Abstract
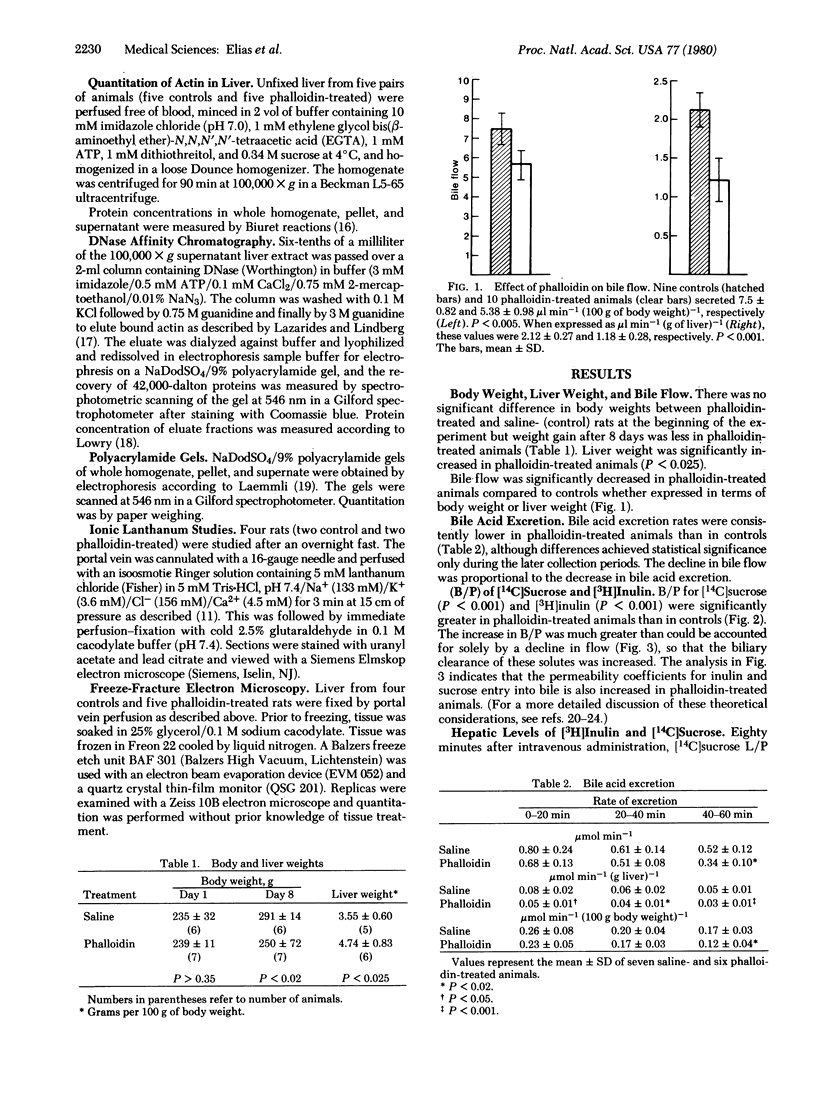
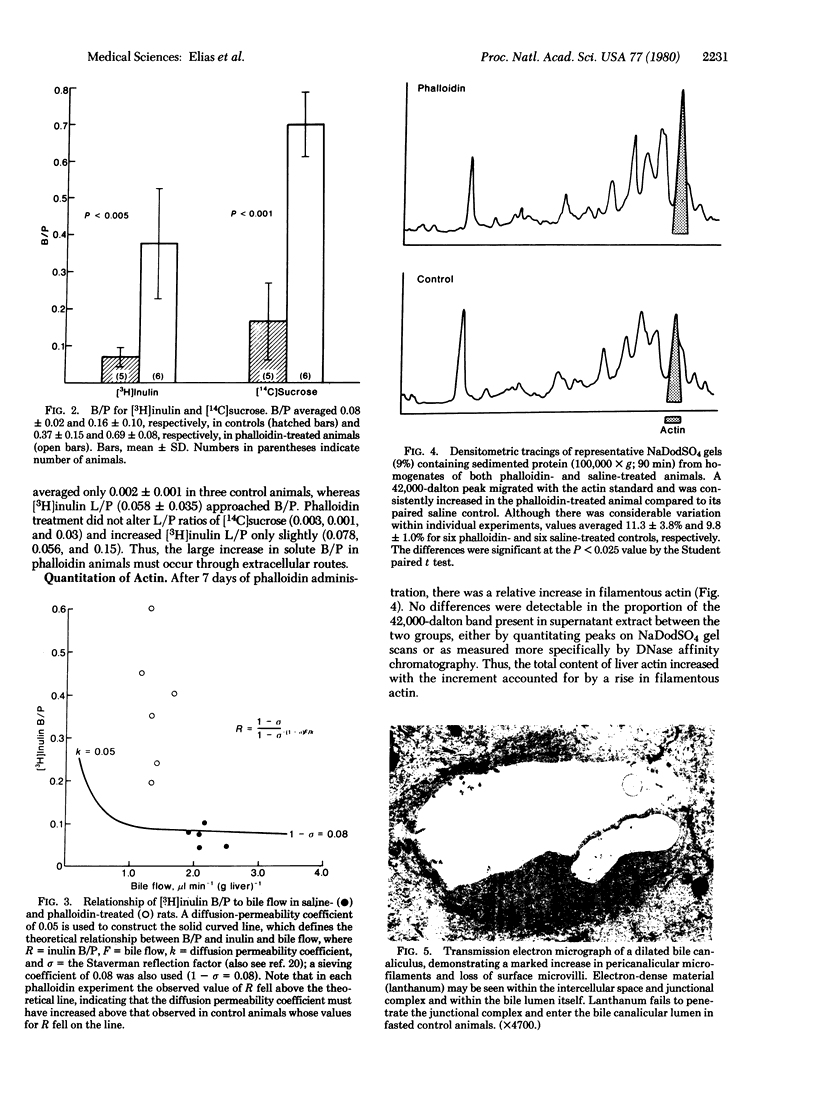
Phalloidin, administered to male rats for 7 days (500 microgram per kg/day), increased the mean hepatic content of filamentous actin. Both bile flow and bile acid excretion diminished proportionally, whereas the bile-to-plasma ratios of [3H]inulin and [14C]sucrose increased significantly from 0.08 and 0.16 in controls to 0.37 and 0.69, respectively, in phalloidin-treated animals. Simultaneously, junctional permeability was altered as noted by the free penetration of ionic lanthanum into the zonula occludens and bile canaliculus. Freeze-fracture replicas of the junctional complex revealed rearrangements of the junctional elements and regions in which only a single element separated the canaliculus from the lateral intercellular space. These findings suggest that microfilaments influence the permeability of "tight junctions" between hepatocytes and that bile constituents might reflux from the canaliculus to the intercellular space in phalloidin-induced cholestasis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer J. L., Elias E., Layden T. J. The paracellular pathway and bile formation. Yale J Biol Med. 1979 Jan-Feb;52(1):61–67. [PMC free article] [PubMed] [Google Scholar]
- Boyer J. L., Scheig R. L., Klatskin G. The effect of sodium taurocholate on the hepatic metabolism of sulfobromophthalein sodium (BSP). The role of bile flow. J Clin Invest. 1970 Feb;49(2):206–215. doi: 10.1172/JCI106229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley S. E., Herz R. Permselectivity of biliary canalicular membrane in rats: clearance probe analysis. Am J Physiol. 1978 Nov;235(5):E570–E576. doi: 10.1152/ajpendo.1978.235.5.E570. [DOI] [PubMed] [Google Scholar]
- Claude P., Goodenough D. A. Fracture faces of zonulae occludentes from "tight" and "leaky" epithelia. J Cell Biol. 1973 Aug;58(2):390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancker P., Löw I., Hasselbach W., Wieland T. Interaction of actin with phalloidin: polymerization and stabilization of F-actin. Biochim Biophys Acta. 1975 Aug 19;400(2):407–414. doi: 10.1016/0005-2795(75)90196-8. [DOI] [PubMed] [Google Scholar]
