Abstract
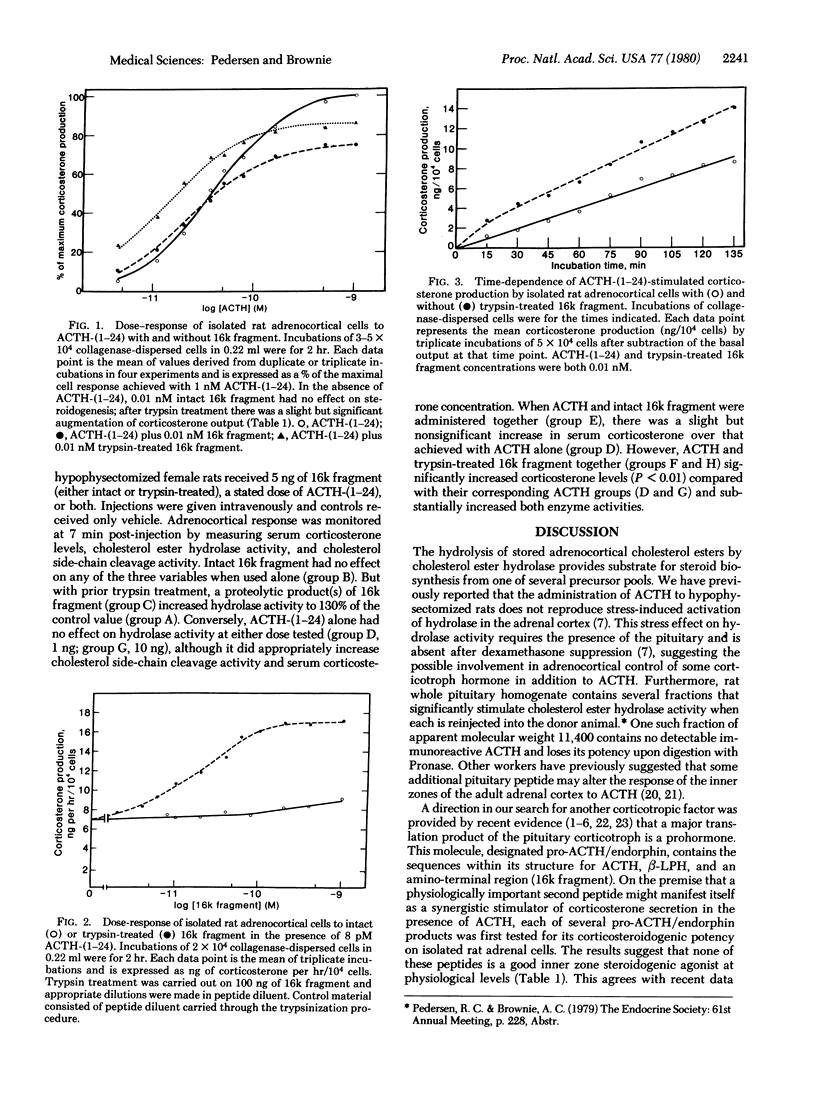
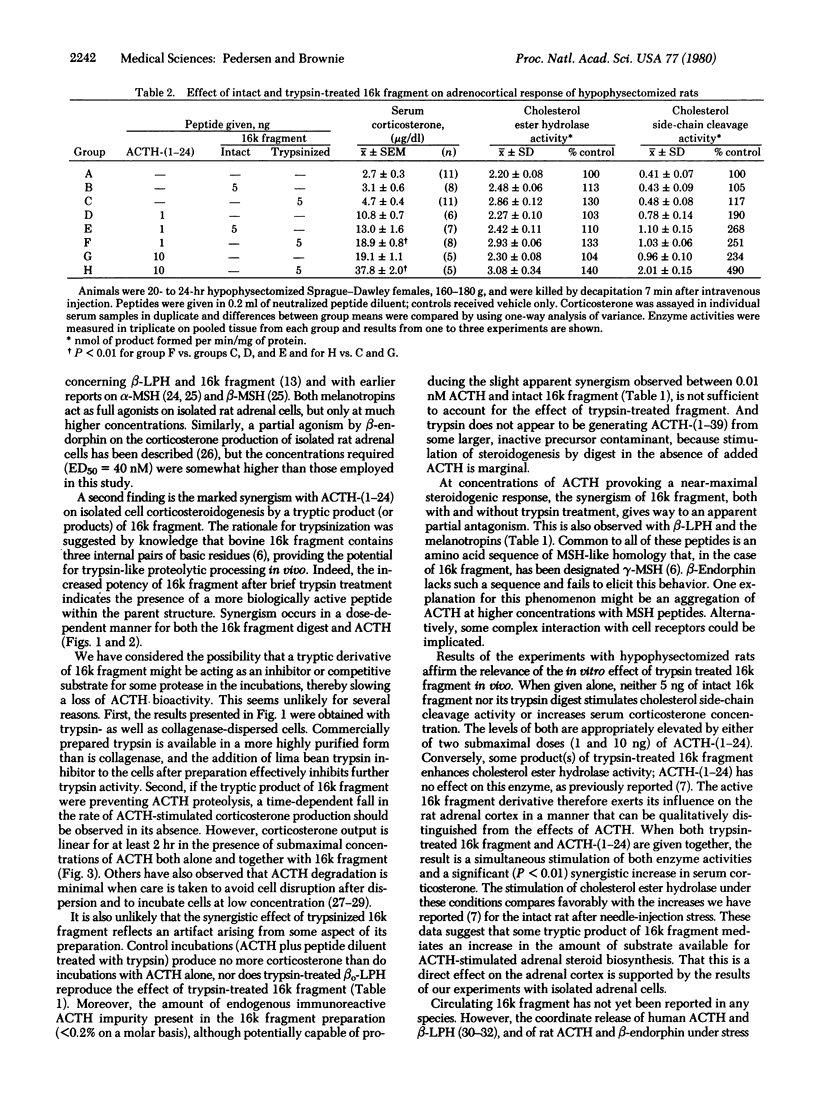
Five peptides derived from pro-corticotropin/endorphin (pro-ACTH/endorphin), the pituitary corticotroph cell prohormone, were bioassayed with isolated rat adrenocortical cells: alpha- and beta-melanotropin, beta-lipotropin, beta-endorphin, and the amino-terminal region of pro-ACTH/endorphin known as "16k fragment." The effect of each on steroidogenesis was measured at potentially physiological concentrations (0.01-1 nM) in both the absence and presence of varying concentrations of ACTH-(1-24). Of the peptides tested, only 16k fragment, the amino-terminal region of pro-ACTH/endorphin, has a slight but significant potentiating effect on ACTH-(1-24) action. Prior treatment of 16k fragment with trypsin for 30 sec dramatically increases this dose-dependent synergism. Experiments performed in vivo with hypophysectomized female rats indicate that the trypsin digest of 16k fragment stimulates cholesterol ester hydrolase (cholesterol esterase; sterol-ester acylhydrolase, EC 3.1.1.13) activity in the adrenal cortex but fails to activate cholesterol side-chain cleavage. The effect of the trypsinized material can therefore be qualitatively distinguished from that of ACTH-(1-24). When both ACTH-(1-24) and the digest are administered together, a synergistic increase in serum corticosterone concentration results. We propose that a portion of 16k fragment molecule may play a hormonal role in the control of adrenocortical steroidogenesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett H. P., Bullock G., Lowry P. J., McMartin C., Peters J. Fate of corticotrophins in an isolated adrenal-cell bioassay and decrease of peptide breakdown by cell purification. Biochem J. 1974 Feb;138(2):185–194. doi: 10.1042/bj1380185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergon L., Gallant S., Brownie A. C. Cholesterol side-chain cleavage activity and levels of high-spin cytochrome P-450 in adrenal-regeneration hypertension (ARH). Endocrinology. 1974 Feb;94(2):336–345. doi: 10.1210/endo-94-2-336. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cooper D. M., Schulster D. Apparent positive cooperativity of ACTH action on adrenocortical cells: the effect of hormone degradation. Mol Cell Endocrinol. 1977 Jan;6(3):211–216. doi: 10.1016/0303-7207(77)90087-9. [DOI] [PubMed] [Google Scholar]
- Crine P., Gianoulakis C., Seidah N. G., Gossard F., Pezalla P. D., Lis M., Chrétien M. Biosynthesis of beta-endorphin from beta-lipotropin and a larger molecular weight precursor in rat pars intermedia. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4719–4723. doi: 10.1073/pnas.75.10.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Analysis of the common precursor to corticotropin and endorphin. J Biol Chem. 1978 Aug 25;253(16):5732–5744. [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Existence of a common precursor to ACTH and endorphin in the anterior and intermediate lobes of the rat pituitary. J Supramol Struct. 1978;8(3):247–262. doi: 10.1002/jss.400080304. [DOI] [PubMed] [Google Scholar]
