



