- De Vos R., Desmet V. J. Morphologic changes of the junctional complex of the hepatocytes in rat liver after bile duct ligation. Br J Exp Pathol. 1978 Apr;59(2):220–227. [PMC free article] [PubMed] [Google Scholar]
- Dubin M., Maurice M., Feldmann G., Erlinger S. Phalloidin-induced cholestasis in the rat: relation to changes in microfilaments. Gastroenterology. 1978 Sep;75(3):450–455. [PubMed] [Google Scholar]
- Edwards J. G., Dysart J. M., Hughes R. C. Cellular adhesiveness reduced in ricin-resistant hamster fibroblasts. Nature. 1976 Nov 4;264(5581):66–68. doi: 10.1038/264066a0. [DOI] [PubMed] [Google Scholar]
- Forker E. L. The effect of estrogen on bile formation in the rat. J Clin Invest. 1969 Apr;48(4):654–663. doi: 10.1172/JCI106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forker E. L. Two sites of bile formation as determined by mannitol and erythritol clearance in the guinea pig. J Clin Invest. 1967 Jul;46(7):1189–1195. doi: 10.1172/JCI105612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend D. S., Gilula N. B. Variations in tight and gap junctions in mammalian tissues. J Cell Biol. 1972 Jun;53(3):758–776. doi: 10.1083/jcb.53.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Montesano R., Tuchweber B., Salas M., Orci L. Phalloidin-induced hyperplasia of actin filaments in rat hepatocytes. Lab Invest. 1975 Nov;33(5):562–569. [PubMed] [Google Scholar]
- Govindan V. M., Faulstich H., Wieland T., Agostini B., Hasselbach W. In-vitro effect of phalloidin on plasma membrane preparation from rat liver. Naturwissenschaften. 1972 Nov;59(11):521–522. doi: 10.1007/BF00609837. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Layden T. J., Boyer J. L. Taurolithocholate-induced cholestasis: taurocholate but not dehydrocholate, reverses cholestasis and bile canalicular membrane injury. Gastroenterology. 1977 Jul;73(1):120–128. [PubMed] [Google Scholar]