- Gasson J. C. Steroidogenic activity of high molecular weight forms of corticotropin. Biochemistry. 1979 Sep 18;18(19):4215–4224. doi: 10.1021/bi00586a028. [DOI] [PubMed] [Google Scholar]
- Gilkes J. J., Bloomfield G. A., Scott A. P., Lowry P. J., Ratcliffe J. G., Landon J., Rees L. H. Development and validation of a radioimmunoassay for peptides related to beta-melanocyte-stimulating hormone in human plasma: the lipotropins. J Clin Endocrinol Metab. 1975 Mar;40(3):450–457. doi: 10.1210/jcem-40-3-450. [DOI] [PubMed] [Google Scholar]
- Guillemin R., Vargo T., Rossier J., Minick S., Ling N., Rivier C., Vale W., Bloom F. beta-Endorphin and adrenocorticotropin are selected concomitantly by the pituitary gland. Science. 1977 Sep 30;197(4311):1367–1369. doi: 10.1126/science.197601. [DOI] [PubMed] [Google Scholar]
- Keutmann H. T., Eipper B. A., Mains R. E. Partial characterization of a glycoprotein comprising the NH2-terminal region of mouse tumor cell pro-adrenocorticotropic hormone/endorphin. J Biol Chem. 1979 Sep 25;254(18):9204–9208. [PubMed] [Google Scholar]
- Kramer R. E., Gallant S., Brownie A. C. The role of cytochrome P-450 in the action of sodium depletion on aldosterone biosynthesis in rats. J Biol Chem. 1979 May 25;254(10):3953–3958. [PubMed] [Google Scholar]
- Krieger D. T., Liotta A., Li C. H. Human plasma immunoreactive beta-lipotropin: correlation with basal and stimulated plasma ACTH concentrations. Life Sci. 1977 Dec 15;21(12):1771–1777. doi: 10.1016/0024-3205(77)90157-6. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A., Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Synthesis and secretion of corticotropins, melanotropins, and endorphins by rat intermediate pituitary cells. J Biol Chem. 1979 Aug 25;254(16):7885–7894. [PubMed] [Google Scholar]
- McMartin C., Purdon G. E., Schenkel L., Desaulles P. A., Maier R., Brugger M., Rittel W., Sieber P. Differences between in-vitro and in-vivo potencies of corticotrophins: an interpretation in terms of metabolic stability. J Endocrinol. 1977 Apr;73(1):79–89. doi: 10.1677/joe.0.0730079. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Pedersen R. C., Brownie A. C. Failure of ACTH to mimic the stress-induced activation of rat adrenocortical cholesterol ester hydrolase in vivo. J Steroid Biochem. 1979 Oct;11(4):1393–1400. doi: 10.1016/0022-4731(79)90112-2. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Khoo J. C., Steinberg D. Cholesterol esterase in rat adipose tissue and its activation by cyclic adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1975 Jun 25;250(12):4505–4511. [PubMed] [Google Scholar]