- Layden T. J., Elias E., Boyer J. L. Bile formation in the rat: the role of the paracellular shunt pathway. J Clin Invest. 1978 Dec;62(6):1375–1385. doi: 10.1172/JCI109258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengsfeld A. M., Löw I., Wieland T., Dancker P., Hasselbach W. Interaction of phalloidin with actin. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2803–2807. doi: 10.1073/pnas.71.7.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw I., Wieland T. The interaction of phalloidin. Some of its derivatives, and of other cyclic peptides with muscle actin as studied by viscosimetry. FEBS Lett. 1974 Aug 30;44(3):340–343. [PubMed] [Google Scholar]
- Martínez-Palomo A., Erlij D. Structure of tight junctions in epithelia with different permeability. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4487–4491. doi: 10.1073/pnas.72.11.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz J., Aoki A., Merlo M., Forssmann W. G. Morphological alterations and functional changes of interhepatocellular junctions induced by bile duct ligation. Cell Tissue Res. 1977 Aug 26;182(3):299–310. doi: 10.1007/BF00219766. [DOI] [PubMed] [Google Scholar]
- Montesano R., Gabbiani G., Perrelet A., Orci L. In vivo induction of tight junction proliferation in rat liver. J Cell Biol. 1976 Mar;68(3):793–798. doi: 10.1083/jcb.68.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M., Chaponnier C., Jeanrenaud B., Gabbiani G. Actin microfilaments, cell shape, and secretory processes in isolated rat hepatocytes. Effect of phalloidin and cytochalasin D. J Cell Biol. 1979 Jun;81(3):592–607. doi: 10.1083/jcb.81.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasberg S. M., Petrunka C. N., Ilson R. G., Paloheimo J. E. Characteristics of inert solute clearance by the monkey liver. Gastroenterology. 1979 Feb;76(2):259–266. [PubMed] [Google Scholar]
- Wheeler H. O., Ross E. D., Bradley S. E. Canalicular bile production in dogs. Am J Physiol. 1968 Apr;214(4):866–874. doi: 10.1152/ajplegacy.1968.214.4.866. [DOI] [PubMed] [Google Scholar]