- Ramachandran J., Suyama A. T. Inhibition of replication of normal adrenocortical cells in culture by adrenocorticotropin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):113–117. doi: 10.1073/pnas.72.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: cell-free synthesis of the precursor and identification of corticotropin peptides in the molecule. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4826–4830. doi: 10.1073/pnas.74.11.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M., Stein S., Udenfriend S. Characterization of pro-opiocortin, a precursor to opioid peptides and corticotropin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):669–671. doi: 10.1073/pnas.75.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILBER R. H., BUSCH R. D., OSLAPAS R. Practical procedure for estimation of corticosterone or hydrocortisone. Clin Chem. 1958 Aug;4(4):278–285. [PubMed] [Google Scholar]
- STUDZINSKI G. P., HAY D. C., SYMINGTON T. Observations on the weight of the human adrenal gland and the effect of preparations of corticotropin of different purity on the weight and morphology of the human adrenal gland. J Clin Endocrinol Metab. 1963 Mar;23:248–254. doi: 10.1210/jcem-23-3-248. [DOI] [PubMed] [Google Scholar]
- Sayers G., Swallow R. L., Giordano N. D. An improved technique for the preparation of isolated rat adrenal cells: a sensitive, accurate and specfic method for the assay of ACTH. Endocrinology. 1971 Apr;88(4):1063–1068. doi: 10.1210/endo-88-4-1063. [DOI] [PubMed] [Google Scholar]
- Seelig S., Lindley B. D., Sayers G. A new approach to the structure-activity relationship for ACTH analogs using isolated adrenal cortex cells. Methods Enzymol. 1975;39:347–359. doi: 10.1016/s0076-6879(75)39031-9. [DOI] [PubMed] [Google Scholar]
- Segal B. M., Christy N. P. Potentiation of the biologic activity of ACTH by human plasma. A preliminary study. J Clin Endocrinol Metab. 1968 Oct;28(10):1465–1472. doi: 10.1210/jcem-28-10-1465. [DOI] [PubMed] [Google Scholar]
- Shanker G., Sharma R. K. beta-Endorphin stimulates corticosterone synthesis in isolated rat adrenal cells. Biochem Biophys Res Commun. 1979 Jan 15;86(1):1–5. doi: 10.1016/0006-291x(79)90373-5. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Nicholson W. E., Orth D. N. The nature of the immunoreactive lipotropins in human plasma and tissue extracts. J Clin Invest. 1978 Jul;62(1):94–104. doi: 10.1172/JCI109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E., Martin R., Voigt K. H. Corticotropin/beta-endorphin precursor: concomitant storage of its fragments in the secretory granules of anterior pituitary corticotropin/endorphin cells. Life Sci. 1979 Sep 24;25(13):1111–1118. doi: 10.1016/0024-3205(79)90132-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]



